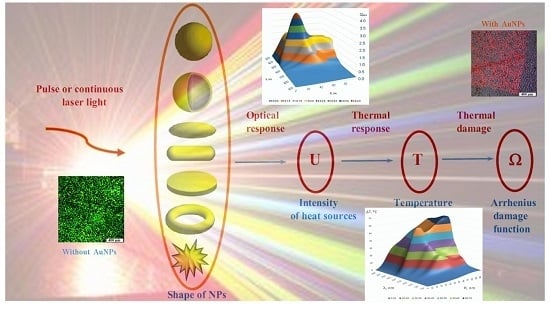
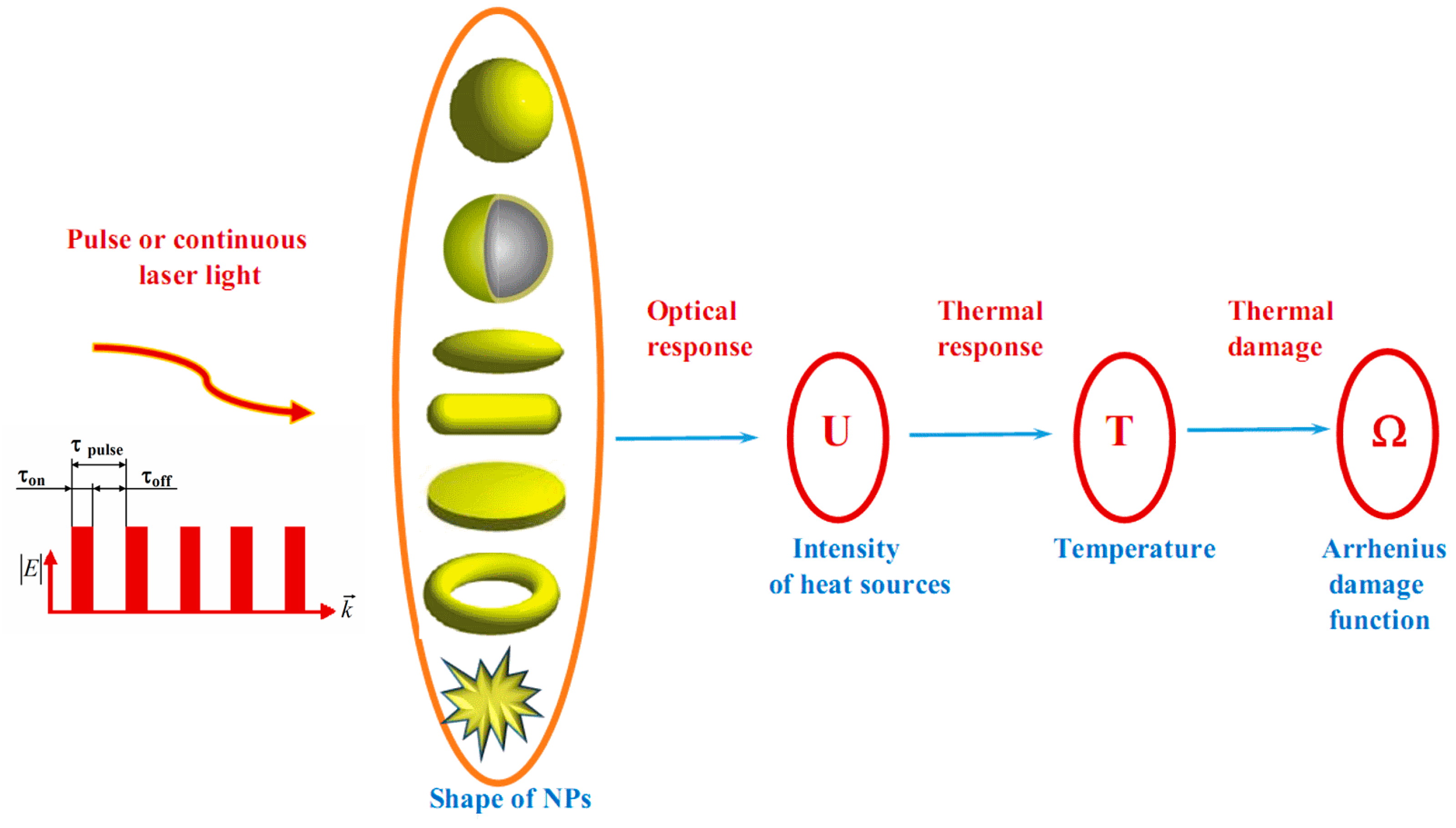
Towards Effective Photothermal/Photodynamic Treatment Using Plasmonic Gold Nanoparticles
Abstract
:1. Introduction
2. Nanocomposites Based on Au Nanoparticles and Nanoclusters
3. Multiscale Mathematical Modeling of Temperature Field of Tissues and Cells Doped by Plasmonic Nanoparticles
- (i)
- Macroscale model is valid for mean temperature fields analysis in the spatially extended regions of tissues doped by assembles of plasmonic nanoparticles. In this case, the values T, c, ρ, k in Equation (1) should be considered as corresponding variables averaged over physically small volumes containing, at the same time, a sufficiently large number of nanoparticles.
- (ii)
- Microscale model is valid for calculation of the small-scale spatial inhomogeneity of the temperature field within a nanoparticle itself and its vicinity. It means the exact local values of the variables T and other variables should be considered in Equation (1). This is important, e.g., for the study of cell membrane optoporation or transfection.
