Abstract
Octamer-binding transcription factor 4 (Oct4) is a member of POU (Pit-Oct-Unc) transcription factor family Class V that plays a crucial role in maintaining the pluripotency and self-renewal of stem cells. Though it has been deeply investigated in mammals, its lower vertebrate homologue, especially in the marine fish, is poorly studied. In this study, we isolated the full-length sequence of Paralichthys olivaceus pou5f3 (Popou5f3), and we found that it is homologous to mammalian Oct4. We identified two transcript variants with different lengths of 3′-untranslated regions (UTRs) generated by alternative polyadenylation (APA). Quantitative real-time RT-PCR (qRT-PCR), in situ hybridization (ISH) and immunohistochemistry (IHC) were implemented to characterize the spatial and temporal expression pattern of Popou5f3 during early development and in adult tissues. Our results show that Popou5f3 is maternally inherited, abundantly expressed at the blastula and early gastrula stages, then greatly diminishes at the end of gastrulation. It is hardly detectable from the heart-beating stage onward. We found that Popou5f3 expression is restricted to the adult gonads, and continuously expresses during oogenesis while its dynamics are downregulated during spermatogenesis. Additionally, numerous cis-regulatory elements (CRE) on both sides of the flanking regions show potential roles in regulating the expression of Popou5f3. Taken together, these findings could further our understanding of the functions and evolution of pou5f3 in lower vertebrates, and also provides fundamental information for stem cell tracing and genetic manipulation in Paralichthys olivaceus.
1. Introduction
Octamer-binding transcription factor 4 (Oct4) is a member of the POU transcription factor family Class V, which is essential for maintaining the pluripotency and self-renewal of stem cells. The basic feature of Oct4 is a highly conserved POU domain with two structurally independent subdomains, POUs and POUh [1]. As with other Oct proteins, Oct4 can recognize and bind to the octamer motif through its DNA-binding segments on the POU domain to activate or inhibit the transcription of downstream target genes [2]. It can also cooperatively interact with Sox2 to form complexes by binding to Oct-Sox motif to drive pluripotent-specific gene expression [3,4]. In fact, pluripotent stem cell identity is governed by the combinatorial control of transcriptional modulators Oct4, Sox2 and Nanog, and imbalance of any of them causes cells to enter a specific differentiation program [5,6,7,8].
In mice, Oct4/Pou5f1 is highly expressed in pluripotent cells and germ cells of the early embryo. The expression of Oct4/Pou5f1 is activated at the four-cell to morula stage and maintained in the inner cell mass (ICM) of the blastocyst while rapidly downregulated in the trophectoderm. After implantation, Oct4/Pou5f1 is differentially expressed in the epiblast, decreased during gastrulation and exclusively confined to the primordial germ cells (PGCs) [9,10,11]. Although Oct4-deficient mouse embryos are able to develop to the blastocyst stage, their ICMs are not pluripotent and differentiate into trophectoderm [12]. A specific expression of Oct4 among mouse adult tissues is observed in the germ stem cells [13,14]. In contrast to the ICM localization of Oct4 in mammals, the homologue of Oct4 in lower teleosts, which was recently renamed pou5f3, is spatially expressed throughout the blastomere from fertilized egg to gastrulae [15,16,17,18,19]. In addition to pluripotency regulation, zebrafish pou5f3 (originally named pou2/spg) also has some new roles as well as partial function loss when compared with other species. It has a different expression pattern after the gastrula stage, which is expressed in developing brain and plays an important role in regionalization [16]. Moreover, the expression of pou5f3 has not been detected in PGCs, suggesting that it is not a necessary factor for PGC development in zebrafish [20]. Though minor differences exist between zebrafish and medaka model fish systems, medaka pou5f3 (previously called oct4/pou2) shows a similar expression pattern with mammalian Oct4 [17,21]. A study found that the expression of pou5f3 in differentiated medaka embryonic stem cells (ESCs) is reduced more than four-fold compared to their level in undifferentiated state ESCs, which reveals its crucial role in maintaining stem cell pluripotency [22]. Moreover, medaka pou5f3 is able to reprogram somatic cells into pluripotent cells [23].
Paralichthys olivaceus, popularly known as Japanese flounder, is a mariculture flatfish species with ecological and economical importance in China. Over years of improved variety selection and breeding, new demands for the protection of genetic resources have been made. Fish embryonic stem (ES) cell lines have been demonstrated to have many common features compared to mammalian ESCs, that can be serially passaged to a stable cell line and differentiate into various types of cells, including germ cells [24]. Thus, pluripotent stem cells are considered to be the best medium for the preservation of improved varieties due to their good biological characteristics. Thus far, several ES (or ES-like) cell lines have been successfully established by using the blastula stage embryos in medaka, gilthead sea bream, sea bass, perch, India carp and Pacific cod [25,26,27,28,29,30]. However, there are as of yet no reports on Japanese flounder stem cells and their manipulation lacks theoretical reference. Therefore, the analysis of stem cell regulators that are homologous to mammals in flounder will be helpful. In this study, we identified and characterized the sequence features and expression patterns of pou5f3 to lay a foundation for further investigation in P. olivaceus.
2. Results
2.1. Molecular Characterization and Homological Analysis of P. olivaceus pou5f3
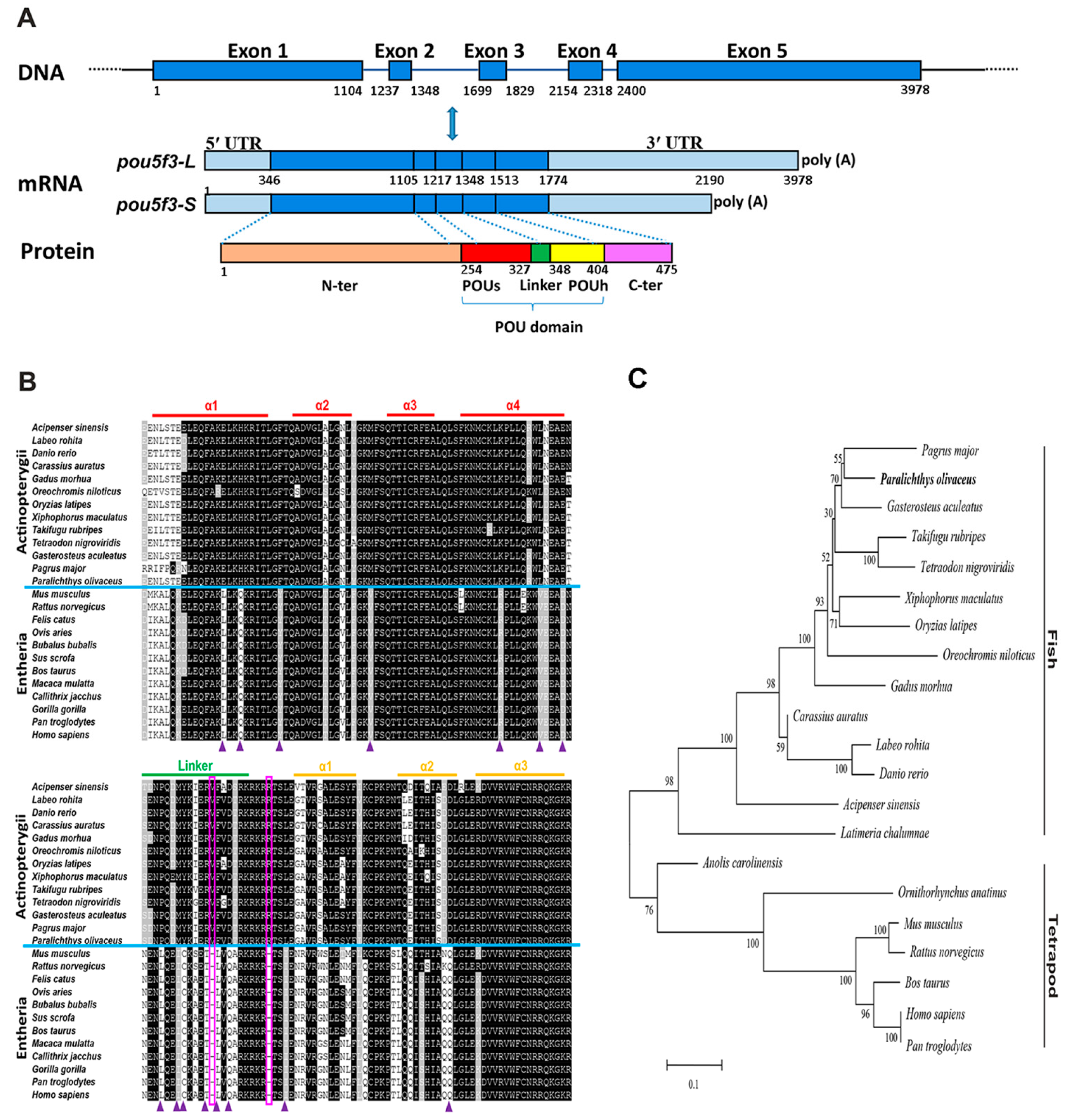
We have cloned P. olivaceus pou5f3 from the blastula-stage embryos through the homology cloning method and the rapid-amplification of cDNA ends (RACE) strategy. It exhibited two transcript isoforms with an identical 5′-UTR (345 bp length) and different lengths of 3′-UTR, which are 874 and 1318 bp long, respectively. The deduced PoPou5f3 contained an open reading frame (ORF) with 1428 bp encoding a protein of 475 amino acids with a calculated molecular mass (MM) of 51.98 kDa and a theoretical isoelectric point (PI) of 6.28 (GenBank accession number: KJ522774) (Figure 1A and Figure S1). Similar to other previously identified Class V POU proteins, the predicted PoPou5f3 possessed a characteristic POU domain consisting of the POU specific domain, the POU homeodomain and a linker region between them (Figure 1 and Figure S2).
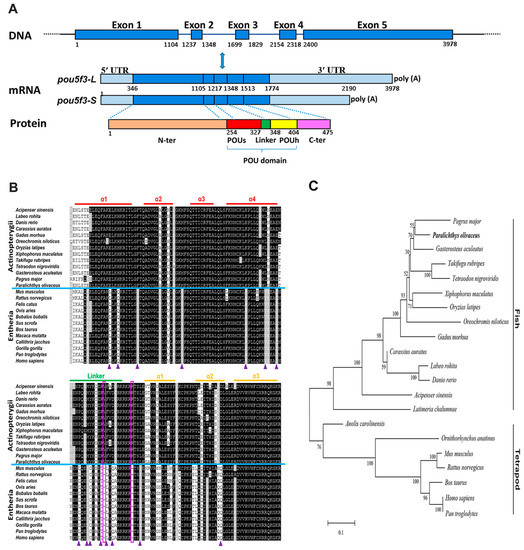
Figure 1.
Sequence analysis of pou5f3 gene in Japanese flounder. (A) The pou5f3 gene is composed of five exons and four introns. Two isoforms of the pou5f3 mRNA transcript (pou5f3-L and pou5f3-S) have different lengths of 3′-UTR. The schematic diagram was drawn to scale and the relative sequence numbers were indicated. UTRs (light blue); exons (dark blue). N-ter, N-terminal domain (orange); POUs, POU specific domain (red); Linker, Linker domain (green); POUh, POU homeodomain (yellow); C-ter, C-terminal domain (purple); (B) Amino acid sequence alignment of the POU domains from POUV proteins in various vertebrates. Identical residues and conservative substitutions are indicated in black and gray, respectively. The upper lines indicate the amino acid sequences stretched for α-helix of the POUs and POUh domains, as well as the linker region. The solid triangles and the two rectangles indicate residues mentioned in subsection 3.1; and (C) Phylogenetic analysis of PoPou5f3 and other POUV proteins. The tree was constructed by MEGA 6.0 using Poisson correction distance based upon the neighbor-joining (NJ) method. The Paralichthys olivaceus Pou5f3 is highlighted in bold. The numbers at the nodes corresponding to the bootstrap support are expressed as the percentage of 1000 replicates. The GenBank accession numbers or Ensembl IDs of analyzed sequences are listed in Table S1.
