Electrochemical Determination of Food Preservative Nitrite with Gold Nanoparticles/p-Aminothiophenol-Modified Gold Electrode
Abstract
:1. Introduction
2. Results
2.1. Fabrication of 4-ATP Polymer Film on an Au Electrode
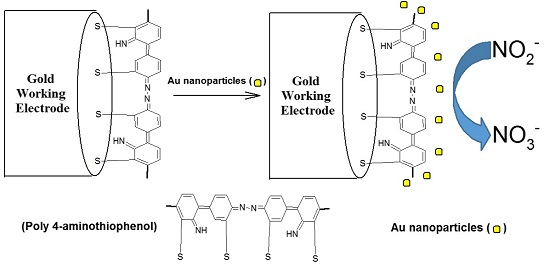
2.2. Electrodeposition of Au-Nanoparticles on a PATP-Coated Au Electrode
2.3. CV Characterization of the Modified Electrodes
2.4. SEM Images of an Au/PATP-Aunano Electrode
2.5. Electrochemical Impedance Spectroscopy (EIS) Applied to Modified Electrodes
2.6. Square Wave Voltammetric Response of the Recommended Sensor Electrode to NO2−
2.7. Colorimetric Sensor Response to NO2−
2.8. Real Sample Analysis
3. Discussion
4. Materials and Methods
4.1. Chemicals, Solutions, and Instruments
4.2. Optimization of Voltammetric Method
4.3. Gold Electrode Pre-Treatment
4.4. Electrochemical Polymerization of PATP Film
4.5. Electrodeposition of Au Nanoparticles on the Polymer Coated Electrode
4.6. Electrochemical Determinaion of NO2−
4.7. Preparation of Colorimetric Sensor for NO2− Determination
4.8. Application of Voltammetric and Colorimetric Sensors to Sausage Samples
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Alahakoon, A.U.; Jayasena, D.D.; Ramachandra, S.; Jo, C. Alternatives to nitrite in processed meat: Up to date. Trends Food Sci. Technol. 2015, 45, 37–49. [Google Scholar] [CrossRef]
- Parthasarathy, D.K.; Bryan, N.S. Sodium nitrite: The “cure” for nitric oxide insufficiency. Meat Sci. 2012, 92, 274–279. [Google Scholar] [CrossRef] [PubMed]
- Pegg, R.B.; Shahidi, F. Nitrite Curing of Meat; Food & Nutrition Press Inc.: Trumbull, CT, USA, 2008; pp. 1–268. [Google Scholar]
- Jo, C.; Ahn, H.J.; Son, J.H.; Lee, J.W.; Byun, M.W. Packaging and irradiation effect on lipid oxidation, color, residual nitrite content, and nitrosamine formation in cooked pork sausage. Food Control 2003, 14, 7–12. [Google Scholar] [CrossRef]
- Moorcroft, M.J.; Davis, J.; Compton, R.G. Detection and determination of nitrate and nitrite: A review. Talanta 2001, 54, 785–803. [Google Scholar] [CrossRef]
- Helaleh, M.I.H.; Korenaga, T. Ion chromatographic method for simultaneous determination of nitrate and nitrite in human saliva. J. Chromatogr. B 2000, 744, 433–437. [Google Scholar] [CrossRef]
- Horikoshi, N.; Nagamoto, S.; Kitagawa, T.; Kohzuma, T. Resonance raman spectroscopic studies of a copper-containing nitrite reductase from the Alcaligenes xylosoxidans NCIB 11015. J. Inorg. Biochem. 1997, 67, 46. [Google Scholar] [CrossRef]
- Nadzhafova, O.; Etienne, M.; Walcarius, A. Direct electrochemistry of hemoglobin and glucose oxidase in electrodeposited sol–gel silica thin films on glassy carbon. Electrochem. Commun. 2007, 9, 1189–1195. [Google Scholar] [CrossRef]
- Griess, P. Bemerkungen zu der Abhandlung der HH. Weselsky und Benedikt “Ueber einige Azoverbindungen”. Ber. Dtsch. Chem. Ges. 1879, 12, 426–428. [Google Scholar] [CrossRef]