4. Arrhenius Damage Integral
5. PPT/PDT Pathogen Killing Using AuNPs
6. Photothermal and Photodynamic Therapy for Transplanted Tumors
7. AuNP Mediated Optoporation
8. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Huang, X.; El-Sayed, M.A. Plasmonic photo-thermal therapy (PPTT). Alex. J. Med. 2011, 47, 1–9. [Google Scholar] [CrossRef]
- Mackey, M.A.; Ali, M.R.K.; Austin, L.A.; Near, R.D.; El-Sayed, M.A. The most effective gold nanorod size for plasmonic photothermal therapy: Theory and in vitro experiments. J. Phys. Chem. B 2014, 118, 1319–1326. [Google Scholar] [CrossRef] [PubMed]
- Bergeron, E.; Boutopoulos, C.; Martel, R.; Torres, A.; Rodriguez, C.; Niskanen, J.; Lebrun, J.-J.; Winnik, F.M.; Sapieha, P.; Meunier, M. Cell-specific optoporation with near-infrared ultrafast laser and functionalized gold nanoparticles. Nanoscale 2015, 7, 17836–17847. [Google Scholar] [CrossRef] [PubMed]
- Krawinkel, J.; Torres-Mapa, M.L.; Werelius, K.; Heisterkamp, A.; Rüttermann, S.; Romanos, G.E.; Gerhardt-Szép, S. Gold nanoparticle-mediated delivery of molecules into primary human gingival fibroblasts using ns-laser pulses: A pilot study. Materials 2016, 9, 397. [Google Scholar] [CrossRef]
- Pissuwan, D.; Cortie, C.H.; Valenzuela, S.M.; Cortie, M.B. Functionalised gold nanoparticles for controlling pathogenic bacteria. Trends Biotechnol. 2009, 28, 207–213. [Google Scholar] [CrossRef] [PubMed]
- Tzarouchis, D.C.; Ylä-Oijala, P.; Ala-Nissila, T.; Sihvola, A. Shape effects on surface plasmons in spherical, cubic, and rod-shaped silver nanoparticles. Appl. Phys. A 2016, 122, 298. [Google Scholar] [CrossRef]
- Dreaden, E.C.; Austin, L.A.; Mackey, M.A.; El-Sayed, M.A. Size matters: Gold nanoparticles in targeted cancer drug delivery. Ther. Deliv. 2012, 3, 457–478. [Google Scholar] [CrossRef] [PubMed]
- Nabil, M.; Decuzzi, P.; Zunino, P. Modelling mass and heat transfer in nano-based cancer hyperthermia. R. Soc. Open Sci. 2015, 2, 150447. [Google Scholar] [CrossRef] [PubMed]
- Peeters, S.; Kitz, M.; Preisser, S.; Wetterwald, A.; Rothen-Rutishauser, B.; Thalmann, G.N.; Brandenberger, C.; Bailey, A.; Frenz, M. Mechanisms of nanoparticle-mediated photomechanical cell damage. Biomed. Opt. Express 2012, 3, 435–446. [Google Scholar] [CrossRef] [PubMed]
- Li, D.; Wang, G.X.; He, Y.L.; Wu, W.J.; Chen, B. A three-temperature model of selective photothermolysis for laser treatment of port wine stain containing large malformed blood vessels. Appl. Therm. Eng. 2014, 65, 308–321. [Google Scholar] [CrossRef]
- Clark, C.D.; Denton, M.L.; Thomas, R.J. Mathematical model that describes the transition from thermal to photochemical damage in retinal pigment epithelial cell culture. J. Biomed. Opt. 2011, 16, 020504. [Google Scholar] [CrossRef] [PubMed]
- Schomaker, M.; Heinemann, D.; Kalies, S.; Willenbrock, S.; Wagner, S.; Nolte, I.; Ripken, T.; Murua Escobar, H.; Meyer, H.; Heisterkamp, A. Characterization of nanoparticle mediated laser transfection by femtosecond laser pulses for applications in molecular medicine. J. Nanobiotechnol. 2015, 13, 10. [Google Scholar] [CrossRef] [PubMed]
- Schomaker, M.; Fehlauer, H.; Bintig, W.; Ngezahayo, A.; Nolte, I.; Murua Escobar, H.; Lubatschowski, H.; Heisterkamp, A. Fs-laser cell perforation using gold nanoparticles of different shapes. Proc. SPIE 2010. [Google Scholar] [CrossRef]
- Khlebtsov, B.N.; Panfilova, E.V.; Khanadeev, V.A.; Bibikova, O.A.; Terentyuk, G.S.; Ivanov, A.; Rumyantseva, V.; Shilov, I.; Ryabova, A.; Loshchenov, V.; et al. Nanocomposites containing silica-coated gold-silver nanocagesand Yb-2,4-dimethoxyhematoporphyrin: Multifunctional capability of IR-luminescence detection, photosensitization, and photothermolysis. ACS Nano 2011, 5, 7077–7089. [Google Scholar] [CrossRef] [PubMed]
- Khlebtsov, N.; Bogatyrev, V.; Dykman, L.; Khlebtsov, B.; Staroverov, S.; Shirokov, A.; Matora, L.; Khanadeev, V.; Pylaev, T.; Tsyganova, N.; et al. Analytical and theranostic applications of gold nanoparticles and multifunctional nanocomposites. Theranostics 2013, 3, 167–180. [Google Scholar] [CrossRef] [PubMed]
- Huang, W.-C.; Tsai, P.-J.; Chen, Y.-C. Functional gold nanoparticles as photothermal agents for selective-killing of pathogenic bacteria. Nanomedicine 2007, 2, 777–787. [Google Scholar] [CrossRef] [PubMed]
- Yin, R.; Agrawal, T.; Khan, U.; Gupta, G.K.; Rai, V.; Huang, Y.-Y.; Hamblin, M.R. Antimicrobial photodynamic inactivation in nanomedicine: Small light strides against bad bugs. Nanomedicine 2015, 10, 2379–2404. [Google Scholar] [CrossRef] [PubMed]
- Yoon, I.; Li, J.Z.; Shim, Y.K. Advance in photosensitizers and light delivery for photodynamic therapy. Clin. Endosc. 2013, 46, 7–23. [Google Scholar] [CrossRef] [PubMed]
- Jang, B.; Park, J.Y.; Tung, C.H.; Kim, I.H.; Choi, Y. Gold nanorod-photosensitizer complex for near-infrared fluorescence imaging and photodynamic/photothermal therapy in vivo. ACS Nano 2011, 5, 1086–1094. [Google Scholar] [CrossRef] [PubMed]