The analysis for sequence homology showed that PoPou5f3 has 37.2% to 81.5% amino acid sequence identities and the conserved POU domain of PoPou5f3 showed 68.5% to 97.4% identities to those from other vertebrates (Table 1). The alignment of PoPou5f3 with other POUV proteins from mammals, avian, reptile, amphibian, and teleosts showed that conservation was high at the POU domain when compared to the full-length protein. Moreover, PoPou5f3 shares a significantly higher sequence identity with other teleosts than tetrapods (Table 1). In addition, there were two one-residue deletions located within the linker region and the POU homeodomain, and several distinct residues in the POU domain when compared with actinopterygii POU5F3 to that of entheria POU5F1 (Figure 1B).

Table 1.
Identity between POU (Pit-Oct-Unc) V proteins.
To evaluate the evolutionary relationship between the predicted PoPou5f3 and other vertebrate orthologues, a phylogenetic tree that contained two main branches, fish Pou5f3 and tetrapod Pou5f1, was constructed with the full-length amino acid sequences using the neighbor-joining method (Figure 1C). Based on our identification, the PoPou5f3 is obviously clustered with the fish Pou5f3 homologues and the relationships displayed in the phylogenetic tree is generally in accordance with classical taxonomy.
2.2. Genomic Organization and Conserved Synteny of Class V POU Family Members
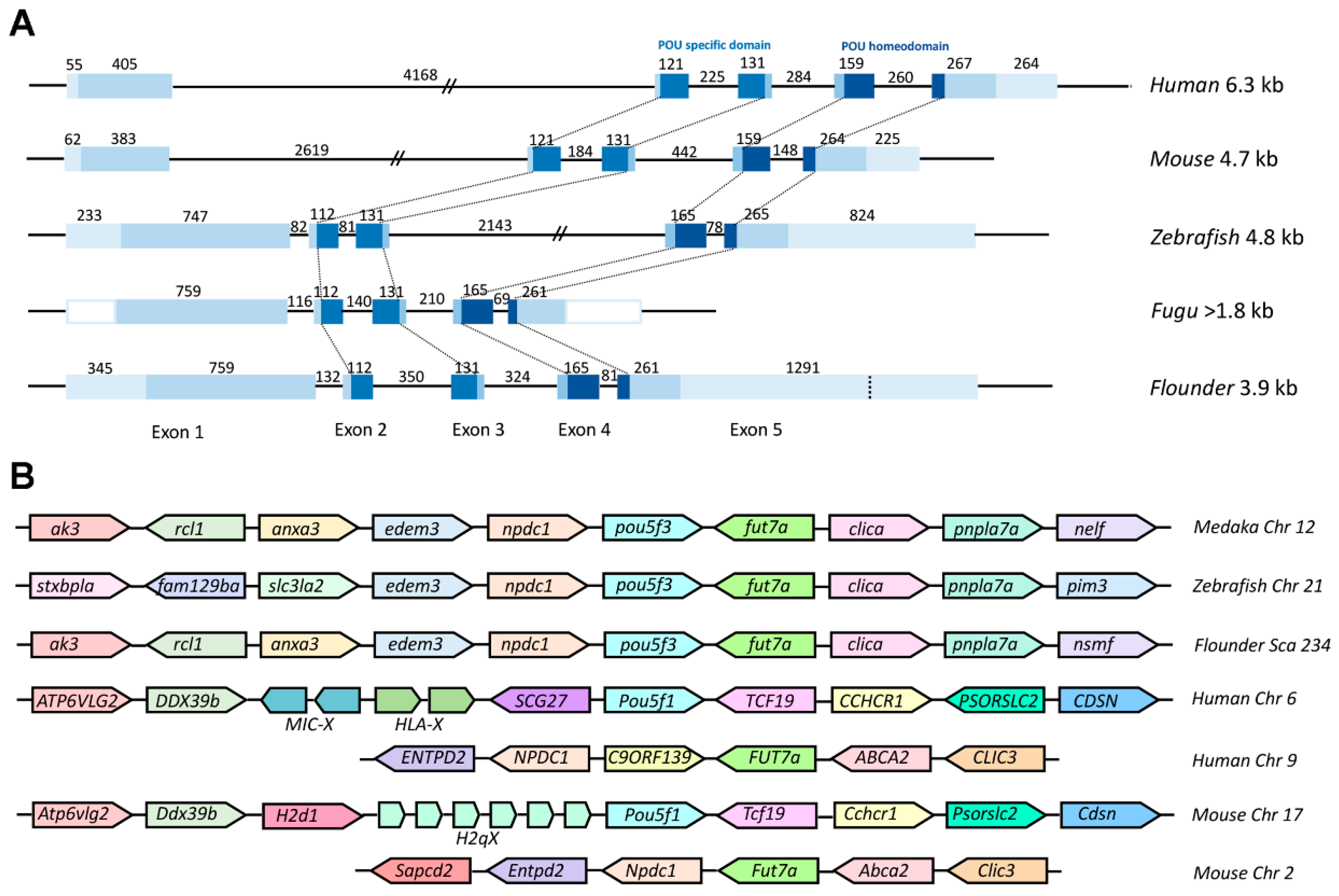
To further explore the evolutionary conservation of Class V POU genes, we performed comparative analysis between Popou5f3 cDNA and its genomic DNA sequence. Our results revealed that it consists of five exons and four introns (Figure 1). All exon-intron boundaries conform to the GT-AG rule (Figure S1). As shown in Figure 2A, Popou5f3 exhibited a similar genomic organization as other teleost and mammal orthologues. Importantly, the POUs and POUh domains are conservatively located within the second to third and fourth to fifth exons in all species despite their different gene sizes. Although the first exons in human and mouse are shorter than those in fugu and flounder, the first intron greatly extended them to have much longer genomic lengths. Consistent with our previously studied nanog gene [31], pou5f3 in zebrafish also showed similar genomic length with mammals rather than other fish due to expanded introns. Popou5f3 was found on scaffold 234 flanked by npdc1 and fut7a according to our up-to-date P. olivaceus whole-genome sequencing data. A cross-species comparison of the chromosomal syntenic relationship showed that Popou5f3-containing region is mostly syntenic to the pou5f3-bearing region of other fish genomes (edem3-npdc1-pou5f3-fut7a-clica) (Figure 2B). However, the human and mouse Pou5f1 were located on the other chromosome between Ddx39b and Tcf19 despite their counterpart of Popou5f3 neighbors (Npdc1 and Fut7a) exhibiting a conserved synteny on human chromosome 9 and mouse chromosome 2.
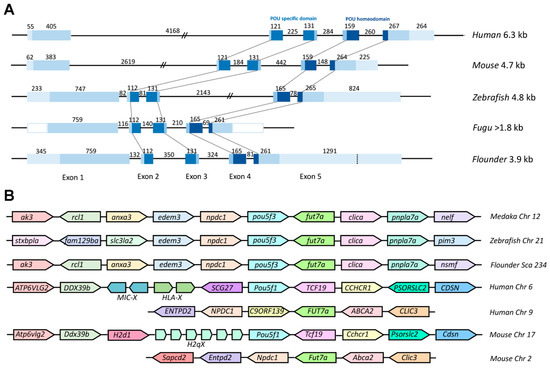
Figure 2.
Gene structure and chromosome synteny of Pou5f1/pou5f3 in vertebrate. (A) Comparison of genomic organizations of Pou5f1/pou5f3 between fish and mammals. Exons are shown in blue box while introns are shown in straight line. The sizes of primary transcripts and each part are indicated by base pair. The 5′- and 3′-UTRs are not known for Fugu. The conserved POUs and POUh domains are connected with dot line among species; and (B) Schematic diagram for the loci of Pou5f1/pou5f3 genes in genomes of fish, human, and mouse from ensemble database. Chr, chromosome; Sca, scaffold.
2.3. Bioinformatics Analysis of Popou5f3 5′ and 3′ Regulatory Sequences
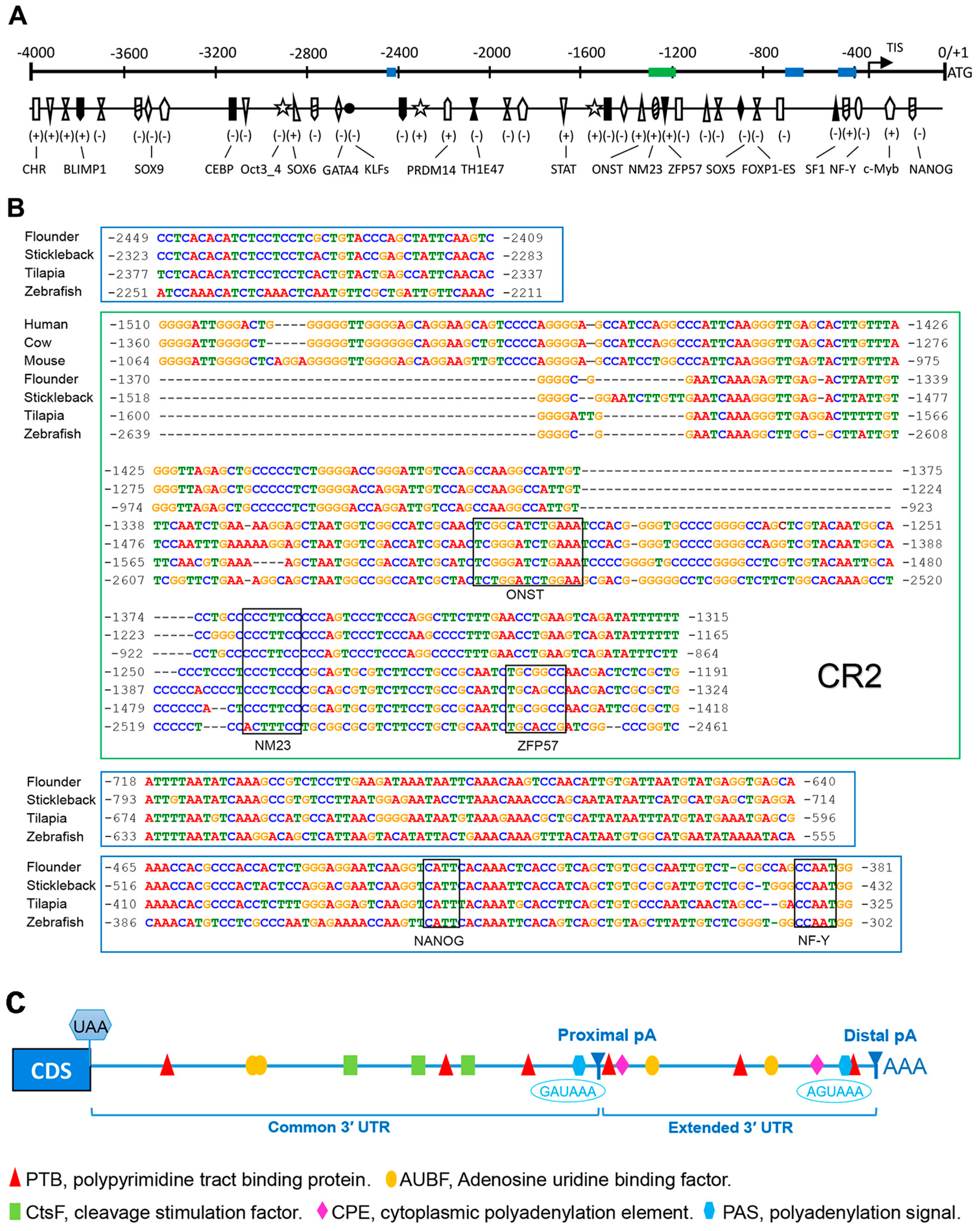
To identify the potential cis-regulatory elements (CREs) regulating Popou5f3 expression, we analyzed about 4000 bp genomic sequences upstream of the ATG codon using several online bioinformatics software. Consistent with the previously identified TATA-less promoter in mouse Pou5f1 [32], we also could not find the typical TATA-box or CAAT-box in the Popou5f3 promoter region. Nevertheless, sequence analysis with the MatInspector program revealed that there are numerous transcription factor binding sites on the regulatory region of Popou5f3, including POU domain class 5 transcription factor1 (Oct 3_4), homeobox protein Nanog (NANOG), PR domain zinc finger protein 14 (PRDM14), transcriptional repressor B lymphocyte-induced maturation protein 1 (BLIMP1), Kruppel-like factors (KLFs), signal transducer and activator of transcription (STAT), cellular and viral myb-like transcriptional regulator (c-Myb), Thing1/E47 heterodimer (ThTH1/E47), alternative splicing variant of forkhead box protein P1 (FOXP1-ES), and composed binding site for Oct4, Sox2, Nanog, Tcf3 and Sall4b in pluripotent cells (ONST) (Figure 3A). Additionally, binding sites for CCAAT/enhancer binding protein (C/EBP), GATA binding protein (GATA4), nuclear factor Y (NF-Y), steroidogenic factor 1 (SF1) and zinc-finger protein 57 (ZFP57) were also identified (Figure 3A). Four conserved regions were predicted among the teleost pou5f3 promoters, but only one of them shared some homology with that of mammal Pou5f1, which is to be the conserved region 2 (CR2) in the mammal Pou5f1 promoter (Figure 3B).