- Wang, H.; Yang, W.; Liang, S.-C.; Zhang, Z.-M.; Zhang, H.-S. Spectrofluorimetric determination of nitrite with 5,6-diamino-1,3-naphthalene disulfonic acid. Anal. Chim. Acta 2000, 419, 169–173. [Google Scholar] [CrossRef]
- Zhang, X.; Wang, H.; Liang, S.-C.; Zhang, H.-S. Spectrofluorimetric determination of nitric oxide at trace levels with 5,6-diamino-1,3-naphthalene disulfonic acid. Talanta 2002, 56, 499–504. [Google Scholar] [CrossRef]
- Dombrowski, L.J.; Pratt, E.J. Fluorometric method for determining nanogram quantities of nitrite ion. Anal. Chem. 1972, 44, 2268–2272. [Google Scholar] [CrossRef]
- Camargo, A.; Aguirre, M.J.; Cheuquepán, W.; Chen, Y.-Y.; Ramírez, G. Electrooxidation of Nitrite Mediated by Cu-x-Tetraaminophenylporphyrin (x = 2, 3, and 4) Glassy Carbon-Modified Electrodes: Effect of Substituent Position. Electroanalysis 2008, 20, 2635–2641. [Google Scholar] [CrossRef]
- Da Rocha, J.R.C.; Angnes, L.; Bertotti, M.; Araki, K.; Toma, H.E. Amperometric detection of nitrite and nitrate at tetraruthenated porphyrin-modified electrodes in a continuous-flow assembly. Anal. Chim. Acta 2002, 452, 23–28. [Google Scholar] [CrossRef]
- Lin, C.-Y.; Balamurugan, A.; Lai, Y.-H.; Ho, K.-C. A novel poly(3,4-ethylenedioxythiophene)/iron phthalocyanine/multi-wall carbon nanotubes nanocomposite with high electrocatalytic activity for nitrite oxidation. Talanta 2010, 82, 1905–1911. [Google Scholar] [CrossRef] [PubMed]
- Armijo, F.; Goya, M.C.; Reina, M.; Canales, M.J.; Arévalo, M.C.; Aguirre, M.J. Electrocatalytic oxidation of nitrite to nitrate mediated by Fe(III) poly-3-aminophenyl porphyrin grown on five different electrode surfaces. J. Mol. Catal. A Chem. 2007, 268, 148–154. [Google Scholar] [CrossRef]
- Da Silva, S.M.; Mazo, L.H. Differential Pulse Voltammetric Determination of Nitrite with Gold Ultramicroelectrode. Electroanalysis 1998, 10, 1200–1203. [Google Scholar] [CrossRef]
- Jiang, Y.N.; Luo, H.Q.; Li, N.B. Determination of nitrite with a nano-gold modified glassy carbon electrode by cyclic voltammetry. Int. J. Environ. Anal. Chem. 2007, 87, 295–306. [Google Scholar] [CrossRef]
- Yang, W.; Bai, Y.; Li, Y.; Sun, C. Amperometric nitrite sensor based on hemoglobin/colloidal gold nanoparticles immobilized on a glassy carbon electrode by a titania sol-gel film. Anal. Bioanal. Chem. 2005, 382, 44–50. [Google Scholar] [CrossRef] [PubMed]
- Milhano, C.; Pletcher, D. The electrodeposition and electrocatalytic properties of copper–palladium alloys. J. Electroanal. Chem. 2008, 614, 24–30. [Google Scholar] [CrossRef]
- Davis, J.; Moorcroft, M.J.; Wilkins, S.J.; Compton, R.G.; Cardosi, M.F. Electrochemical detection of nitrate and nitrite at a copper modified electrode. Analyst 2000, 125, 737–741. [Google Scholar] [CrossRef]
- Agboola, B.O.; Ozoemena, K.I.; Nyokong, T. Electrochemical properties of benzylmercapto and dodecylmercapto tetra substituted nickel phthalocyanine complexes: Electrocatalytic oxidation of nitrite. Electrochim. Acta 2006, 51, 6470–6478. [Google Scholar] [CrossRef]