- Lin, J.; Wang, S.; Huang, P.; Wang, Z.; Chen, S.; Niu, G.; Li, W.; He, J.; Cui, D.; Lu, G.; et al. Photosensitizer-loaded gold vesicles with strong plasmonic coupling effect for imaging-guided photothermal/photodynamic therapy. ACS Nano 2013, 7, 5320–5329. [Google Scholar] [CrossRef] [PubMed]
- Abadeer, N.S.; Murphy, C.J. Recent progress in cancer thermal therapy using gold nanoparticles. J. Phys. Chem. C 2016, 120, 4691–4716. [Google Scholar] [CrossRef]
- Weissleder, R.A. Clearer vision for in vivo imaging. Nat. Biotechnol. 2001, 19, 316–317. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.; El-Sayed, I.H.; Qian, W.; El-Sayed, M.A. Cancer cell imaging and photothermal therapy in the near-infrared region by using gold nanorods. J. Am. Chem. Soc. 2006, 128, 2115–2120. [Google Scholar] [CrossRef] [PubMed]
- Hirsch, L.R.; Stafford, R.J.; Bankson, J.A.; Sershen, S.R.; Rivera, B.; Price, R.E.; Hazle, J.D.; Halas, N.J.; West, J.L. Nanoshell-mediated near-infrared thermal therapy of tumors under magnetic resonance guidance. Proc. Natl. Acad. Sci. USA 2003, 100, 13549–13554. [Google Scholar] [CrossRef] [PubMed]
- Skrabalak, S.E.; Chen, J.; Au, L.; Lu, X.; Li, X.; Xia, Y. Gold nanocages for biomedical applications. Adv. Mater. 2007, 19, 3177–3184. [Google Scholar] [CrossRef] [PubMed]
- Bagley, A.F.; Hill, S.; Rogers, G.S.; Bhatia, S.N. Plasmonic photothermal heating of intraperitoneal tumors through the use of an implanted near-infrared source. ACS Nano 2013, 7, 8089–8097. [Google Scholar] [CrossRef] [PubMed]
- Shanmugam, V.; Selvakumar, S.; Yeh, C.-S. Near-infrared light-responsive nanomaterials in cancer therapeutics. Chem. Soc. Rev. 2014, 43, 6254–6287. [Google Scholar] [CrossRef] [PubMed]
- Hauck, T.S.; Jennings, T.L.; Yatsenko, T.; Kumaradas, J.C.; Chan, W.C.W. Enhancing the toxicity of cancer chemotherapeutics with gold nanorod hyperthermia. Adv. Mater. 2008, 20, 3832–3838. [Google Scholar] [CrossRef]
- Sanchez, C.; Belleville, P.; Popall, M.; Nicole, L. Applications of advanced hybrid organic-inorganic nanomaterials: From laboratory to market. Chem. Soc. Rev. 2011, 40, 696–753. [Google Scholar] [CrossRef] [PubMed]
- Jiao, P.F.; Zhou, H.Y.; Chen, L.X.; Yan, B. Cancer-targeting multifunctionalized gold nanoparticles in imaging and therapy. Curr. Med. Chem. 2011, 18, 2086–2102. [Google Scholar] [CrossRef] [PubMed]
- Liang, R.; Wei, M.; Evans, D.G.; Duan, X. Inorganic nanomaterials for bioimaging, targeted drug delivery and therapeutics. Chem. Commun. 2014, 50, 14071–14081. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.; Zhang, X.; Pan, Y.; Miao, X.; Su, Z.; Wang, C.; Li, X. Fabrication of doxorubicin functionalized gold nanorod probes for combined cancer imaging and drug delivery. Dalton Trans. 2011, 40, 9789–9794. [Google Scholar] [CrossRef] [PubMed]
- Shen, J.; Kim, H.-C.; Mu, C.; Gentile, E.; Mai, J.; Wolfram, J.; Ji, L.; Ferrari, M.; Mao, Z.; Shen, H. Multifunctional gold nanorods for siRNA gene silencing and photothermal therapy. Adv. Healthc. Mater. 2014, 10, 1629–1637. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Tang, H.Y.; Yang, W.L.; Chen, J.Y. Aluminum phthalocyanine and gold nanorod conjugates: The combination of photodynamic therapy and photothermal therapy to kill cancer cells. J. Porphyr. Phthalocyanines 2012, 16, 802–808. [Google Scholar] [CrossRef]
- Zhang, Y.; Qian, J.; Wang, D.; Wang, Y.; He, S. Multifunctional gold nanorods with ultrahigh stability and tunability for in vivo fluorescence imaging, SERS detection, and photodynamic therapy. Angew. Chem. Int. Ed. 2013, 52, 1148–1151. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Huang, P.; Nie, L.; Xing, R.; Liu, D.; Wang, Z.; Lin, J.; Chen, S.; Niu, G.; Lu, G.; et al. Single continuous wave laser induced photodynamic/plasmonic photothermal therapy using photosensitizer-functionalized gold nanostars. Adv. Mater. 2013, 25, 3055–3061. [Google Scholar] [CrossRef] [PubMed]
- Khlebtsov, B.N.; Tuchina, E.S.; Khanadeev, V.A.; Panfilova, E.V.; Petrov, P.O.; Tuchin, V.V.; Khlebtsov, N.G. Enhanced photoinactivation of Staphylococcus aureus with nanocomposites containing plasmonic particles and hematoporphyrin. J. Biophotonics 2013, 6, 338–351. [Google Scholar] [CrossRef] [PubMed]
- Terentyuk, G.; Panfilova, E.; Khanadeev, V.; Chumakov, D.; Genina, E.; Bashkatov, A.; Tuchin, V.; Bucharskaya, A.; Maslyakova, G.; Khlebtsov, N.; et al. Gold nanorods with hematoporphyrin-loaded silica shell for dual-modality photodynamic and photothermal treatment of tumors in vivo. Nano Res. 2014, 7, 325–337. [Google Scholar] [CrossRef]
- Jin, R. Atomically precise metal nanoclusters: Stable sizes and optical properties. Nanoscale 2015, 7, 1549–1565. [Google Scholar] [CrossRef] [PubMed]