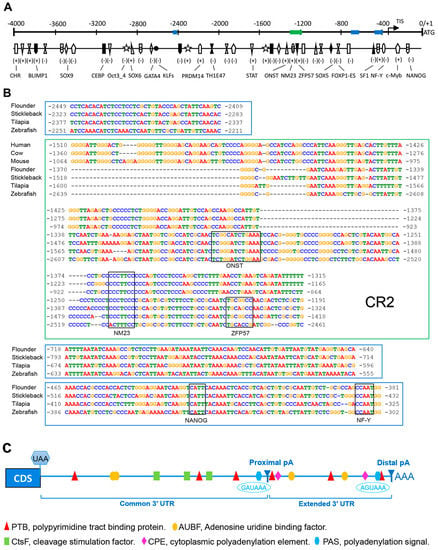
Figure 3.
The 5′ and 3′ flanking sequences features of Popou5f3 gene. (A) Sketch map of potential transcription factor (TF) binding sites in the upstream region. The map is drawn to scale with the start codon ATG designated as +1. The plus-minus sign indicates the TF binding strand. The blue and green panes represent the conserved regions among species corresponding to the sequences shown in (B). TIS: transcriptional initiation site; (B) The 5′ flanking consensus regions of Pou5f1/pou5f3 orthologues among different species with the MEME and Dialign softwares. The genomic sequences were acquired from the Ensembl database and the transcript IDs are listed in Table S2. The binding sites for TFs common for all species (ONST, NM23, ZFP57, NANOG, and NF-Y) are annotated with black boxes above the aligned sequences; and (C) Sketch map of potential binding sites in the downstream region. The cleavage sites are shown as proximal pA and distal pA, respectively. Two non-canonical polyadenylation signals (blue hexagons) are positioned at 816 and 1265 bp downstream of the coding region. Binding sites for PTB (UCUUU), AUBF (AUUUA), CtsF (UUUUU), and CPE (UUUUAU) are represented relative to their locations with a red triangle, yellow oval, green rectangle, and pink rhombus, respectively.
We obtained two transcript isoforms with different 3′-UTRs, which are generated by alternative polyadenylation (APA). In consequence, Popou5f3 contained two poly (A) sites in the 3′ exon, distinguished as proximal- and distal-pA on the basis of their positions relative to the coding region. Meanwhile, we found that Popou5f3 3′-UTR has a common region and an extended region. Sequence analysis revealed that Popou5f3 transcripts lacs the canonical polyadenylation signal (AATAAA). However, putative non-canonical polyadenylation signals are predicted upstream of the proximal and distal poly (A) sites, which were GAUAAA and AGUAAA, respectively. Other cis-acting regulatory elements were also identified within the common and extended 3′-UTRs, such as binding sites for polypyrimidine tract binding protein (PTB), adenosine uridine binding factor (AUBF), cleavage stimulation factor (CtsF), and cytoplasmic polyadenylation element (CPE) (Figure 3C).
2.4. Spatiotemporal Expression of Popou5f3 during Early Embryogensis
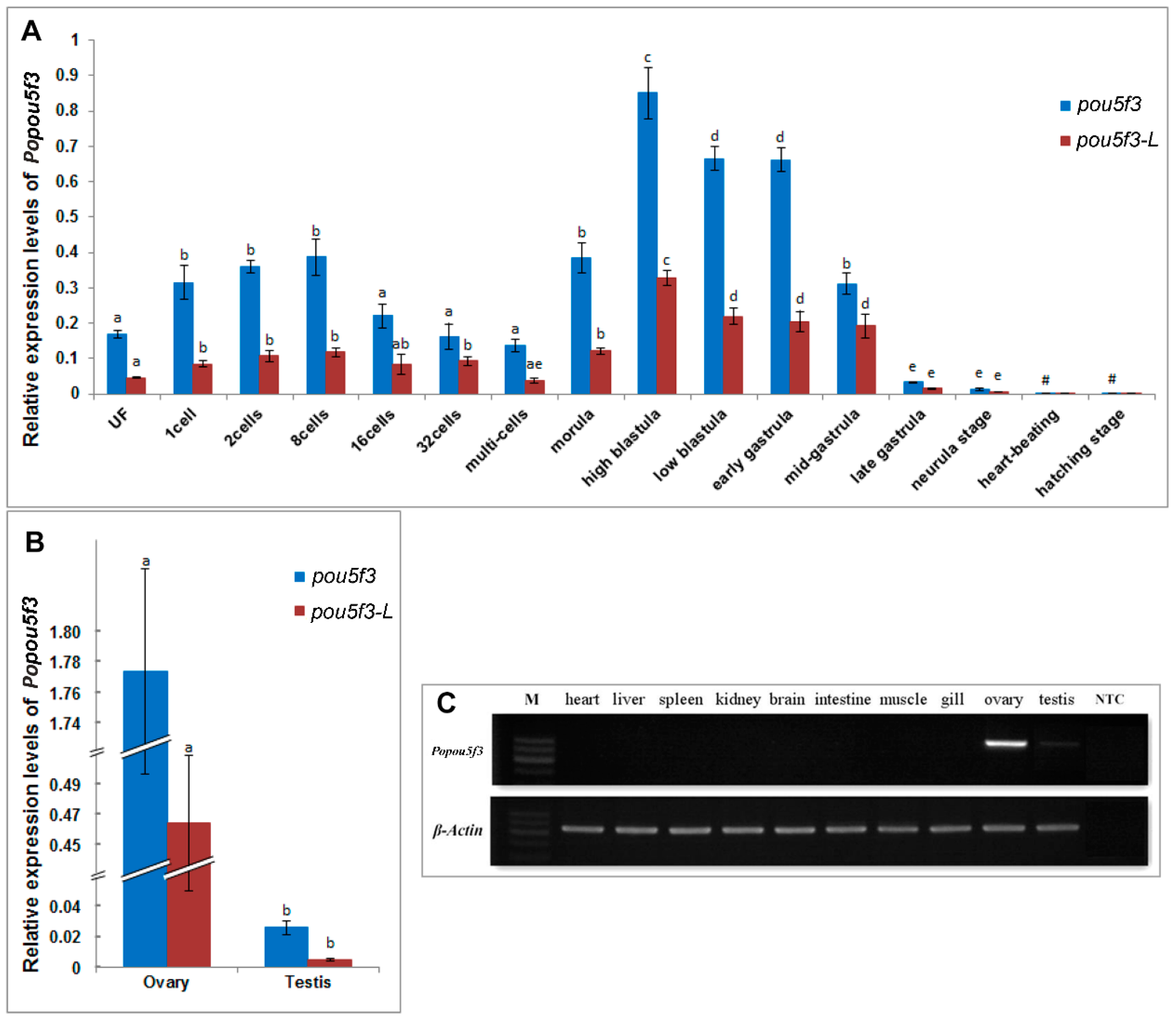
Next, we investigated the expression pattern of Popou5f3 during early embryonic development. Real-time RT-PCR analysis showed that Popou5f3 is to be maternally expressed. The transcripts of Popou5f3 were present from the fertilized egg to the gastrula stage of embryo (Figure 4A). With the cleavage proceeding, the amount of transcripts were increased from the one-cell to eight-cell stage and decreased from the 16-cell stage to multi-cells because of decay of maternally deposited transcripts. Its level was up-regulated again from the morula stage on, which is probably attributed to the activation of zygotic gene expression. We observed that Popou5f3 reaches its highest expression level at the high-blastula stage; although its level decreases, it remains relatively high until the mid-gastrula stage, and then greatly diminishes at the end of the gastrulation stage. From the heart-beating stage onward, Popou5f3 is barely detectable. Next, we analyzed the expression profile of the long transcript of Popou5f3, which we named Popou5f3-L, with specific-primers located within the extended 3’-UTR. The level of the short transcript Popou5f3-S was estimated by subtraction of the total amounts of Popou5f3 to Popou5f3-L. Overall, Popou5f3-L exhibited a similar expression pattern with the total Popou5f3 transcripts and Popou5f3-S showed a higher expression level than Popou5f3-L (Figure 4A).
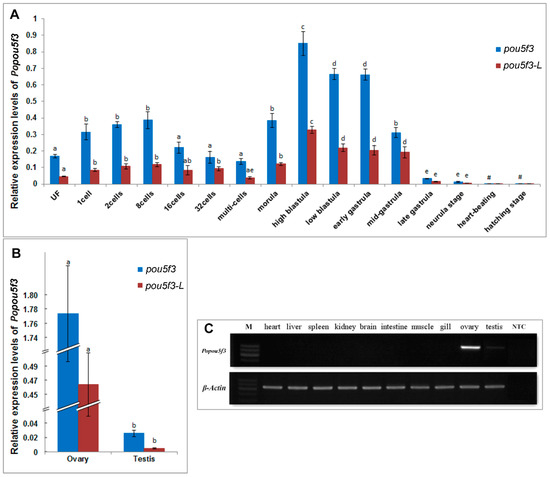
Figure 4.
Quantitative analyses of the expression profiles of Popou5f3. (A) Relative expression levels of Popou5f3 and the long transcript Popou5f3-L during early embryo development; (B) Relative expression levels of Popou5f3 and Popou5f3-L in adult gonads. 18S RNA and UbcE are chosen as the reference genes. Data are represented as mean ± SEM (n = 3). Groups marked with different letters are statistically different (p < 0.05); and (C) Tissue distribution of Popou5f3 analyzed by semi-quantitative RT-PCR using β-actin as a reference gene. NTC, negative control without template.