- Kozub, B.R.; Rees, N.V.; Compton, R.G. Electrochemical determination of nitrite at a bare glassy carbon electrode; why chemically modify electrodes? Sens. Actuators B 2010, 143, 539–546. [Google Scholar] [CrossRef]
- Cui, L.; Zhu, J.; Meng, X.; Yin, H.; Pan, X.; Ai, S. Controlled chitosan coated Prussian blue nanoparticles with the mixture of graphene nanosheets and carbon nanoshperes as a redox mediator for the electrochemical oxidation of nitrite. Sens. Actuators B 2012, 161, 641–647. [Google Scholar] [CrossRef]
- Li, X.-R.; Liu, J.; Kong, F.-Y.; Liu, X.-C.; Xu, J.-J.; Chen, H.-Y. Potassium-doped graphene for simultaneous determination of nitrite and sulfite in polluted water. Electrochem. Commun. 2012, 20, 109–112. [Google Scholar] [CrossRef]
- Mani, V.; Periasamy, A.P.; Chen, S.-M. Highly selective amperometric nitrite sensor based on chemically reduced graphene oxide modified electrode. Electrochem. Commun. 2012, 17, 75–78. [Google Scholar] [CrossRef]
- Wen, Z.-H.; Kang, T.-F. Determination of nitrite using sensors based on nickel phthalocyanine polymer modified electrodes. Talanta 2004, 62, 351–355. [Google Scholar] [CrossRef] [PubMed]
- Caro, C.A.; Bedioui, F.; Zagal, J.H. Electrocatalytic oxidation of nitrite on a vitreous carbon electrode modified with cobalt phthalocyanine. Electrochim. Acta 2002, 47, 1489–1494. [Google Scholar] [CrossRef]
- Abbaspour, A.; Mehrgardi, M.A. Electrocatalytic activity of Ce(III)–EDTA complex toward the oxidation of nitrite ion. Talanta 2005, 67, 579–584. [Google Scholar] [CrossRef] [PubMed]
- Pournaghi-Azar, M.H.; Dastangoo, H. Electrocatalytic oxidation of nitrite at an aluminum electrode modified by a chemically deposited palladium pentacyanonitrosylferrate film. J. Electroanal. Chem. 2004, 567, 211–218. [Google Scholar] [CrossRef]
- Winnischofer, H.; de Souza Lima, S.; Araki, K.; Toma, H.E. Electrocatalytic activity of a new nanostructured polymeric tetraruthenated porphyrin film for nitrite detection. Anal. Chim. Acta 2003, 480, 97–107. [Google Scholar] [CrossRef]
- Cardoso, W.S.; Gushikem, Y. Electrocatalytic oxidation of nitrite on a carbon paste electrode modified with Co(II) porphyrin adsorbed on SiO2/SnO2/Phosphate prepared by the sol–gel method. J. Electroanal. Chem. 2005, 583, 300–306. [Google Scholar] [CrossRef]
- Dai, Z.; Xu, X.; Ju, H. Direct electrochemistry and electrocatalysis of myoglobin immobilized on a hexagonal mesoporous silica matrix. Anal. Biochem. 2004, 332, 23–31. [Google Scholar] [CrossRef] [PubMed]
- Strehlitz, B.; Gründig, B.; Schumacher, W.; Kroneck, P.M.H.; Vorlop, K.-D.; Kotte, H. A Nitrite Sensor Based on a Highly Sensitive Nitrite Reductase Mediator-Coupled Amperometric Detection. Anal. Chem. 1996, 68, 807–816. [Google Scholar] [CrossRef] [PubMed]
