Implication of the Receptor Tyrosine Kinase AXL in Head and Neck Cancer Progression
Abstract
:1. Introduction
2. Results
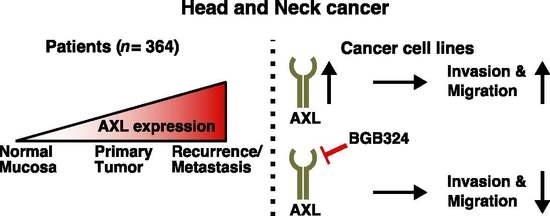
2.1. Analysis of AXL Expression in Patients with HNSCC
2.2. Effect of AXL Overexpression
2.3. Effect of AXL Inhibition
3. Discussion
4. Materials and Methods
4.1. Immunohistochemical Staining
4.2. Cell Lines
4.3. Generation of AXL Overexpressing Cells
4.4. Proliferation
4.5. Migration and Invasion
4.6. Western Blot
4.7. Statistics
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
Abbreviations
| AXL | AXL receptor tyrosine kinase |
| EGFR | Epidermal growth factor receptor |
| HNSCC | Head and neck squamous cell carcinoma |
| IHC | Immunohistochemistry |
| RTK | Receptor tyrosine kinase |
| SI | Average sample staining intensity |
| TMA | Tissue microarray |
References
- Jemal, A.; Bray, F.; Center, M.M.; Ferlay, J.; Ward, E.; Forman, D. Global cancer statistics. CA Cancer J. Clin. 2011, 61, 69–90. [Google Scholar] [CrossRef] [PubMed]
- Leemans, C.R.; Braakhuis, B.J.M.; Brakenhoff, R.H. The molecular biology of head and neck cancer. Nat. Rev. Cancer 2011, 11, 9–22. [Google Scholar] [CrossRef] [PubMed]
- Barnes, C.J.; Ohshiro, K.; Rayala, S.K.; El-Naggar, A.K.; Kumar, R. Insulin-like growth factor receptor as a therapeutic target in head and neck cancer. Clin. Cancer Res. 2007, 13, 4291–4299. [Google Scholar] [CrossRef] [PubMed]
- Kim, C.-H.; Koh, Y.W.; Han, J.H.; Kim, J.W.; Lee, J.S.; Baek, S.J.; Hwang, H.S.; Choi, E.C. c-Met expression as an indicator of survival outcome in patients with oral tongue carcinoma. Head Neck 2010, 32, 1655–1664. [Google Scholar] [CrossRef] [PubMed]
- Goerner, M.; Seiwert, T.Y.; Sudhoff, H. Molecular targeted therapies in head and neck cancer—An update of recent developments. Head Neck Oncol. 2010, 2, 8. [Google Scholar] [CrossRef] [PubMed]
- Göke, F.; Franzen, A.; Hinz, T.K.; Marek, L.A.; Yoon, P.; Sharma, R.; Bode, M.; von Maessenhausen, A.; Lankat-Buttgereit, B.; Göke, A.; et al. FGFR1 Expression Levels Predict BGJ398 Sensitivity of FGFR1-Dependent Head and Neck Squamous Cell Cancers. Clin. Cancer Res. 2015, 21, 4356–4364. [Google Scholar] [CrossRef] [PubMed]
- Temam, S.; Kawaguchi, H.; El-Naggar, A.K. Epidermal growth factor receptor copy number alterations correlate with poor clinical outcome in patients with head and neck squamous cancer. J. Clin. Oncol. 2007. [Google Scholar] [CrossRef] [PubMed]
- Ang, K.K.; Berkey, B.A.; Tu, X.; Zhang, H.-Z.; Katz, R.; Hammond, E.H.; Fu, K.K.; Milas, L. Impact of epidermal growth factor receptor expression on survival and pattern of relapse in patients with advanced head and neck carcinoma. Cancer Res. 2002, 62, 7350–7356. [Google Scholar] [PubMed]
- Bonner, J.A.; Harari, P.M.; Giralt, J.; Azarnia, N.; Shin, D.M.; Cohen, R.B.; Jones, C.U.; Sur, R.; Raben, D.; Jassem, J.; et al. Radiotherapy plus cetuximab for squamous-cell carcinoma of the head and neck. N. Engl. J. Med. 2006, 354, 567–578. [Google Scholar] [CrossRef] [PubMed]
- Cripps, C.; Winquist, E.; Devries, M.C.; Stys-Norman, D.; Gilbert, R.; Head and Neck Cancer Disease Site Group. Epidermal growth factor receptor targeted therapy in stages III and IV head and neck cancer. Curr. Oncol. 2010, 17, 37–48. [Google Scholar] [CrossRef] [PubMed]
- Machiels, J.; Haddad, R.I.; Fayette, J.; Licitra, L.F. Afatinib versus methotrexate as second-line treatment in patients with recurrent or metastatic squamous-cell carcinoma of the head and neck progressing on or after platinum-based therapy (LUX-Head & Neck 1): An open-label, randomised phase 3 trial. Lancet 2015, 16, 583–594. [Google Scholar] [PubMed]