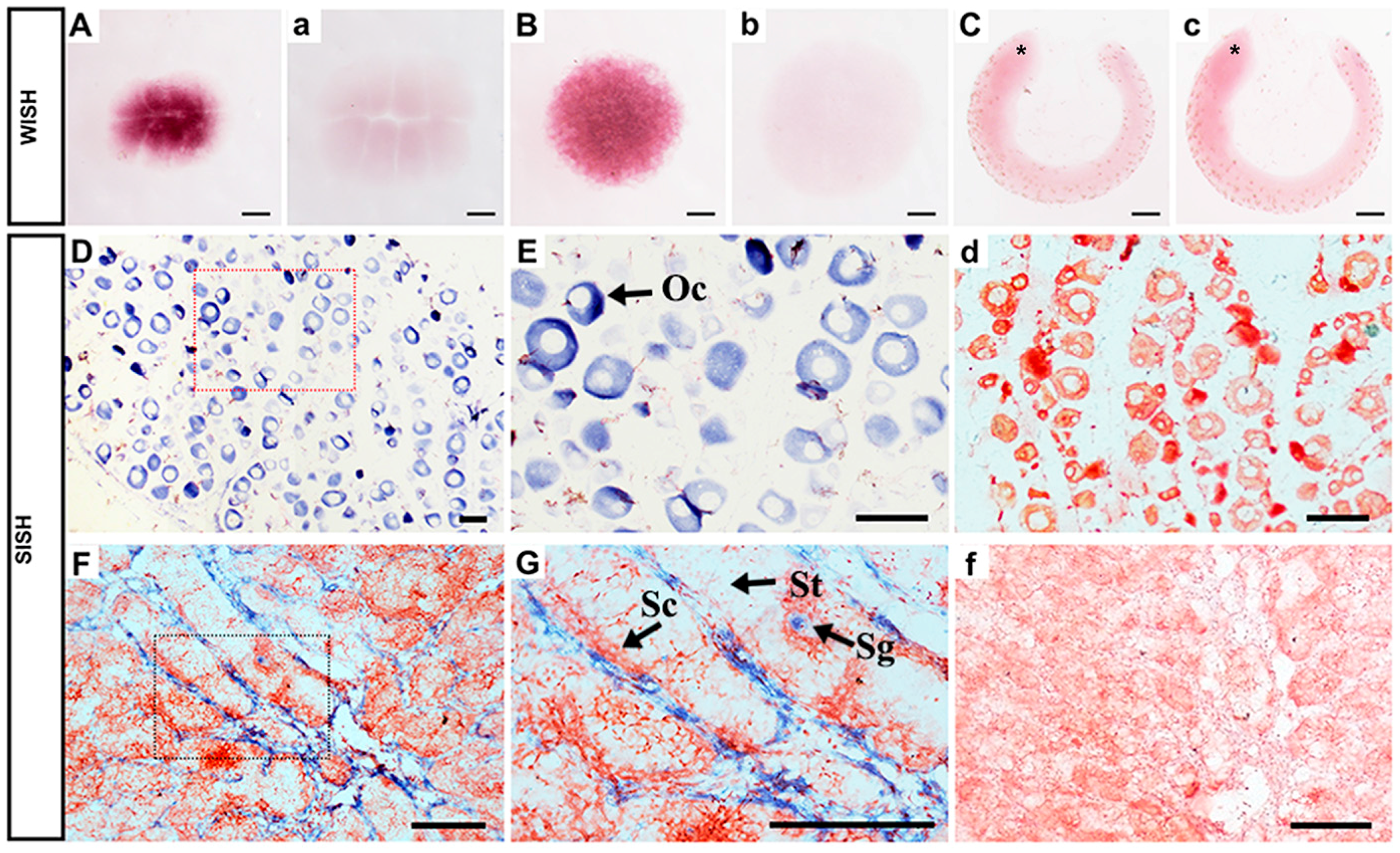
To further investigate Popou5f3 expression in more detail, whole-mount in situ hybridization (WISH) was performed using digoxigenin (DIG)-labeled antisense and sense probes on three selected early developmental stage embryos. A strong signal was detected at the eight-cell stage (Figure 5A), which suggests its maternal expression. At the blastula stage, the signal was present in all blastomeres (Figure 5B). At the tail-bud stage, a period between the neural and heart-beating stages, the signal was not detectable in the embryo, which is consistent with the qRT-PCR data (Figure 5C). Together, this data confirm the results of qRT-PCR. For all studied stages, no signal was observed in embryos hybridized with the sense probe (Figure 5a–c).
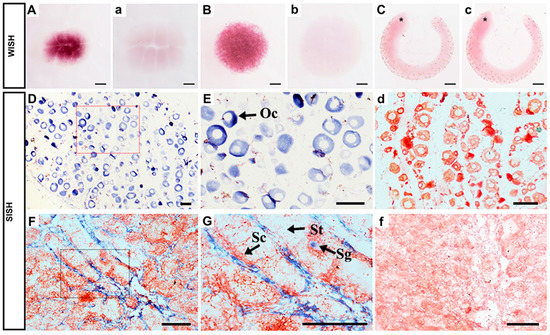
Figure 5.
Expression of Popou5f3 during early embryogenesis and in adult gonads analyzed by in situ hybridization (ISH). The positive signals with antisense probe hybridization were stained with dark purple or blue, while the negative controls with sense probe (a–c,d,f) were not stained. (A) eight cells, (B) blastula stage, (C) tail-bud stage. Popou5f3 mRNA transcripts were located in the cytoplasm of oocytes in ovary (D,E). In testis, Popou5f3 expressed in spermatogonia, but no signals in spermatocytes or spermatids (F,G). (E,G) are magnification of (D,F). Asterisks (*) indicate the head of the embryo. Oc, oocytes; Sg, Sc, and St are represent spermatogonia, spermatocytes, and spermatids, respectively. Scale bars, 100 μm.
2.5. Gonad-Specific Expression of Popou5f3 in Adult Tissues
To determine the distribution of Popou5f3 in adult tissues, semiquantitative-RT-PCR was first performed using β-actin as an internal control. The results showed that Popou5f3 mRNA transcripts were exclusively restricted to the gonadal tissues, but absent in any somatic organs examined, such as the heart, liver, spleen, kidney, brain, intestine, muscle and gill (Figure 4C). Additionally, strong expression was observed in the ovary while a faint band was detectable in the testis. Then, the expression pattern was quantitated and confirmed by qRT-PCR. The female gonad had a dominantly higher expression level of Popou5f3 than the male gonad (Figure 4B). Furthermore, Popou5f3-S showed about 2−5-fold expression level to Popou5f3-L.
To define the cellular localization of Popou5f3 transcripts and PoPou5f3 protein in ovary and testis, ISH and immunohistochemistry (IHC) analyses on paraffin sections of these organs were performed. ISH results showed that the ovary was in development stage II, which was composed of a large number of oocytes. The expression of Popou5f3 in oocytes was consistent with its maternal expression profile (Figure 5D). Similar with the cellular localization of zebrafish pou5f3 in oocytes [33], Popou5f3 mRNA transcripts were also observed throughout the cytoplasm (Figure 5E). In testis, the hybridization signal was only detected in spermatogonia, with no signal in spermatocytes or spermatids (Figure 5F,G), nor in the negative controls (Figure 5d,f).
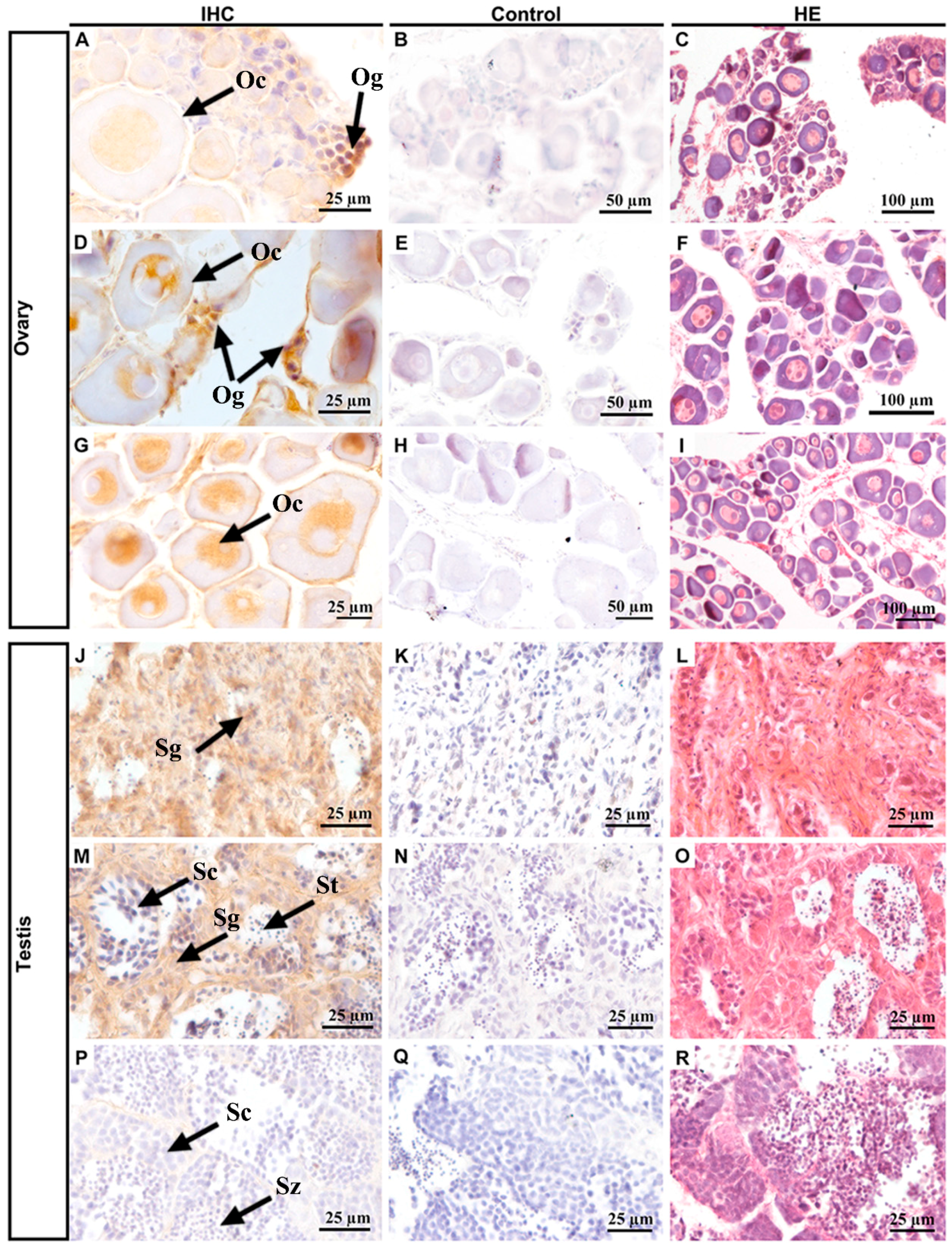
Next, we collected samples at different stages of gonadal development to investigate the cellular localization of PoPou5f3 (Figure 6). The initial observation data and subsequent hematoxylin and eosin (HE) staining showed that the ovaries in panels A–C were 1 ± 0.1 cm long, and had cylindrical rods and a certain number of oogonia and primary oocytes. The ovaries in panels D–F were 2.5 ± 0.5 cm long, triangularly shaped, containing a small number of oogonia and many oocytes. The ovaries in panels G–I were 4.0 ± 0.5 cm long, bearing a slender trident shape, with a large number of primary oocytes. The testes in panels J–L were 0.5 ± 0.1 cm long, resembled thin strips, and were mainly composed of spermatogia with a large size and small nuclear. The testes in panels M–O were 2.0 ± 0.5 cm long, thin strips, and contained a lot of spermatogonia, primary spermatocytes and spermatids. The testes in panels P–R were 5.0 ± 0.5 cm long, of the fleshy lobular type, with plenty of spermatocytes, spermatids and spermatozoa. When compared with those of spermatogonia, the size of primary spermatocytes decreased and the nucleocytoplasmic ratio increased. IHC results showed that PoPou5f3 is located in the nucleus of oogonia and oocytes, especially with a strong signal in oogonia (Figure 6A,D,J). But in the testis, the PoPou5f3 signal was enriched in spermatogonia and became undetectable when spermatogenesis proceeded (Figure 6J,M,P). Taken together, these results indicate that the expression of Popou5f3 is specific to gonadal tissues and it peaks in oogonia and spermatogonia.