- Cabral, R.M.; Baptista, P.V. Anti-cancer precision theranostics: A focus on multifunctional gold nanoparticles. Expert Rev. Mol. Diagn. 2014, 14, 1041–1052. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Li, S.; Li, B.; Ren, X.; Li, S.; Mahounga, D.M.; Cui, S.; Gu, Y.; Achilefu, S. Folate-modified gold nanoclusters as near-infrared fluorescent probes for tumor imaging and therapy. Nanoscale 2012, 4, 6050–6064. [Google Scholar] [CrossRef] [PubMed]
- Ding, C.; Tian, Y. Gold nanocluster-based fluorescence biosensor for targeted imaging in cancer cells and ratiometric determination of intracellular pH. Biosens. Bioelectron. 2015, 65, 183–190. [Google Scholar] [CrossRef] [PubMed]
- Vankayala, R.; Kuo, C.-L.; Nuthalapati, K.; Chiang, C.-S.; Hwang, K. Nucleus-targeting gold nanoclusters for simultaneous in vivo fluorescence imaging, gene delivery, and NIR-light activated photodynamic therapy. Adv. Funct. Mater. 2015, 25, 5934–5945. [Google Scholar] [CrossRef]
- Zhang, C.; Li, C.; Liu, Y.; Zhang, J.; Bao, C.; Liang, S.; Wang, Q.; Yang, Y.; Fu, H.; Wang, K.; et al. Gold nanoclusters-based nanoprobes for simultaneous fluorescence imaging and targeted photodynamic therapy with superior penetration and retention behavior in tumors. Adv. Funct. Mater. 2015, 25, 1314–1325. [Google Scholar] [CrossRef]
- Pustovalov, V. Analytical modeling of the light-to-heat conversion by nanoparticle ensemble under radiation action. Adv. Mater. Sci. Appl. 2015, 4, 10–22. [Google Scholar] [CrossRef]
- Jasi’nski, M.; Majchrzak, E.; Turchan, L. Numerical analysis of the interactions between laser and soft tissues using generalized dual-phase lag equation. Appl. Math. Model. 2016, 40, 750–762. [Google Scholar] [CrossRef]
- Yakunin, A.N.; Avetisyan, Y.A.; Tuchin, V.V. Quantification of laser local hyperthermia induced by gold plasmonic nanoparticles. J. Biomed. Opt. 2015, 20, 051030. [Google Scholar] [CrossRef] [PubMed]
- Pustovalov, V.K.; Astafyeva, L.G.; Fritzsche, W. Plasmonic and thermooptical properties of spherical metallic nanoparticles for their thermoplasmonic and photonic applications. J. Nanopart. Res. 2014, 2014, 893459. [Google Scholar] [CrossRef]
- Pustovalov, V.K.; Astafyeva, L.G.; Fritzsche, W. Selection of thermo-optical parameter of nanoparticles for achievement of their maximal thermal energy under optical irradiation. Nano Energy 2013, 2, 1137–1141. [Google Scholar] [CrossRef]
- Mezeme, M.E.; Brosseau, C. Engineering nanostructures with enhanced thermoplasmonic properties for biosensing and selective targeting applications. Phys. Rev. E Stat. Nonlinear Soft Matter Phys. 2013, 87, 012722. [Google Scholar] [CrossRef] [PubMed]
- Letfullin, R.R.; George, T.F. Plasmonic nanomaterials for nanomedicine. In Handbook of Nanomaterials; Springer: Berlin, Germany, 2013; pp. 1063–1098. [Google Scholar]
- Yakunin, A.N.; Avetisyan, Y.A.; Bykov, A.A.; Tuchin, V.V. Spatio-temporal thermal processes induced by pulsed laser irradiation of medium doped by nanoparticles. In Proceedings of the IEEE International Conference on BioPhotonics (BioPhotonics), Florence, Italy, 20–22 May 2015; pp. 157–162.
- Avetisyan, Y.A.; Yakunin, A.N.; Tuchin, V.V. Novel thermal effect at nanoshell heating by pulsed laser irradiation: Hoop-shaped hot zone. J. Biophotonics 2012, 5, 734–744. [Google Scholar] [CrossRef] [PubMed]
- Avetisyan, Y.A.; Yakunin, A.N.; Tuchin, V.V. Thermal energy transfer by plasmon-resonant composite nanoparticles at pulse laser irradiation. Appl. Opt. 2012, 51, C88–C94. [Google Scholar] [CrossRef] [PubMed]
- Avetisyan, Y.A.; Yakunin, A.N.; Tuchin, V.V. On the problem of local tissue hyperthermia control: Multiscale modelling of pulsed laser radiation action on a medium with embedded nanoparticles. Quantum Electron. 2011, 40, 1081–1088. [Google Scholar] [CrossRef]
- Richardson, H.H.; Carlson, M.T.; Tandler, P.J.; Hernandez, P.; Govorov, A.O. Experimental and theoretical studies of light-to-heat conversion and collective heating effects in metal nanoparticle solutions. Nano Lett. 2009, 9, 1139–1146. [Google Scholar] [CrossRef] [PubMed]
- Tiwari, P.M.; Vig, K.; Dennis, V.A.; Singh, S.A. Functionalized gold nanoparticles and their biomedical applications. Nanomaterials 2011, 1, 31–63. [Google Scholar] [CrossRef]
- Rotello, V.M. Gold nanoparticles: Preparation, properties, and applications in bionanotechnology. Nanoscale 2012, 4, 1871–1880. [Google Scholar]
- Yeh, Y.-C.; Creran, B.; Dreaden, E.; Alkilany, A.M.; Huang, X.; Murphy, C.J.; El-Sayed, M.A. The golden age: Gold nanoparticles for biomedicine. Chem. Soc. Rev. 2012, 41, 2740–2779. [Google Scholar]
- Raghavendra, R.; Arunachalam, K.; Annamalai, S.K.; Arunachalam, A.M. Diagnostics and therapeutic application of gold nanoparticles. Int. J. Pharm. Pharm. Sci. 2014, 6, 74–87. [Google Scholar]