- Golub, E.; Pelossof, G.; Freeman, R.; Zhang, H.; Willner, I. Electrochemical, Photoelectrochemical, and Surface Plasmon Resonance Detection of Cocaine Using Supramolecular Aptamer Complexes and Metallic or Semiconductor Nanoparticles. Anal. Chem. 2009, 81, 9291–9298. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.; Yang, G.; Meng, W.; Wu, L.; Zhu, A.; Jiao, X.A. An electrochemical impedimetric immunosensor for label-free detection of Campylobacter jejuni in diarrhea patients’ stool based on O-carboxymethylchitosan surface modified Fe3O4 nanoparticles. Biosens. Bioelectron. 2010, 25, 1204–1211. [Google Scholar] [CrossRef] [PubMed]
- Lin, H.; Ji, X.; Chen, Q.; Zhou, Y.; Banks, C.E.; Wu, K. Mesoporous-TiO2 nanoparticles based carbon paste electrodes exhibit enhanced electrochemical sensitivity for phenols. Electrochem. Commun. 2009, 11, 1990–1995. [Google Scholar] [CrossRef]
- Tel-Vered, R.; Yildiz, H.B.; Willner, I. Enhanced photocurrents generated by supramolecular relay/CdS-nanoparticle/electron-donor structures on gold electrodes. Adv. Mater. 2009, 21, 716–720. [Google Scholar] [CrossRef]
- Huang, X.; Li, Y.; Chen, Y.; Wang, L. Electrochemical determination of nitrite and iodate by use of gold nanoparticles/poly(3-methylthiophene) composites coated glassy carbon electrode. Sens. Actuators B 2008, 134, 780–786. [Google Scholar] [CrossRef]
- Saber-Tehrani, M.; Pourhabib, A.; Husain, S.W.; Arvand, M. A Simple and Efficient Electrochemical Sensor for Nitrite Determination in Food Samples Based on Pt Nanoparticles Distributed Poly(2-aminothiophenol) Modified Electrode. Food Anal. Methods 2013, 6, 1300–1307. [Google Scholar] [CrossRef]
- Zhang, L.; Jia, J.; Zou, X.; Dong, S. Simultaneous Determination of Dopamine and Ascorbic Acid at an In-Site Functionalized Self-Assembled Monolayer on Gold Electrode. Electroanalysis 2004, 16, 1413–1418. [Google Scholar] [CrossRef]
- Gopalan, A.I.; Lee, K.-P.; Manesh, K.M.; Santhosh, P.; Kim, J.H.; Kang, J.S. Electrochemical determination of dopamine and ascorbic acid at a novel gold nanoparticles distributed poly(4-aminothiophenol) modified electrode. Talanta 2007, 71, 1774–1781. [Google Scholar] [CrossRef] [PubMed]
- Lukkari, J.; Kleemola, K.; Meretoja, M.; Ollonqvist, T.; Kankare, J. Electrochemical Post-Self-Assembly Transformation of 4-Aminothiophenol Monolayers on Gold Electrodes. Langmuir 1998, 14, 1705–1715. [Google Scholar] [CrossRef]
- Gopalan, A.I.; Lee, K.-P.; Manesh, K.M.; Santhosh, P.; Kim, J.H. Gold nanoparticles dispersed into poly(aminothiophenol) as a novel electrocatalyst—Fabrication of modified electrode and evaluation of electrocatalytic activities for dioxygen reduction. J. Mol. Catal. A Chem. 2006, 256, 335–345. [Google Scholar] [CrossRef]
- Arbizzani, C.; Catellani, M.; Mastragostino, M.; Mingazzini, C. N- and P-doped Polydithieno[3,4-B:3′,4′-D] thiophene: A narrow band gap polymer for redox supercapacitors. Electrochim. Acta 1995, 40, 1871–1876. [Google Scholar] [CrossRef]
- Zhou, M.; Zhai, Y.; Dong, S. Electrochemical Sensing and Biosensing Platform Based on Chemically Reduced Graphene Oxide. Anal. Chem. 2009, 81, 5603–5613. [Google Scholar] [CrossRef] [PubMed]