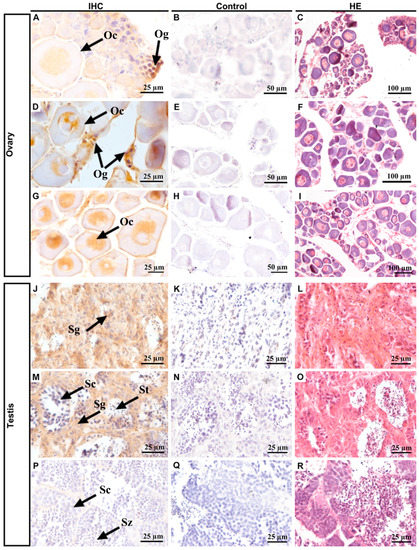
Figure 6.
Immnohistochemisiry of localization of PoPou5f3 in P. olivaceus ovaries and testes at different stages. Positive signals of anti-PoPou5f3 immunolabeling are shown in tawny (ovaries: (A,D,G); testes: (J,M,P)). Negative controls with the rabbit preimmune serum (ovaries: (B,E,H); testes: (K,N,Q)) and hematoxylin and eosin (HE, ovaries: (C,F,I); testes: (L,O,R)) staining of the corresponding sections were included. Og, oogonia; Oc, oocytes; Sg, spermatogonia; Sc, spermatocytes; St, spermatids; Sz, spermatozoa. Scale bars are indicated in the lower right corner of each photo.
3. Discussion
Pou5f1 is an important marker gene of stem cells. In the past 20 years, the homologues of Pou5f1 in mammals as well as in other vertebrates, including human [34], bovine [35], brushtail possum [36,37], rhesus monkey [38], platypus [39], vole [40], chicken [41], xenopus [42], zebrafish [21,43], medaka [17,44], Chinese sturgeon [45], goldfish [18], and Nile tilapia [19], have been identified. Several research studies have revealed their critical roles during early embryonic development and their importance in the maintenance of stem cell pluripotency. In this study, we have cloned and characterized Popou5f3 from the marine fish Japanese flounder, analyzed its sequence features and expression patterns, which lays a foundation for further functional study on stem cell tracing and manipulations in this species.
3.1. Popou5f3 Is the Homologue of Mammalian POU5F1
Previously, the names of the homologues of mouse Pou5f1/Oct4 in teleost fish were not unified, such as the originally named pou2/spg, then pou5f1 in zebrafish [20,21,46], oct4 in medaka and annual killifish [17,44,47], pou2 in Gadus morhua, Chinese sturgeon, and goldfish [18,30,45], which creates confusion in their naming. However, sequence and synteny conservation analyses indicate that zebrafish pou2 is not the true orthologue of mammalian POU5F1, but is closely related to mammalian POU5F3. Due to this reason, the zebrafish nomenclature committee renamed it zebrafish pou5f3 [48,49]. In addition, scholars recommend the application of this nomenclature to all vertebrate orthologues of POU5F3 [49,50].
In our study, we have cloned the complete sequence of pou5f3 in Japanese flounder and proven it to be the homologue of mammalian POU5F1. In the studies about the origin of POU5F1, it is suggested that the two copies of POU5F1 and POU2 (now called POU5F3) existing in marsupials and monotremes originated from the Pou2 in lower vertebrates [39,48,49]. Furthermore, POU5F1 is only present in eutherian mammals; however, there are many POU5F1 pseudogenes [51]. Indubitably, complex evolutionary events, such as gene duplication, loss, chromosome rearrangement, etc., have occurred in the process of POU5F1/POU5F3 evolution. In our study, we found only one gene copy of Popou5f3 via genomic sequence searching, which is coincident with other identified teleost pou5f3 [19,44]. Moreover, Popou5f3 has a conserved chromosome synteny and a high sequence similarity with medaka and zebrafish pou5f3. Therefore, we conclude that Popou5f3 is the orthologue of teleost pou5f3.The protein encoded by Popou5f3 has a characteristic POU domain, which shares high sequence identities over 68.5%, with other representative species and it has a similar three-dimensional structure to the POU domain of mouse Pou5f1 (Figure S3). Among different species, Pou5f1/pou5f3 have the same genomic organization composed of five exons and four introns, and the same positions of the POU domain in the gene [32,52]. All these indicate that the POU domain is primarily conserved in evolutionary development. Studies have demonstrated that the POU domains of mammalian Pou5f1 as well as zebrafish Pou5f3 can bind to the octamer motif containing a conserved ATGCAAAT sequence and activate the transcription of the target gene or itself [52]. For Pou5f1, it can further interact with the HMG domain of Sox2 to form a complex to regulate the pluripotency maintenance of stem cells [4,53], which illustrates its functional importance. Based on the sequence and structural similarity, we speculate that Popou5f3 may have similar functions as other species. However, the extent of conserved functions of PoPou5f3 still needs further investigation.
3.2. The Potential Cis-Regulatory Elements (CREs) in Regulating Popou5f3 Expression
CRE regions of DNA typically regulate the nearby gene expression by functioning as binding sites for transcription factors and may be located either at 5’ or 3’ to the coding sequence of the gene. As vital components of genetic regulatory networks, CREs also have an important evolutionary role [54,55]. Thus, it is essential to characterize the regulatory regions of Popou5f3 for a better understanding of the regulation of its expression and function. For this purpose, we first obtained the flanking sequences of Popou5f3 and analyzed these sequences with a number of potential cis-elements for TF binding. In mice, the transcriptional initiation of Oct4/Pou5f1 is regulated by a germ cell (GC)-rich proximal promoter sequence that lacks the TATA-box element, and its expression is also regulated by the proximal and distal enhancer elements within the upstream region [56]. Moreover, a variety of TFs can be combined with the corresponding cis-elements on human Oct4 promoter region to regulate its gene expression [57]. For example, in retinoic acid-induced stem cells, transcription Specificity Protein 1 (Sp1) regulates Oct3/4 gene expression by binding to the corresponding locus on the promoter, which results in stem cell differentiation [58]. The germ cell nuclear factor (GCNF) can also be combined with the corresponding site on the promoter to induce gene silencing [59]. In this study, we cloned the complete Popou5f3 promoter sequence through two rounds of chromosome walking. Consistent with the report in mice [56], we also did not find traditional TATA box element in Popou5f3. Nevertheless, the transcription factors and related signal pathways involved in regulating stem cell pluripotency, such as FOXP1-ES, LEF1/TCF, PRDM14, STAT, etc. [60,61,62,63,64], were predicted to be abundant in the regulatory region of the gene. In the mammalian Oct4 gene promoters, there are four conserved regions (CR1-CR4), which are involved in the regulation of gene expression [56,65,66]. However, the promoter sequence of pou5f3 in teleost, such as flounder, stickleback, tilapia and zebrafish, is less conservative when compared to those of mammals, as it has only one region sharing some similarity with mammalian CR2. Besides, three additional conserved regions and potential conserved cis-elements among different species were identified, which suggests that these sequences may be involved in regulating the transcription and expression of Popou5f3. In zebrafish, Pou5f3 has been demonstrated to be able to bind to the octamer sequences in upstream DNA to positively regulate its expression through an autoregulatory loop [52]. This provides a worthy place to begin exploring the regulation mechanism for Popou5f3 since we have also identified potential Oct binding sites in the upstream region. On the other hand, through in vitro experiments, Hong et al. [67] have found that the mouse Oct4 gene promoter can be activated in the medaka embryonic stem cells. This indicates that some of the cis-elements and regulatory sequences involved in regulation of the pluripotency specific gene expression are conserved between mammals and teleost fish. Actually, this part of the results is only from bioanalytical level analysis and prediction. Thus, we need further experiments in order to verify whether it has a regulatory function.
It is well known that different spliced variants of pou5f3/OCT4 had been identified and their functionality with distinct capacities was analyzed both in mammals and teleost [21,68,69]. However, we did not find any alternative splicing in Popou5f3. Interestingly, we have found the presence of APA, and it generated two kinds of Popou5f3 transcripts with different 3’-UTR length, which have not been reported in this gene of other species yet. Recently, a great deal of attention has been paid to APA due to its central role in post-transcriptional gene regulation, modulation of genes that are critical for stem cell function, development and disease [70,71,72]. Mechanically, changes of binding sites for RNA binding protein or microRNA on 3′-UTR can affect the stability and localization as well as the translation efficiency of mRNA. The cleavage and polyadenylation specificity factor (CPSF)-associated protein, Fip1, is able to activate the ESC-specific APA profiles to ensure the optimal expression of self-renewal genes [73]. Moreover, the APA-mediated shorter 3′-UTR shift of key myogenic factor, Pax3, can render itself resistant to regulation by miR-206, thus maintaining the muscle stem cell function [72]. With regard to Popou5f3, multiple cis-elements for mRNA 3′-processing proteins such as PTB, AUBF, CtsF, and CPE were identified, which might be involved in post-transcriptional regulation of Popou5f3. Nowadays, the mechanism and regulation of non-coding RNAs, especially microRNAs, have been extensively and deeply investigated in mammals, whereas study in teleost fish is still in its infancy stage. Here, we provide new ideas and directions for future study.
3.3. Popou5f3 Is Abundant at Blastula-Stage Embryos and Restricted in Adult Gonads
As the expression of Pou5f1 mRNA is closely related to the cell fates, the Pou5f1 homologue has been regarded as a master regulator of self-renewal in pluripotent stem cells as well as cell reprogramming in mammals [23,50,74]. Nevertheless, a considerable difference in expression pattern of pou5f3 exists among lower vertebrates. In zebrafish, pou5f3 was maternally expressed, increased to reach its peak at the gastrula stage, restricted in the neural plate and afterwards confined to the caudal end of the spinal cord, showing vital roles during development, such as brain regionalization, mesendoderm specification and endoderm formation [20,21,43,75]. Certain similarity was found in Nile tilapia pou5f3, in that it is expressed until the blastula stage and transiently appears in the brain region before hatching [19]. On the other hand, the Atlantic cod pou5f3 (originally called ac-Pou2) showed high expression levels at early development stages before gastrulation as well as the ESC isolated from blastula stage eggs, which illustrates its role in marking the undifferentiated blastula cells in vivo and in vitro [30]. The same expression profiles of medaka pou5f3 during embryogenesis coupled with functional verification demonstrated thoroughly that it is essential for pluripotency maintenance [17,44]. For adults, though we did not observe its expression in brain tissue, the mRNA and protein distribution of Popou5f3 are similar with those of medaka pou5f3 in gonads, which is specific to germ cells. Moreover, Popou5f3 was maternally inherited, abundantly expressed at the blastula stage, then gradually diminished to barely detectable by the end of the gastrula stage, a period in which cells are differentiated into three embryonic germ layers. Generally, Popou5f3 showed a more similar expression pattern with medaka rather than zebrafish. Combined with our previous findings on another two pluripotent-associated genes (Ponanog and Posox2), it appears that these three genes might form an interactive network to regulate the pluripotent state of blastula cells and subsequent germ layer differentiation in P. olivaceus [31,76,77]. Currently, we are focusing our next studies on finding whether Popou5f3 is expressed in primordial germ cells and the functions of the two different-length transcripts of Popou5f3.