- Kuo, W.; Chang, C.N.; Chang, Y.T.; Yeh, C.S. Antimicrobial gold nanorods with dual-modality photodynamic inactivation and hyperthermia. Chem. Сommun. 2009, 32, 4853–4855. [Google Scholar] [CrossRef] [PubMed]
- Bresee, J.; Maier, K.E.; Boncella, M.E.; Melander, C.; Feldheim, D.L. Growth inhibition of Staphylococcus aureus by mixed monolayer gold nanoparticles. Small 2011, 7, 2027–2031. [Google Scholar] [CrossRef] [PubMed]
- Embleton, M.L.; Nair, S.P.; Cookson, B.D.; Wilson, M. Selective lethal photosensitization of methicillin-resistant Staphylococcus aureus using an IgG-tin (IV) chlorin e6 conjugate. Microb. Drug Resist. 2004, 10, 92. [Google Scholar] [CrossRef] [PubMed]
- Perni, S.; Piccirillo, C.; Kafizas, A.; Uppal, M.; Pratten, J.; Wilson, M.; Parkin, I.P. Antibacterial activity of light-activated silicone containing methylene blue and gold nanoparticles of different sizes. J. Clust. Sci. 2010, 21, 427–438. [Google Scholar] [CrossRef]
- Fazaeli, Y.; Amini, M.M.; Ashourion, H.; Heydari, H.; Majdabadi, A.; Jalilian, A.R.; Abolmaali, S. Grafting of a novel gold (III) complex on nanoporous MCM-41 and evaluation of its toxicity in Saccharomyces cerevisiae. Int. J. Nanomed. 2011, 6, 3251–3257. [Google Scholar]
- Zhu, X.; Xie, Y.; Zhang, Y.; Huang, H.; Huang, S.; Hou, L.; Zhang, H.; Li, Z.; Shi, J.; Zhang, Z. Photoinactivation of Candida albicans and Escherichia coli using aluminium phthalocyanine on gold nanoparticles. J. Biomater. Appl. 2014, 29, 769–779. [Google Scholar] [CrossRef] [PubMed]
- Mohd, A.S.; Tufail, S.; Khan, A.A.; Owais, M. Gold nanoparticle-photosensitizer conjugate based photodynamic inactivation of biofilm producing cells: Potential for treatment of C. albicans infection in BALB/c Mice. PLoS ONE 2015, 10, 1–20. [Google Scholar]
- Nombona, N.; Antunes, E.; Chidawanyika, W.; Kleyi, P.; Tshentu, Z.; Nyokong, T. Synthesis, photophysics and photochemistry of phthalocyanine-E-polylysine conjugates in the presence of metal nanoparticles against Staphylococcus aureus. J. Photochem. Photobiol. A 2012, 233, 24–33. [Google Scholar] [CrossRef]
- Jijie, R.; Dumych, T.; Chengnan, L.; Bouckaert, J.; Turcheniuk, K.; Hage, C.-H.; Heliot, L.; Cudennec, B.; Dumitrascu, N.; Boukherroub, R.; et al. Particle-based photodynamic therapy based on indocyanine green modified plasmonic nanostructures for inactivation of a Crohn’s disease-associated Escherichia coli strain. J. Mater. Chem. B 2016, 4, 2598–2605. [Google Scholar] [CrossRef]
- Ratto, F.; Tuchina, E.S.; Khlebtsov, B.N.; Centi, S.; Matteini, P.; Rossi, F.; Fusi, F.; Khlebtsov, N.G.; Pini, R.; Tuchin, V.V. Combined near infrared photothermolysis and photodynamic therapy by association of gold nanoparticles and an organic dye. In Proceedings of the Plasmonics in Biology and Medicine VIII, San Francisco, CA, USA, 22 January 2011; Volume 7911, p. 79111C.
- Tuchina, E.S.; Tuchin, V.V.; Khlebtsov, B.N.; Khlebtsov, N.G. Phototoxic effect of conjugates of plasmon-resonance nanoparticles with indocyanine green dye on Staphylococcus aureus induced by IR laser radiation. Quantum Electron. 2011, 41, 354–359. [Google Scholar] [CrossRef]
- Tuchina, E.S.; Petrov, P.O.; Kozina, K.V.; Ratto, F.; Centi, S.; Pini, R.; Tuchin, V.V. Using gold nanorods labelled with antibodies in photothermal impact of IR laser radiation on Staphylococcus aureus. Quantum Electron. 2014, 44, 683–688. [Google Scholar] [CrossRef]
- Tuchina, E.S.; Petrov, P.O.; Ratto, F.; Centi, S.; Pini, R.; Tuchin, V.V. The action of NIR (808 nm) laser radiation and gold nanorods labeled with IgA and IgG human antibodies on methicillin-resistant and methicillin sensitive strains of Staphylococcus aureus. In Proceedings of the Biophotonics and Immune Responses X; SPIE: Bellingham, WA, USA, 2015; Volume 9324, p. 93240X. [Google Scholar]
- Khlebtsov, B.N.; Tuchina, E.S.; Tuchin, V.V.; Khlebtsov, N.G. Multifunctional Au nanoclusters for targeted bioimaging and enhanced photodynamic inactivation of Staphylococcus aureus. RSC Adv. 2015, 5, 61639–61649. [Google Scholar] [CrossRef]
- Omar, G.S.; Wilson, M.; Nair, S.P. Lethal photosensitization of wound-associated microbes using indocyanine green and near-infrared light. BMC Microbiol. 2008, 8, 111–120. [Google Scholar] [CrossRef] [PubMed]
- Josefsen, L.B.; Boyle, R.W. Unique diagnostic and therapeutic roles of porphyrins and phthalocyanines in photodynamic therapy, imaging and theranostics. Theranostics 2012, 2, 916–966. [Google Scholar] [CrossRef] [PubMed]
- Xie, J.; Zheng, Y.; Ying, J.Y. Protein-directed synthesis of highly fluorescent gold nanoclusters. J. Am. Chem. Soc. 2009, 131, 888–889. [Google Scholar] [CrossRef] [PubMed]
- Kennedy, L.C.; Bickford, L.R.; Lewinski, N.A.; Coughlin, A.J.; Hu, Y.; Day, E.S.; West, J.L.; Drezek, R.A. A new era for cancer treatment: Gold-nanoparticle-mediated thermal therapies. Small 2011, 7, 169–183. [Google Scholar] [CrossRef] [PubMed]