- Gu, H.-Y.; Yu, A.-M.; Chen, H.-Y. Direct electron transfer and characterization of hemoglobin immobilized on a Au colloid–cysteamine-modified gold electrode. J. Electroanal. Chem. 2001, 516, 119–126. [Google Scholar] [CrossRef]
- Girija, T.C.; Sangaranarayanan, M.V. Investigation of polyaniline-coated stainless steel electrodes for electrochemical supercapacitors. Synth. Met. 2006, 156, 244–250. [Google Scholar] [CrossRef]
- Bradbury, C.R.; Zhao, J.; Fermin, D.J. Distance-Independent Charge-Transfer Resistance at Gold Electrodes Modified by Thiol Monolayers and Metal Nanoparticles. J. Phys. Chem. C 2008, 112, 10153–10160. [Google Scholar] [CrossRef]
- Analytical Methods Committee. Recommendations for the definition, estimation and use of the detection limit. Analyst 1987, 112, 199–204. [Google Scholar]
- Adekunlea, A.S.; Pillaya, J.; Ozoemena, K.I. Probing the electrochemical behaviour of SWCNT-cobalt nanoparticles and their electrocatalytic activities towards the detection of nitrite at acidic and physiological pH conditions. Electrochim. Acta 2010, 55, 4319–4327. [Google Scholar] [CrossRef]
- Miao, P.; Shen, M.; Ning, L.M.; Chen, G.F.; Yin, Y.M. Functionalization of platinum nanoparticles for electrochemical detection of nitrite. Anal. Bioanal. Chem. 2011, 399, 2407–2411. [Google Scholar] [CrossRef] [PubMed]
- Zhao, K.; Song, H.Y.; Zhuang, S.Q.; Dai, L.M.; He, P.G.; Fang, Y.Z. Determination of nitrite with the electrocatalytic property to the oxidation of nitrite on thionine modified aligned carbon nanotubes. Electrochem. Commun. 2007, 9, 65–70. [Google Scholar] [CrossRef]
- Liu, Y.; Gu, H.-Y. Amperometric detection of nitrite using a nanometer-sized gold colloid modified pretreated glassy carbon electrode. Microchim. Acta 2008, 162, 101–106. [Google Scholar] [CrossRef]
- Fanning, J.C. The chemical reduction of nitrate in aqueous solution. Coord. Chem. Rev. 2000, 199, 159–179. [Google Scholar] [CrossRef]
- Tütem, E.; Apak, R.; Baykut, F. Spectrophotometric determination of trace amounts of copper(I) and reducing agents with neocuproine in the presence of copper(II). Analyst 1991, 116, 89–94. [Google Scholar] [CrossRef]
- Marco, A.; Navarro, J.L.; Flores, M. The influence of nitrite and nitrate on microbial, chemical and sensory parameters of slow dry fermented sausage. Meat Sci. 2006, 73, 660–673. [Google Scholar] [CrossRef] [PubMed]
- Noel, P.; Briand, E.; Dumont, J.P. Role of nitrite in flavor development in uncooked cured meat-products—Sensory assessment. Meat Sci. 1990, 28, 1–8. [Google Scholar] [CrossRef]
- Skjelvale, R.; Tjaberg, T.B. Comparison of salami sausage produced with and without addition of sodium nitrite and sodium nitrate. J. Food Sci. 1974, 39, 520–524. [Google Scholar] [CrossRef]
- Yang, C.; Lu, Q.; Hu, S. A Novel Nitrite Amperometric Sensor and Its Application in Food Analysis. Electroanalysis 2006, 18, 2188–2193. [Google Scholar] [CrossRef]
- Wang, P.; Mai, Z.; Daia, Z.; Li, Y.; Zou, X. Construction of Au nanoparticles on choline chloride modified glassy carbon electrode for sensitive detection of nitrite. Biosens. Bioelectron. 2009, 24, 3242–3247. [Google Scholar] [CrossRef] [PubMed]