4. Experimental Section
4.1. Animals and Handling
The Japanese flounder (P. olivaceus) used in this study was sampled from local mariculture farms in Haiyang City, China. All handling of the samples were conducted and approved by the Institutional Animal Care and Use Committee of the Ocean University of China and the China Government Principles for the Utilization and Care of Vertebrate Animals Used in Testing, Research and Training (State Science and Technology Commission of the People’s Republic of China for No. 2, 31 October 1988).
Fertilized eggs were obtained by artificial fertilization and they were incubated in clean sea water with continuous aeration at 17 ± 1 °C. During the development stage, embryos were observed under a microscope and three pools of embryos that came from mixed families were collected separately at a particular stage [78]. There were 16embryonic stages (unfertilized egg (UF, 0 h), one-cell (0.5 h after fertilization (haf)), two-cell (1.8 haf), eight-cell (3.5 haf), 16-cell (4.6 haf), 32-cell (5.3 haf), multi-cells (10.3 haf), morula (10.6 haf), high blastula (17 haf), low blastula (17.5 haf), early gastrula (20.1 haf), mid-gastrula (25.6 haf), late gastrula (28.5 haf), neurula (30.6 haf), heart-beating (67.1 haf), and hatching stage (93 haf)) that were selected for this study. The embryos were sampled by immersion in 1 mL RNAwait liquid (Solarbio, Beijing, China), and then immediately stored at −80 °C until use. Tissue samples of the heart, liver, spleen, kidney, brain, intestine, muscle, gill, and gonads (ovary and testis) were collected from six randomly selected healthy adults (three females and three males), immediately frozen in liquid nitrogen, and stored at −80 °C until use. Embryos (at eight-cell, high blastula, and tail-bud stages (42 haf)) and gonad (ovary and testis) samples used for ISH were immediately fixed in 4% paraformaldehyde (PFA)−phosphate buffered saline (PBS) (4% PFA overnight at 4 °C, dehydrated in a gradient-increasing methanol, and stored in 100% methanol at −20 °C. The embryos were microscopically dissected to remove the envelopes before dehydration. Gonads used for immunohistochemistry were collected from 4-, 10- and 16-month-old fish samples, respectively, and fixed by immersion in fresh Bouin′s fixative for 20 h, then dehydrated and stored in 100% ethanol.
4.2. Genomic DNA, Total RNA Isolation and cDNA Synthesis
Genomic DNA was extracted from the muscle tissue via the phenol-chloroform procedure. Total RNA was isolated separately from the sampled embryos and tissues using Trizol reagent (Invitrogen, CA, USA) according to the manufacturer’s protocol and treated with RNase-free DNase I (Takara, Dalian, China) to remove contaminating genomic DNA and stored at −80 °C. The quantity and quality of genomic DNA and total RNA were determined by agarose gel electrophoresis and Nanophotometer Pearl (Implen GmbH, Munich, Germany). The first-strand cDNA was synthesized using M-MLV reverse transcriptase (Takara, Dalian, China) as described in the protocol. The corresponding cDNA was stored at −20 °C.
4.3. Cloning and Sequencing of Popou5f3
The semi-nested degenerate primers, pou5f3-core-Fw and pou5f3-core-Rv-1/2 (Table 2) were used to amplify Popou5f3 cDNA fragment. They were designed according to the evolutionary conserved domains of pou5f3 in other fish species obtained from the NCBI. The untranslated regions of Popou5f3 cDNA were obtained by 5′ and 3′ RACE with the SMART™ RACE cDNA Amplification kit (Clontech, CA, USA) and by using the gene-specific primers 5’-RACE-pou5f3 and 3′-RACE-pou5f3 (Table 2). The genomic DNA sequence were obtained by primers pou5f3-full-length-Fw/Rv (Table 2). The promoter region was amplified by two rounds of PCR strategy using six gene-specific primers (Table 2, pou5f3-Pro-SP1/2/3-1st and pou5f3-Pro-SP1/2/3-2nd) and four shorter arbitrary degenerates (AP1/2/3/4) supplied with the Genome Walking kit (Takara, Dalian, China) under the manufacturer’s instructions.

Table 2.
Sequences of the primers used in this study.
4.4. Sequence Analysis
The exon and intron boundaries were determined by alignment of the obtained cDNA sequence with the generated genome sequence. NCBI′s online ORF Finder and DNASTAR were employed to predict ORF for translated peptide product. The POU domains were identified using the simple modular architecture research tool (SMART) and InterProScan search software. The multiple alignments of Pou5f3 were generated with ClustalW and the phylogenetic tree was constructed using the MEGA 5.2 program by the neighbor-joining method with 1000 bootstrap replicates. Bioinformatic analysis and potential transcription factor binding sites within the 5′ regulatory region of Popou5f3 was performed with online program MatInspector. The conserved promoter sequences between fish and mammals were analyzed by the MEME software and the Dialign software from the Genomarix suite. The websites of the software mentioned above were listed in Table 3.

Table 3.
The websites of the online software used in this study.
4.5. Quantitative Expression Analysis of Popou5f3
Semiquantitative-RT-PCR was carried out to determine the tissue distribution of Popou5f3 using β-actin as an endogenous reference. The primers, pou5f3-SRT-Fw and pou5f3-SRT-Rv (Table 2), located in the exon 1 and exon 2, respectively, with a 605 bp of product, were used for semi-RT-PCR. qRT-PCR was used to quantify the expression levels of Popou5f3 at different developmental stages. The differences between the two sexes were identified using Light-Cycler Roche 480 (Roche Applied Science, Mannheim, Germany) according to the method we described previously [31,79]. Since we had found two transcript isoforms but could not design appropriate primers to distinguish them exactly, we used primers pou5f3-RT-Fw/Rv and pou5f3-L-RT-Fw/Rv (Table 2) for qRT-PCR to amplify the total pou5f3 and pou5f3-L. These two primer pairs are located within exon 1 and the pou5f3-L specific 3′-UTR region, respectively.
4.6. In Situ Hybridization Analysis
Digoxigenin-labelled RNA sense and antisense probes were synthesized using a DIG RNA labelling kit (Roche, Mannheim, Germany) with pou5f3-ISH-Fw/Rv (Table 2) specific primers spanning the partial 5′-UTR and N-terminal coding region of Popou5f3 cDNA. RNA whole-mount ISH for embryos and section ISH for gonadal tissues were performed as previously described [31,79].
4.7. Immunohistochemistry and Histological Analysis
Full-length cDNA fragment encoding 475 amino acids of PoPou5f3 was fused to the His tag by insertion between the EcoRI-NotI sites in pET-32a (+) (Novagen, Darmstadt, Germany). The recombinant fusion protein was expressed in E. coli Transetta (DE3) (Transgene, Beijing, China) and purified using the Ni-NTA His·Bind® Resins (Novagen, Darmstadt, Germany) according to the manufacturer′s protocol. Purified protein was sent to Shanghai Shenggong Biological Engineering Co., Ltd. (Shanghai, China) for the production of the polyclonal antibody against PoPou5f3. The antiserum was stored in aliquot at −20 °C. The immunohistochemical staining for Pou5f3 was performed using a standard indirect peroxidase method. Briefly, the dewaxed and rehydrated sections were incubated with 3% H2O2 at room temperature for 15 min to inhibit the endogenous peroxidase. Subsequently, the sections were heated in ethylenediaminetetraacetic acid (EDTA) buffer with an oven at 85 °C for 40 min to unmask the antigen, followed by blocking with 2% non-fat milk at room temperature for 2 h. The incubation with the primary anti-Pou5f3 antibody with a dilution of 1:500 was performed overnight at 4 °C. Pre-immune rabbit serum was used for the negative control group. Subsequently, the secondary horseradish peroxidase (HRP)-conjugated goat anti-rabbit IgG antibody (1:5000) was applied, and then visualized using 3,3′-diaminobenzidine (DAB). Between all steps the sections were thoroughly washed with PBST. Finally, sections were lightly counterstained with haematoxylin and mounted in resin. For histological analysis, sections were stained with Hematoxylin and eosin (HE). Nikon Eclipse Ti-U microscope (Nikon, Tokyo, Japan) was used to observe and photograph the results.
4.8. Statistical Analysis
All data were represented as mean ± standard error of the mean (SEM). Statistical differences were calculated using one-way ANOVA followed by Duncan′s test with SPSS 20.0 software. p < 0.05 were considered statistically significant.
5. Conclusions
In conclusion, we have identified and characterized the structure and expression profiles of pou5f3 in P. olivaceus. Evidence has been gathered from gene structure, chromosome synteny and phylogenetic position and prove that Popou5f3 is the homologue of mammalian Pou5f1. Plenty of cis-regulatory elements are predicted in the flanking regions, which could be involved in the regulation of Popou5f3 expression. A very high and specific expression level of Popou5f3 at blastula stage during embryonic development and adult germ stem cells suggests its potential roles in lineage formation and pluripotency maintenance. Significantly, this is the first report showing that Popou5f3 has two transcripts with different lengths of 3′-UTR generated by alternative polyadenylation (APA). These findings may extend the understanding of the function and evolution of pou5f3 in lower vertebrates. Moreover, our study provides fundamental information for stem cell tracing and genetic manipulation in Paralichthys olivaceus.
Supplementary Materials
Supplementary materials can be found at www.mdpi.com/1422-0067/18/1/231/s1.
Acknowledgments
This work was supported by the National Natural Science Foundation of China (31272646), Qingdao Postdoctoral Application Research Funded Project (2015143) and China Postdoctoral Science Foundation Funded Project (2016M592132).