- Sau, T.K.; Rogach, A.L.; Jackel, F.; Klar, T.A.; Feldmann, J. Properties and applications of colloidal nonspherical noble metal nanoparticles. Adv. Mater. 2010, 22, 1805–1825. [Google Scholar] [CrossRef] [PubMed]
- Xia, Y.; Li, W.; Cobley, C.M.; Chen, J.; Xia, X.; Zhang, Q.; Yang, M.; Cho, E.C.; Brown, P.K. Gold nanocages: From synthesis to theranostic applications. Acc. Chem. Res. 2011, 44, 914–924. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J. Biomedical applications of shape-controlled plasmonic nanostructures: A case study of hollow gold nanospheres for photothermal ablation therapy of cancer. J. Phys. Chem. Lett. 2010, 1, 686–695. [Google Scholar] [CrossRef]
- Gobin, A.M.; Lee, M.H.; Halas, N.J.; James, W.D.; Drezek, R.A.; West, J.L. Near-infrared resonant nanoshells for combined optical imaging and photothermal cancer therapy. Nano Lett. 2007, 7, 1929–1934. [Google Scholar] [CrossRef] [PubMed]
- Maltzahn, G.; Park, J.-H.; Agrawal, A.; Bandaru, N.K.; Das, S.K.; Sailor, M.J.; Bhatia, S.N. Computationally guided photothermal tumor therapy using long-circulating gold nanorod antennas. Cancer Res. 2009, 69, 3892–3900. [Google Scholar] [CrossRef] [PubMed]
- Hirn, S.; Semmler-Behnke, M.; Schleh, C.; Wenk, A.; Lipka, J.; Schäffler, M.; Takenaka, S.; Möller, W.; Schmid, G.; Simon, U.; et al. Particle size-dependent and surface charge-dependent biodistribution of gold nanoparticles after intravenous administration. Eur. J. Pharm. Biopharm. 2011, 77, 407–416. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Bai, R.; Yang, R.; Liu, J.; Tang, J.; Liu, Y.; Li, J.; Chaic, Z.; Chen, C. Size- and surface chemistry-dependent pharmacokinetics and tumor accumulation of engineered gold nanoparticles after intravenous administration. Metallomics 2015, 7, 516–524. [Google Scholar] [CrossRef] [PubMed]
- Terentyuk, G.S.; Maslyakova, G.N.; Khlebtsov, N.G.; Akchurin, G.G.; Tuchin, V.V.; Maksimova, I.L.; Khlebtsov, B.N.; Suleymanova, L.V. Laser-induced tissue hyperthermia mediated by gold nanoparticles: Toward cancer phototherapy. J. Biomed. Opt. 2009. [Google Scholar] [CrossRef] [PubMed]
- Bucharskaya, A.B.; Maslyakova, G.N.; Afanasyeva, G.A.; Terentyuk, G.S.; Navolokin, N.A.; Zlobina, O.V.; Chumakov, D.S.; Bashkatov, A.N.; Genina, E.A.; Khlebtsov, N.G.; et al. The morpho-functional assessment of plasmonic photothermal therapy effects on transplanted liver tumor. J. Innov. Opt. Health Sci. 2015, 8, 1541004. [Google Scholar] [CrossRef]
- Heinemann, D.; Schomaker, M.; Kalies, S.; Schieck, M.; Carlson, R.; Escobar, H.M.; Ripken, T.; Meyer, H.; Heisterkam, A. Gold nanoparticle mediated laser transfection for efficient siRNA mediated gene knock down. PLoS ONE 2013, 8, e58604. [Google Scholar] [CrossRef] [PubMed]
- Terentyuk, G.S.; Akchurin, G.G.; Maksimova, I.L.; Maslyakova, G.N.; Khlebtsov, N.G.; Tuchin, V.V. Cancer laser thermotherapy mediated by plasmonic nanoparticles. In Handbook of Photonics for Biomedical Science; Tuchin, V.V., Ed.; CRC Press, Taylor & Francis Group: London, UK, 2010; pp. 763–797. [Google Scholar]
- Zharov, V.P.; Galitovsky, V.; Viegas, M. Photothermal detection of local thermal effects during selective nanophotothermolysis. Appl. Phys. Lett. 2003, 83, 4897–4899. [Google Scholar] [CrossRef]
- Dressler, C.; Schwandt, D.; Beuthan, J.; Mildaziene, V.; Zabarylo, U.; Minet, O. Thermally induced changes of optical and vital parameters in human cancer cells. Laser Phys. Lett. 2010, 7, 817–823. [Google Scholar] [CrossRef]
- Pustovalov, V.K. Modeling of the processes of laser-nanoparticle interaction taking into account temperature dependences of parameters. Laser Phys. 2011, 21, 906–912. [Google Scholar] [CrossRef]
- Hleb, E.Y.; Hafner, J.H.; Myers, J.N.; Hanna, E.Y.; Rostro, B.C.; Zhdanok, S.A.; Lapotko, D.O. LANTCET: Elimination of solid tumor cells with photothermal bubbles generated around clusters of gold nanoparticles. Nanomedicine 2008, 3, 647–667. [Google Scholar] [CrossRef] [PubMed]
- Lukianova-Hleb, E.; Hu, Y.; Latterini, L.; Tarpani, L.; Lee, S.; Drezek, R.A.; Hafner, J.H.; Lapotko, D.O. Plasmonic nanobubbles as transient vapor nanobubbles generated around plasmonic nanoparticles. ACS Nano 2010, 4, 2109–2123. [Google Scholar] [CrossRef] [PubMed]
- Arita, Y.; Ploschner, M.; Antkowiak, M.; Gunn-Moore, F.; Dholakia, K. Laser-induced breakdown of an optically trapped gold nanoparticle for single cell transfection. Opt. Lett. 2013, 38, 3402–3405. [Google Scholar] [CrossRef] [PubMed]
- Qin, Z.P.; Bischof, J.C. Thermophysical and biological responses of gold nanoparticle laser heating. Chem. Soc. Rev. 2012, 41, 1191–1217. [Google Scholar] [CrossRef] [PubMed]
- Lukianova-Hleb, E.Y.; Mutonga, M.B.G.; Lapotko, D.O. Cell-specific multifunctional processing of heterogeneous cell systems in a single laser pulse treatment. ACS Nano 2012, 6, 10973–10981. [Google Scholar] [CrossRef] [PubMed]