- Özkan, S.A.; Uslu, B.; Zuman, P. Electrochemical reduction and oxidation of the antibiotic cefepime at a carbon electrode. Anal. Chim. Acta 2002, 457, 265–274. [Google Scholar] [CrossRef]
- Üzer, A.; Can, Z.; Akın, İ.; Ercağ, E.; Apak, R. 4-Aminothiophenol Functionalized Gold Nanoparticle-Based Colorimetric Sensor for the Determination of Nitramine Energetic Materials. Anal. Chem. 2014, 86, 351–356. [Google Scholar] [CrossRef] [PubMed]
- Çelik, S.E.; Özyürek, M.; Güçlü, K.; Apak, R. Solvent effects on the antioxidant capacity of lipophilic and hydrophilic antioxidants measured by CUPRAC, ABTS/persulphate and FRAP methods. Talanta 2010, 81, 1300–1309. [Google Scholar] [CrossRef] [PubMed]









| Electrode Material | Method | LOD µM | Linear Range | Reference |
|---|---|---|---|---|
| EPPGE/SWCNT/Co and CoOx NP | Chronoamperometry | 5.6 | 32.3–189 µM | [51] |
| GE/AEBA/DPAN/PtNPs | Amperometry | 5 | 1–10 µM | [52] |
| ACNTs/thionin modified | Differential Pulse Voltammetry (DPV) | 1.12 | 3 × 10−6–5 × 10−4 mol·L−1 | [53] |
| GE/PATP-Ptnano | Cyclic Voltammetry (CV) | 1 | 3 µmol·L−1–1 mmol·L−1 | [40] |
| PGCE/nanometer-sized gold colloid/ethylenediamine | Amperometry | 45 | 1.3 × 10−4–4.4 × 10−2 mol·L−1 | [54] |
| GE/p-ATP-Aunano | Square Wave Voltammetry (SWV) | 2.6 | 0.5–50 mg·L−1 | Üzer et al. (Proposed method) |
| Method | Brand “A” | Brand “B” | Brand “C” |
|---|---|---|---|
| Voltammetric | 22.48 mg·L−1 | 5.83 mg·L−1 | 9.47 mg·L−1 |
| Colorimetric | 17.64 mg·L−1 | 5.18 mg·L−1 | 7.71 mg·L−1 |
| Method | Mean Conc. (mg·L−1) | SD (σ) | S a,b | t a,b | ttable b | F b | Ftable b |
|---|---|---|---|---|---|---|---|
| Voltammetric | 5.83 | 0.522 | - | - | - | - | - |
| Colorimetric | 5.18 | 0.276 | 0.418 | 2.402 | 3.355 | 0.278 | 6.39 |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Üzer, A.; Sağlam, Ş.; Can, Z.; Erçağ, E.; Apak, R. Electrochemical Determination of Food Preservative Nitrite with Gold Nanoparticles/p-Aminothiophenol-Modified Gold Electrode. Int. J. Mol. Sci. 2016, 17, 1253. https://doi.org/10.3390/ijms17081253
Üzer A, Sağlam Ş, Can Z, Erçağ E, Apak R. Electrochemical Determination of Food Preservative Nitrite with Gold Nanoparticles/p-Aminothiophenol-Modified Gold Electrode. International Journal of Molecular Sciences. 2016; 17(8):1253. https://doi.org/10.3390/ijms17081253
Chicago/Turabian StyleÜzer, Ayşem, Şener Sağlam, Ziya Can, Erol Erçağ, and Reşat Apak. 2016. "Electrochemical Determination of Food Preservative Nitrite with Gold Nanoparticles/p-Aminothiophenol-Modified Gold Electrode" International Journal of Molecular Sciences 17, no. 8: 1253. https://doi.org/10.3390/ijms17081253
APA StyleÜzer, A., Sağlam, Ş., Can, Z., Erçağ, E., & Apak, R. (2016). Electrochemical Determination of Food Preservative Nitrite with Gold Nanoparticles/p-Aminothiophenol-Modified Gold Electrode. International Journal of Molecular Sciences, 17(8), 1253. https://doi.org/10.3390/ijms17081253