Author Contributions
Jinning Gao contributed to the experimental process, data analysis and manuscript writing; Quanqi Zhang and Xubo Wang conceived and designed the experiments; all authors have read and approved the final manuscript.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Herr, W.; Cleary, M.A. The POU domain: Versatility in transcriptional regulation by a flexible two-in-one DNA-binding domain. Genes Dev. 1995, 9, 1679–1693. [Google Scholar] [CrossRef] [PubMed]
- Jerabek, S.; Merino, F.; Scholer, H.R.; Cojocaru, V. OCT4: Dynamic DNA binding pioneers stem cell pluripotency. Biochim. Biophys. Acta 2014, 1839, 138–154. [Google Scholar] [CrossRef] [PubMed]
- Yuan, H.; Corbi, N.; Basilico, C.; Dailey, L. Developmental-specific activity of the FGF-4 enhancer requires the synergistic action of Sox2 and Oct3. Genes Dev. 1995, 9, 2635–2645. [Google Scholar] [CrossRef] [PubMed]
- Chew, J.L.; Loh, Y.H.; Zhang, W.; Chen, X.; Tam, W.L.; Yeap, L.S.; Li, P.; Ang, Y.S.; Lim, B.; Robson, P.; et al. Reciprocal transcriptional regulation of Pou5f1 and Sox2 via the Oct4/Sox2 complex in embryonic stem cells. Mol. Cell. Biol. 2005, 25, 6031–6046. [Google Scholar] [CrossRef] [PubMed]
- Chambers, I.; Tomlinson, S.R. The transcriptional foundation of pluripotency. Development 2009, 136, 2311–2322. [Google Scholar] [CrossRef] [PubMed]
- Niwa, H. How is pluripotency determined and maintained? Development 2007, 134, 635–646. [Google Scholar] [CrossRef] [PubMed]
- Boyer, L.A.; Lee, T.I.; Cole, M.F.; Johnstone, S.E.; Levine, S.S.; Zucker, J.P.; Guenther, M.G.; Kumar, R.M.; Murray, H.L.; Jenner, R.G.; et al. Core transcriptional regulatory circuitry in human embryonic stem cells. Cell 2005, 122, 947–956. [Google Scholar] [CrossRef] [PubMed]
- Loh, Y.-H.; Wu, Q.; Chew, J.-L.; Vega, V.B.; Zhang, W.; Chen, X.; Bourque, G.; George, J.; Leong, B.; Liu, J. The Oct4 and Nanog transcription network regulates pluripotency in mouse embryonic stem cells. Nat. Genet. 2006, 38, 431–440. [Google Scholar] [CrossRef] [PubMed]
- Palmieri, S.L.; Peter, W.; Hess, H.; Scholer, H.R. Oct4 transcription factor is differentially expressed in the mouse embryo during establishment of the first two extraembryonic cell lineages involved in implantation. Dev. Biol. 1994, 166, 259–267. [Google Scholar] [CrossRef] [PubMed]
- Kehler, J.; Tolkunova, E.; Koschorz, B.; Pesce, M.; Gentile, L.; Boiani, M.; Lomeli, H.; Nagy, A.; McLaughlin, K.J.; Scholer, H.R.; et al. Oct4 is required for primordial germ cell survival. EMBO Rep. 2004, 5, 1078–1083. [Google Scholar] [CrossRef] [PubMed]
- Boiani, M.; Eckardt, S.; Scholer, H.R.; McLaughlin, K.J. Oct4 distribution and level in mouse clones: Consequences for pluripotency. Genes Dev. 2002, 16, 1209–1219. [Google Scholar] [CrossRef] [PubMed]
- Nichols, J.; Zevnik, B.; Anastassiadis, K.; Niwa, H.; Klewe-Nebenius, D.; Chambers, I.; Scholer, H.; Smith, A. Formation of pluripotent stem cells in the mammalian embryo depends on the POU transcription factor Oct4. Cell 1998, 95, 379–391. [Google Scholar] [CrossRef]
- Rosner, M.H.; Vigano, M.A.; Ozato, K.; Timmons, P.M.; Poirier, F.; Rigby, P.W.; Staudt, L.M. A POU-domain transcription factor in early stem cells and germ cells of the mammalian embryo. Nature 1990, 345, 686–692. [Google Scholar] [CrossRef] [PubMed]
- Scholer, H.R.; Dressler, G.R.; Balling, R.; Rohdewohld, H.; Gruss, P. Oct4: A germline-specific transcription factor mapping to the mouse t-complex. EMBO J. 1990, 9, 2185–2195. [Google Scholar] [PubMed]
- Yeom, Y.I.; Ha, H.S.; Balling, R.; Scholer, H.R.; Artzt, K. Structure, expression and chromosomal location of the Oct4 gene. Mech. Dev. 1991, 35, 171–179. [Google Scholar] [PubMed]
- Belting, H.G.; Hauptmann, G.; Meyer, D.; Abdelilah-Seyfried, S.; Chitnis, A.; Eschbach, C.; Soll, I.; Thisse, C.; Thisse, B.; Artinger, K.B.; et al. Spiel ohne grenzen/pou2 is required during establishment of the zebrafish midbrain-hindbrain boundary organizer. Development 2001, 128, 4165–4176. [Google Scholar] [PubMed]
- Sanchez-Sanchez, A.V.; Camp, E.; Garcia-Espana, A.; Leal-Tassias, A.; Mullor, J.L. Medaka Oct4 is expressed during early embryo development, and in primordial germ cells and adult gonads. Dev. Dyn. 2010, 239, 672–679. [Google Scholar] [CrossRef] [PubMed]
- Marandel, L.; Labbe, C.; Bobe, J.; Jammes, H.; Lareyre, J.J.; Le Bail, P.Y. Do not put all teleosts in one net: Focus on the sox2 and pou2 genes. Comp. Biochem. Physiol. Part B Biochem. Mol. Biol. 2013, 164, 69–79. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.; Yang, Z.; Linyan, L.; Zhenhua, F.; Linyan, Z.; Zhijian, W.; Ling, W.; Deshou, W.; Jing, W. Characterization of the POU5F1 homologue in nile tilapia: From expression pattern to biological activity. Stem Cells Dev. 2016, 25, 1386–1395. [Google Scholar]
- Lunde, K.; Belting, H.-G.; Driever, W. Zebrafish pou5f1/pou2, homolog of mammalian Oct4, functions in the endoderm specification cascade. Curr. Biol. 2004, 14, 48–55. [Google Scholar] [CrossRef] [PubMed]
- Takeda, H.; Matsuzaki, T.; Oki, T.; Miyagawa, T.; Amanuma, H. A novel POU domain gene, zebrafish pou2: Expression and roles of two alternatively spliced twin products in early development. Genes Dev. 1994, 8, 45–59. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Manali, D.; Wang, T.; Bhat, N.; Hong, N.; Li, Z.; Wang, L.; Yan, Y.; Liu, R.; Hong, Y. Identification of pluripotency genes in the fish medaka. Int. J. Biol. Sci. 2011, 7, 440. [Google Scholar] [CrossRef] [PubMed]
- Tapia, N.; Reinhardt, P.; Duemmler, A.; Wu, G.; Arauzo-Bravo, M.J.; Esch, D.; Greber, B.; Cojocaru, V.; Rascon, C.A.; Tazaki, A.; et al. Reprogramming to pluripotency is an ancient trait of vertebrate Oct4 and Pou2 proteins. Nat. Commun. 2012, 3, 1279. [Google Scholar] [CrossRef] [PubMed]
- Hong, Y.; Gui, J.; Chen, S.; Deng, J.; Manfred, S. Embryonic stem cells in fish. Acta Zoolog. Sin. 2002, 49, 281–294. [Google Scholar]
- Hong, Y.; Schartl, M. Establishment and growth responses of early medakafish (Oryzias latipes) embryonic cells in feeder layer-free cultures. Mol. Mar. Biol. Biotechnol. 1996, 5, 93–104. [Google Scholar]
- Béjar, J.; Hong, Y.; Alvarez, M.C. An ES-like cell line from the marine fish Sparus aurata: Characterization and chimaera production. Transgenic Res. 2002, 11, 279–289. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.-L.; Sha, Z.-X.; Ye, H.-Q. Establishment of a pluripotent embryonic cell line from sea perch (Lateolabrax japonicus) embryos. Aquaculture 2003, 218, 141–151. [Google Scholar] [CrossRef]
- Parameswaran, V.; Shukla, R.; Bhonde, R.; Hameed, A.S. Development of a pluripotent ES-like cell line from asian sea bass (Lates calcarifer)—An oviparous stem cell line mimicking viviparous ES cells. Mar. Biotechnol. 2007, 9, 766–775. [Google Scholar] [CrossRef] [PubMed]
- Dash, C.; Routray, P.; Tripathy, S.; Verma, D.K.; Guru, B.C.; Meher, P.K.; Nandi, S.; Eknath, A.E. Derivation and characterization of embryonic stem-like cells of Indian major carp Catla catla. J. Fish Biol. 2010, 77, 1096–1113. [Google Scholar] [CrossRef] [PubMed]
- Holen, E.; Kausland, A.; Skjaerven, K. Embryonic stem cells isolated from atlantic cod (Gadus morhua) and the developmental expression of a stage-specific transcription factor ac-pou2. Fish Physiol. Biochem. 2010, 36, 1029–1039. [Google Scholar] [CrossRef] [PubMed]
- Gao, J.; Wang, J.; Jiang, J.; Fan, L.; Wang, W.; Liu, J.; Zhang, Q.; Wang, X. Identification and characterization of a nanog homolog in Japanese flounder (Paralichthys olivaceus). Gene 2013, 531, 411–421. [Google Scholar] [CrossRef] [PubMed]
- Okazawa, H.; Okamoto, K.; Ishino, F.; Ishino-Kaneko, T.; Takeda, S.; Toyoda, Y.; Muramatsu, M.; Hamada, H. The Oct3 gene, a gene for an embryonic transcription factor, is controlled by a retinoic acid repressible enhancer. EMBO J. 1991, 10, 2997–3005. [Google Scholar] [PubMed]
- Howley, C.; Ho, R.K. mRNA localization patterns in zebrafish oocytes. Mech. Dev. 2000, 92, 305–309. [Google Scholar] [CrossRef]
- Takeda, J.; Seino, S.; Bell, G.I. Human Oct3 gene family: cDNA sequences, alternative splicing, gene organization, chromosomal location, and expression at low levels in adult tissues. Nucleic Acids Res. 1992, 20, 4613–4620. [Google Scholar] [CrossRef] [PubMed]
- Van Eijk, M.; Van Rooijen, M.; Modina, S.; Scesi, L.; Folkers, G.; Van Tol, H.; Bevers, M.; Fisher, S.; Lewin, H.; Rakacolli, D. Molecular cloning, genetic mapping, and developmental expression of bovine POU5F1. Biol. Reprod. 1999, 60, 1093–1103. [Google Scholar] [CrossRef] [PubMed]
- Frankenberg, S.; Tisdall, D.; Selwood, L. Identification of a homologue of POU5F1 (OCT3/4) in a marsupial, the brushtail possum. Mol. Reprod. Dev. 2001, 58, 255–261. [Google Scholar] [CrossRef]
- Frankenberg, S.; Pask, A.; Renfree, M.B. The evolution of class V POU domain transcription factors in vertebrates and their characterisation in a marsupial. Dev. Biol. 2010, 337, 162–170. [Google Scholar] [CrossRef] [PubMed]
- Mitalipov, S.M.; Kuo, H.C.; Hennebold, J.D.; Wolf, D.P. Oct4 expression in pluripotent cells of the rhesus monkey. Biol. Reprod. 2003, 69, 1785–1792. [Google Scholar] [CrossRef] [PubMed]
- Niwa, H.; Sekita, Y.; Tsend-Ayush, E.; Grützner, F. Platypus Pou5f1 reveals the first steps in the evolution of trophectoderm differentiation and pluripotency in mammals. Evol. Dev. 2008, 10, 671–682. [Google Scholar] [CrossRef] [PubMed]
- Medvedev, S.P.; Shevchenko, A.I.; Elisaphenko, E.A.; Nesterova, T.B.; Brockdorff, N.; Zakian, S.M. Structure and expression pattern of Oct4 gene are conserved in vole Microtus rossiaemeridionalis. BMC Genom. 2008, 9, 162. [Google Scholar] [CrossRef] [PubMed]