- Boulais, E.; Lachaine, R.; Hatef, A.; Meunier, M. Plasmonics for pulsed-laser cell nanosurgery: Fundamentals and applications. J. Photochem. Photobiol. C Photochem. Rev. 2013, 17, 26–49. [Google Scholar] [CrossRef]
- Umebayashi, Y.; Miyamoto, Y.; Wakita, M.; Kobayashi, A.; Nishisaka, T. Elevation of plasma membrane permeability on laser irradiation of extracellular latex particles. J. Biochem. 2003, 134, 219–224. [Google Scholar] [CrossRef] [PubMed]
- Pitsillides, C.M.; Joe, E.K.; Wei, X.; Anderson, R.R.; Lin, C.P. Selective cell targeting with light-absorbing microparticles and nanoparticles. Biophys. J. Cell 2003, 84, 4023–4032. [Google Scholar] [CrossRef]
- Schomaker, M.; Killian, D.; Willenbrock, S.; Heinemann, D.; Kalies, S.; Ngezahayo, A.; Nolte, I.; Ripken, T.; Junghanß, C.; Meyer, H.; et al. Biophysical effects in off-resonant gold nanoparticle mediated (GNOME) laser transfection of cell lines, primary- and stem cells using fs laser pulses. J. Biophotonics 2015, 8, 646–658. [Google Scholar] [CrossRef] [PubMed]
- Kalies, S.; Antonopoulos, G.C.; Rakoski, M.S.; Heinemann, D.; Schomaker, M.; Ripken, T.; Meyer, H. Investigation of biophysical mechanisms in gold nanoparticle mediated laser manipulation of cells using a multimodal holographic and fluorescence imaging setup. PLoS ONE 2015, 10, e0124052. [Google Scholar] [CrossRef] [PubMed]
- Kalies, S.; Keil, S.; Sender, S.; Hammer, S.C.; Antonopoulos, G.C.; Schomaker, M.; Ripken, T.; Escobar, H.M.; Meyer, H.; Heinemann, D. Characterization of the cellular response triggered by gold nanoparticle–mediated laser manipulation. J. Biomed. Opt. 2015. [Google Scholar] [CrossRef] [PubMed]
- Yao, C.; Rahmanzadeh, R.; Endl, E.; Zhang, Z.; Gerdes, J.; Hüttmann, G. Elevation of plasma membrane permeability by laser irradiation of selectively bound nanoparticles. J. Biomed. Opt. 2005, 10, 064012. [Google Scholar] [CrossRef] [PubMed]
- Yao, C.; Qu, X.; Zhang, Z.; Hüttmann, G.; Rahmanzadeh, R. Influence of laser parameters on nanoparticle-induced membrane permeabilization. J. Biomed. Opt. 2009, 14, 054034. [Google Scholar] [CrossRef] [PubMed]
- Baumgart, J.; Humbert, L.; Boulais, É.; Lachaine, R.; Lebrun, J.J.; Meunier, M. Off-resonance plasmonic enhanced femtosecond laser optoporation and transfection of cancer cells. Biomaterials 2012, 33, 2345–2350. [Google Scholar]
- St-Louis Lalonde, B.; Boulais, É.; Lebrun, J.-J.; Meunier, M. Visible and near infrared resonance plasmonic enhanced nanosecond laser optoporation of cancer cells. Biomed. Opt. Express 2013, 4, 490–499. [Google Scholar] [CrossRef] [PubMed]
- Bartczak, D.; Muskens, O.L.; Millar, T.M.; Sanchez-Elsner, T.; Kanaras, A.G. Laser-induced damage and recovery of plasmonically targeted human endothelial cells. Nano Lett. 2011, 20, 1358–1363. [Google Scholar] [CrossRef] [PubMed]
- Palankar, R.; Pinchasik, B.-E.; Khlebtsov, B.N.; Kolesnikova, T.A.; Mohwald, H.; Winterhalter, M.; Skirtach, A.G. Nanoplasmonically-induced defects in lipid membrane monitored by ion current: transient nanopores versus membrane rupture. Nano Lett. 2014, 14, 4273–4279. [Google Scholar] [CrossRef] [PubMed]
- Bibikova, O.; Singh, P.; Popov, A.; Akchurin, G.; Skovorodkin, I.; Khanadeev, V.; Kinnunen, M.; Bogatyrev, V.; Khlebtsov, N.; Vainio, S.; et al. The effect of laser irradiation on living cells incubated with gold nanoparticles. Proc. SPIE 2015. [Google Scholar] [CrossRef]
- Bibikova, O.; Singh, P.; Popov, A.; Akchurin, G.; Skaptsov, A.; Skovorodkin, I.; Khanadeev, V.; Mikhalevich, D.; Kinnunen, M.; Akchurin, G.; et al. Shape-dependent interaction of plasmonic gold nanoparticles with cultured cells at laser exposure. J. Biomed. Opt. 2016. submitted. [Google Scholar]
- Yuan, H.; Khoury, C.G.; Hwang, H.; Wilson, C.M.; Grant, G.A.; Vo-Dinh, T. Gold nanostars: surfactant-free synthesis, 3D modelling, and two-photon photoluminescence imaging. Nanotechnology 2012, 23, 075102. [Google Scholar] [CrossRef] [PubMed]
- Grabar, K.C.; Freeman, R.G.; Hommer, M.B.; Natan, M.J. Preparation and characterization of Au colloid monolayers. Anal. Chem. 1995, 67, 735–743. [Google Scholar] [CrossRef]
- Khlebtsov, B.; Khanadeev, V.; Pylaev, T.; Khlebtsov, N. New T-matrix solvable model for nanorods: TEM-based ensemble simulations supported by experiments. J. Phys. Chem. C 2011, 115, 6317–6323. [Google Scholar] [CrossRef]
- Khlebtsov, B.; Khanadeev, V.; Panfilova, E.; Pylaev, T.; Bibikova, O.; Staroverov, S.; Bogatyrev, V.; Dykman, L.; Khlebtsov, N. New types of nanomaterials: Powders of gold nanospheres, nanorods, nanostars and gold-silver nanocages. Nanotechnol. Russ. 2013, 8, 209–219. [Google Scholar] [CrossRef]
- Khlebtsov, N.; Dykman, L. Biodistribution and toxicity of engineered gold nanoparticles: A review of in vitro and in vivo studies. Chem. Soc. Rev. 2011, 40, 1647–1671. [Google Scholar] [CrossRef] [PubMed]
- Fan, Z.; Liu, H.; Mayer, M.; Deng, C.X. Spatiotemporally controlled single cell sonoporation. Proc. Natl. Acad. Sci. 2012, 109, 16486–16491. [Google Scholar] [CrossRef] [PubMed]
- Bibikova, O.; Popov, A.; Skovorodkin, I.; Prilepskyi, A.; Pylaev, T.; Bykov, A.; Staroverov, S.; Bogatyrev, V.; Tuchin, V.; Kinnunen, M.; et al. Plasmon-resonant gold nanoparticles with variable morphology as optical labels and drug carriers for cytological research. Proc. SPIE 2013. [Google Scholar] [CrossRef]
- Bibikova, O.; Popov, A.; Bykov, A.; Fales, A.; Yuan, H.; Skovorodkin, I.; Kinnunen, M.; Vainio, S.; Vo-Dinh, T.; Tuchin, V.; et al. Plasmon-resonant gold nanostars with variable size as contrast agents for imaging applications. IEEE J. Sel. Top. Quantum Electron. 2016, 22, 1–8. [Google Scholar] [CrossRef]
- Jain, P.K.; Lee, K.S.; El-Sayed, I.H.; El-Sayed, M.A. Calculated absorption and scattering properties of gold nanoparticles of different size, shape, and composition: Applications in biological imaging and biomedicine. J. Phys. Chem. B 2006, 110, 7238–7248. [Google Scholar] [CrossRef] [PubMed]
- Kalies, S.; Heinemann, D.; Schomaker, M.; Gentemann, L.; Meyer, H.; Ripken, T. Immobilization of gold nanoparticles on cell culture surfaces for safe and enhanced gold nanoparticle-mediated laser transfection. J. Biomed. Opt. 2014, 19, 70505. [Google Scholar] [CrossRef] [PubMed]
- Heinemann, D.; Kalies, S.; Schomaker, M.; Ertmer, W.; Escobar, H.M.; Meyer, H.; Ripken, T. Delivery of proteins to mammalian cells via gold nanoparticle mediated laser transfection. Nanotechnology 2014, 25, 245101. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.-C.; Lin, Y.-P.; Wang, C.-W.; Tzeng, H.-C.; Wu, C.-H.; Chen, Y.-C.; Chen, C.-P.; Chen, L.-C.; Wu, Y.-C. DNA-gold nanorod conjugates for remote control of localized gene expression by near infrared irradiation. J. Am. Chem. Soc. 2006, 128, 3709–3715. [Google Scholar] [CrossRef] [PubMed]
- Antkowiak, M.; Dholakia, K.; Gunn-Moore, F. Optical transfection of mammalian cells. In Understanding Biophotonics: Fundamentals, Advances and Applications; Tsia, K., Ed.; CRC Press, Taylor & Francis Group: London, UK, 2014; pp. 693–734. [Google Scholar]
- Gu, L.; Koymen, A.R.; Mohanty, S.K. Crystalline magnetic carbon nanoparticle assisted photothermal delivery into cells using CW near-infrared laser beam. Sci. Rep. 2014, 4, 5106. [Google Scholar] [CrossRef] [PubMed]
- Arita, Y.; Torres-Mapa, M.L.; Lee, W.M.; Čižmár, T.; Campbell, P.; Gunn, F.J.; Dholakia, K. Spatially optimized gene transfection by laser-induced breakdown of optically trapped nanoparticles. Appl. Phys. Lett. 2011, 98, 093702. [Google Scholar] [CrossRef]











| Abbr. | Nanoparticle Shape | Photosensitizer (PS) | Functional Component | Average Size, nm | Type of Radiation | Maximal Inhibition of S. aureus 209 P after 30 Min-Light Exposure; CFU, % (Reference) |
|---|---|---|---|---|---|---|
| AuNRd1 | Nanorods | ICG | – | 30 × 10 | 808 nm, 50 mW/cm2 | 65 [70] |
| AuNS | Nanoshells | ICG | – | 140 | 805 nm, 46 mW/cm2 | 55 [71] |
| AuNCg | Nanocages | ICG | – | 53 | 808 nm, 60 mW/cm2 | 64 [71] |
| AuNR2 | Nanorods | HP | – | 50 × 10 | 808 nm, 100 mW/cm2 | 90 [37] |
| AuNCg2 | Nanocages | HP | – | 50 | 625 nm, 100 mW/cm2 | 97 [37] |
| AuNR3 | Nanorods | – | FcIgA, FcIgG | 45 × 13 | 808 nm, 100 mW/cm2 | 95 [72,73] |
| AuNCl | Nanoclusters | PhS | BSA + IgG | 1.8 (25 Au atoms) | 660 nm, 50 mW/cm2 | 90 [74] |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bucharskaya, A.; Maslyakova, G.; Terentyuk, G.; Yakunin, A.; Avetisyan, Y.; Bibikova, O.; Tuchina, E.; Khlebtsov, B.; Khlebtsov, N.; Tuchin, V. Towards Effective Photothermal/Photodynamic Treatment Using Plasmonic Gold Nanoparticles. Int. J. Mol. Sci. 2016, 17, 1295. https://doi.org/10.3390/ijms17081295
Bucharskaya A, Maslyakova G, Terentyuk G, Yakunin A, Avetisyan Y, Bibikova O, Tuchina E, Khlebtsov B, Khlebtsov N, Tuchin V. Towards Effective Photothermal/Photodynamic Treatment Using Plasmonic Gold Nanoparticles. International Journal of Molecular Sciences. 2016; 17(8):1295. https://doi.org/10.3390/ijms17081295
Chicago/Turabian StyleBucharskaya, Alla, Galina Maslyakova, Georgy Terentyuk, Alexander Yakunin, Yuri Avetisyan, Olga Bibikova, Elena Tuchina, Boris Khlebtsov, Nikolai Khlebtsov, and Valery Tuchin. 2016. "Towards Effective Photothermal/Photodynamic Treatment Using Plasmonic Gold Nanoparticles" International Journal of Molecular Sciences 17, no. 8: 1295. https://doi.org/10.3390/ijms17081295
APA StyleBucharskaya, A., Maslyakova, G., Terentyuk, G., Yakunin, A., Avetisyan, Y., Bibikova, O., Tuchina, E., Khlebtsov, B., Khlebtsov, N., & Tuchin, V. (2016). Towards Effective Photothermal/Photodynamic Treatment Using Plasmonic Gold Nanoparticles. International Journal of Molecular Sciences, 17(8), 1295. https://doi.org/10.3390/ijms17081295