- Lavial, F.; Acloque, H.; Bertocchini, F.; Macleod, D.J.; Boast, S.; Bachelard, E.; Montillet, G.; Thenot, S.; Sang, H.M.; Stern, C.D.; et al. The Oct4 homologue PouV and nanog regulate pluripotency in chicken embryonic stem cells. Development 2007, 134, 3549–3563. [Google Scholar] [CrossRef] [PubMed]
- Morrison, G.M.; Brickman, J.M. Conserved roles for oct4 homologues in maintaining multipotency during early vertebrate development. Development 2006, 133, 2011–2022. [Google Scholar] [CrossRef] [PubMed]
- Khan, A.; Nakamoto, A.; Tai, M.; Saito, S.; Nakayama, Y.; Kawamura, A.; Takeda, H.; Yamasu, K. Mesendoderm specification depends on the function of Pou2, the class V POU-type transcription factor, during zebrafish embryogenesis. Dev. Growth Differ. 2012, 54, 686–701. [Google Scholar] [CrossRef] [PubMed]
- Liu, R.; Li, M.; Li, Z.; Hong, N.; Xu, H.; Hong, Y. Medaka Oct4 is essential for pluripotency in blastula formation and ES cell derivation. Stem Cell Rev. 2015, 11, 11–23. [Google Scholar] [CrossRef] [PubMed]
- Ye, H.; Du, H.; Chen, X.H.; Cao, H.; Liu, T.; Li, C.J. Identification of a pou2 ortholog in chinese sturgeon, Acipenser sinensis and its expression patterns in tissues, immature individuals and during embryogenesis. Fish Physiol. Biochem. 2012, 38, 929–942. [Google Scholar] [CrossRef] [PubMed]
- Burgess, S.; Reim, G.; Chen, W.; Hopkins, N.; Brand, M. The zebrafish spiel-ohne-grenzen (spg) gene encodes the POU domain protein Pou2 related to mammalian Oct4 and is essential for formation of the midbrain and hindbrain, and for pre-gastrula morphogenesis. Development 2002, 129, 905–916. [Google Scholar] [PubMed]
- Wagner, J.T.; Podrabsky, J.E. Gene expression patterns that support novel developmental stress buffering in embryos of the annual killifish Austrofundulus limnaeus. EvoDevo 2015, 6, 2. [Google Scholar] [CrossRef] [PubMed]
- Frankenberg, S.; Renfree, M.B. On the origin of POU5F1. BMC Biol. 2013, 11, 56. [Google Scholar] [CrossRef] [PubMed]
- Frankenberg, S.R.; Frank, D.; Harland, R.; Johnson, A.D.; Nichols, J.; Niwa, H.; Scholer, H.R.; Tanaka, E.; Wylie, C.; Brickman, J.M. The POU-er of gene nomenclature. Development 2014, 141, 2921–2923. [Google Scholar] [CrossRef] [PubMed]
- Onichtchouk, D. Evolution and functions of Oct4 homologs in non-mammalian vertebrates. Biochim. Biophys. Acta 2016, 1859, 770–779. [Google Scholar] [CrossRef] [PubMed]
- Liedtke, S.; Enczmann, J.; Waclawczyk, S.; Wernet, P.; Kogler, G. Oct4 and its pseudogenes confuse stem cell research. Cell Stem Cell 2007, 1, 364–366. [Google Scholar] [CrossRef] [PubMed]
- Parvin, M.S.; Okuyama, N.; Inoue, F.; Islam, M.E.; Kawakami, A.; Takeda, H.; Yamasu, K. Autoregulatory loop and retinoic acid repression regulate pou2/pou5f1 gene expression in the zebrafish embryonic brain. Dev. Dyn. 2008, 237, 1373–1388. [Google Scholar] [CrossRef] [PubMed]
- Rodda, D.J.; Chew, J.L.; Lim, L.H.; Loh, Y.H.; Wang, B.; Ng, H.H.; Robson, P. Transcriptional regulation of nanog by Oct4 and Sox2. J. Biol. Chem. 2005, 280, 24731–24737. [Google Scholar] [CrossRef] [PubMed]
- McGregor, A.P.; Orgogozo, V.; Delon, I.; Zanet, J.; Srinivasan, D.G.; Payre, F.; Stern, D.L. Morphological evolution through multiple cis-regulatory mutations at a single gene. Nature 2007, 448, 587–590. [Google Scholar] [CrossRef] [PubMed]
- Stern, D.L.; Frankel, N. The structure and evolution of cis-regulatory regions: The shavenbaby story. Philos. Trans. R. Soc. Lond. 2013, 368, 20130028. [Google Scholar] [CrossRef] [PubMed]
- Nordhoff, V.; Hubner, K.; Bauer, A.; Orlova, I.; Malapetsa, A.; Scholer, H.R. Comparative analysis of human, bovine, and murine Oct4 upstream promoter sequences. Mamm. Genome 2001, 12, 309–317. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.M.; Do, H.J.; Oh, J.H.; Kim, J.H.; Choi, S.Y.; Cha, K.Y.; Chung, H.M.; Kim, J.H. Characterization of putative cis-regulatory elements that control the transcriptional activity of the human Oct4 promoter. J. Cell. Biochem. 2005, 96, 821–830. [Google Scholar] [CrossRef] [PubMed]
- Minucci, S.; Botquin, V.; Yeom, Y.I.; Dey, A.; Sylvester, I.; Zand, D.J.; Ohbo, K.; Ozato, K.; Scholer, H.R. Retinoic acid-mediated down-regulation of Oct3/4 coincides with the loss of promoter occupancy in vivo. EMBO J. 1996, 15, 888–899. [Google Scholar] [PubMed]
- Gu, P.; LeMenuet, D.; Chung, A.C.; Mancini, M.; Wheeler, D.A.; Cooney, A.J. Orphan nuclear receptor GCNF is required for the repression of pluripotency genes during retinoic acid-induced embryonic stem cell differentiation. Mol. Cell. Biol. 2005, 25, 8507–8519. [Google Scholar] [CrossRef] [PubMed]
- Akagi, T.; Usuda, M.; Matsuda, T.; Ko, M.S.; Niwa, H.; Asano, M.; Koide, H.; Yokota, T. Identification of Zfp-57 as a downstream molecule of STAT3 and Oct3/4 in embryonic stem cells. Biochem. Biophys. Res. Commun. 2005, 331, 23–30. [Google Scholar] [CrossRef] [PubMed]
- Gabut, M.; Samavarchi-Tehrani, P.; Wang, X.; Slobodeniuc, V.; O’Hanlon, D.; Sung, H.K.; Alvarez, M.; Talukder, S.; Pan, Q.; Mazzoni, E.O.; et al. An alternative splicing switch regulates embryonic stem cell pluripotency and reprogramming. Cell 2011, 147, 132–146. [Google Scholar] [CrossRef] [PubMed]
- Kim, C.G.; Chung, I.Y.; Lim, Y.; Lee, Y.H.; Shin, S.Y. A Tcf/Lef element within the enhancer region of the human NANOG gene plays a role in promoter activation. Biochem. Biophys. Res. Commun. 2011, 410, 637–642. [Google Scholar] [CrossRef] [PubMed]
- Tsuneyoshi, N.; Sumi, T.; Onda, H.; Nojima, H.; Nakatsuji, N.; Suemori, H. PRDM14 suppresses expression of differentiation marker genes in human embryonic stem cells. Biochem. Biophys. Res. Commun. 2008, 367, 899–905. [Google Scholar] [CrossRef] [PubMed]
- Xu, R.H.; Sampsell-Barron, T.L.; Gu, F.; Root, S.; Peck, R.M.; Pan, G.; Yu, J.; Antosiewicz-Bourget, J.; Tian, S.; Stewart, R.; et al. NANOG is a direct target of TGFβ/activin-mediated SMAD signaling in human ESCs. Cell Stem Cell 2008, 3, 196–206. [Google Scholar] [CrossRef] [PubMed]
- He, H.; McHaney, M.; Hong, J.; Weiss, M.L. Cloning and characterization of 3.1 kb promoter region of the Oct4 gene from the Fischer 344 rat. Open Stem Cell J. 2009, 1, 30–39. [Google Scholar] [CrossRef] [PubMed]
- Kobolak, J.; Kiss, K.; Polgar, Z.; Mamo, S.; Rogel-Gaillard, C.; Tancos, Z.; Bock, I.; Baji, A.G.; Tar, K.; Pirity, M.K.; et al. Promoter analysis of the rabbit POU5F1 gene and its expression in preimplantation stage embryos. BMC Mol. Biol. 2009, 10, 88. [Google Scholar] [CrossRef] [PubMed]
- Hong, Y.; Winkler, C.; Liu, T.; Chai, G.; Schartl, M. Activation of the mouse Oct4 promoter in medaka embryonic stem cells and its use for ablation of spontaneous differentiation. Mech. Dev. 2004, 121, 933–943. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Dai, J. Concise review: Isoforms of Oct4 contribute to the confusing diversity in stem cell biology. Stem Cells 2010, 28, 885–893. [Google Scholar] [PubMed]
- Hwang, J.Y.; Oh, J.N.; Lee, D.K.; Choi, K.H.; Park, C.H.; Lee, C.K. Identification and differential expression patterns of porcine Oct4 variants. Reproduction 2015, 149, 55–66. [Google Scholar] [CrossRef] [PubMed]
- Nimura, K.; Yamamoto, M.; Takeichi, M.; Saga, K.; Takaoka, K.; Kawamura, N.; Nitta, H.; Nagano, H.; Ishino, S.; Tanaka, T.; et al. Regulation of alternative polyadenylation by Nkx2–5 and Xrn2 during mouse heart development. eLife 2016, 5, e16030. [Google Scholar] [CrossRef] [PubMed]
- Miles, W.O.; Lembo, A.; Volorio, A.; Brachtel, E.; Tian, B.; Sgroi, D.; Provero, P.; Dyson, N. Alternative polyadenylation in triple-negative breast tumors allows NRAS and c-JUN to bypass PUMILIO post-transcriptional regulation. Cancer Res. 2016, 76. [Google Scholar] [CrossRef] [PubMed]
- Boutet, S.C.; Cheung, T.H.; Quach, N.L.; Liu, L.; Prescott, S.L.; Edalati, A.; Iori, K.; Rando, T.A. Alternative polyadenylation mediates microRNA regulation of muscle stem cell function. Cell Stem Cell 2012, 10, 327–336. [Google Scholar] [CrossRef] [PubMed]
- Lackford, B.; Yao, C.; Charles, G.M.; Weng, L.; Zheng, X.; Choi, E.A.; Xie, X.; Wan, J.; Xing, Y.; Freudenberg, J.M.; et al. Fip1 regulates mRNA alternative polyadenylation to promote stem cell self-renewal. EMBO J. 2014, 33, 878–889. [Google Scholar] [CrossRef] [PubMed]
- Shi, G.; Jin, Y. Role of Oct4 in maintaining and regaining stem cell pluripotency. Stem Cell Res. Ther. 2010, 1, 39. [Google Scholar] [CrossRef] [PubMed]
- Reim, G.; Brand, M. Spiel-ohne-grenzen/pou2 mediates regional competence to respond to Fgf8 during zebrafish early neural development. Development 2002, 129, 917–933. [Google Scholar] [PubMed]
- Gao, J.; Wang, Z.; Shao, K.; Fan, L.; Yang, L.; Song, H.; Liu, M.; Wang, Z.; Wang, X.; Zhang, Q. Identification and characterization of a Sox2 homolog in the Japanese flounder Paralichthys olivaceus. Gene 2014, 544, 165–176. [Google Scholar] [CrossRef] [PubMed]
- Thomson, M.; Liu, S.J.; Zou, L.N.; Smith, Z.; Meissner, A.; Ramanathan, S. Pluripotency factors in embryonic stem cells regulate differentiation into germ layers. Cell 2011, 145, 875–889. [Google Scholar] [CrossRef] [PubMed]
- Tian, Y.; Chen, S.; Yan, A.; Ji, X. Study on the embryonic development of Paralichthys olivaceus. J. Fish. China 2004, 28, 609–615. [Google Scholar]
- Gao, J.; Li, P.; Zhang, W.; Wang, Z.; Wang, X.; Zhang, Q. Molecular cloning, promoter analysis and expression profiles of the Sox3 gene in Japanese flounder, Paralichthys olivaceus. Int. J. Mol. Sci. 2015, 16, 27931–27944. [Google Scholar] [CrossRef] [PubMed]
© 2017 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).