- Verma, A.; Warner, S.L.; Vankayalapati, H.; Bearss, D.J.; Sharma, S. Targeting AXL and MER kinases in cancer. Mol. Cancer Ther. 2011, 10, 1763–1773. [Google Scholar] [CrossRef] [PubMed]
- Koorstra, J.-B.M.; Karikari, C.A.; Feldmann, G.; Bisht, S.; Rojas, P.L.; Offerhaus, G.J.A.; Alvarez, H.; Maitra, A. The AXL receptor tyrosine kinase confers an adverse prognostic influence in pancreatic cancer and represents a new therapeutic target. Cancer Biol. Ther. 2009, 8, 618–626. [Google Scholar] [CrossRef] [PubMed]
- He, L.; Zhang, J.; Jiang, L.; Jin, C.; Zhao, Y.; Yang, G.; Jia, L. Differential expression of AXL in hepatocellular carcinoma and correlation with tumor lymphatic metastasis. Mol. Carcinog. 2010, 49, 882–891. [Google Scholar] [CrossRef] [PubMed]
- Vajkoczy, P.; Knyazev, P.; Kunkel, A.; Capelle, H.-H.; Behrndt, S.; von Tengg-Kobligk, H.; Kiessling, F.; Eichelsbacher, U.; Essig, M.; Read, T.-A.; et al. Dominant-negative inhibition of the AXL receptor tyrosine kinase suppresses brain tumor cell growth and invasion and prolongs survival. Proc. Natl. Acad. Sci. USA 2006, 103, 5799–5804. [Google Scholar] [CrossRef] [PubMed]
- Shieh, Y.-S.; Lai, C.-Y.; Kao, Y.-R.; Shiah, S.-G.; Chu, Y.-W.; Lee, H.-S.; Wu, C.-W. Expression of AXL in lung adenocarcinoma and correlation with tumor progression. Neoplasia 2005, 7, 1058–1064. [Google Scholar] [PubMed]
- Tai, K.-Y.; Shieh, Y.-S.; Lee, C.S.; Shiah, S.-G.; Wu, C.-W. AXL promotes cell invasion by inducing MMP-9 activity through activation of NF-κB and BRG-1. Oncogene 2008, 27, 4044–4055. [Google Scholar] [CrossRef] [PubMed]
- Brand, T.M.; Iida, M.; Stein, A.P.; Corrigan, K.L.; Braverman, C.M.; Coan, J.P.; Pearson, H.E.; Bahrar, H.; Fowler, T.L.; Bednarz, B.P.; et al. AXL Is a Logical Molecular Target in Head and Neck Squamous Cell Carcinoma. Clin. Cancer Res. 2015, 21, 2601–2612. [Google Scholar] [CrossRef] [PubMed]
- A Study of BGB324 in Combination with Erlotinib in Patients with Non-Small Cell Lung Cancer. Available online: https://clinicaltrials.gov/ct2/show/NCT02424617 (accessed on 2 November 2016).
- Multicenter Open-label Study of BGB324 as a Single Agent and in Combination with Cytarabine in Patients with AML. Available online: https://clinicaltrials.gov/ct2/show/NCT02488408 (accessed on 2 November 2016).
- Rheinwald, J.G.; Beckett, M.A. Tumorigenic keratinocyte lines requiring anchorage and fibroblast support cultured from human squamous cell carcinomas. Cancer Res. 1981, 41, 1657–1663. [Google Scholar] [PubMed]
- Kawamata, H.; Nakashiro, K.; Uchida, D.; Harada, K.; Yoshida, H.; Sato, M. Possible contribution of active MMP2 to lymph-node metastasis and secreted cathepsin L to bone invasion of newly established human oral-squamous-cancer cell lines. Int. J. Cancer 1997, 70, 120–127. [Google Scholar] [CrossRef]
- Liu, L.; Greger, J.; Shi, H.; Liu, Y.; Greshock, J.; Annan, R.; Halsey, W.; Sathe, G.M.; Martin, A.-M.; Gilmer, T.M. Novel mechanism of lapatinib resistance in HER2-positive breast tumor cells: Activation of AXL. Cancer Res. 2009, 69, 6871–6878. [Google Scholar] [CrossRef] [PubMed]
- Giles, K.M.; Kalinowski, F.C.; Candy, P.A.; Epis, M.R.; Zhang, P.M.; Redfern, A.D.; Stuart, L.M.; Goodall, G.J.; Leedman, P.J. AXL Mediates Acquired Resistance of Head and Neck Cancer Cells to the Epidermal Growth Factor Receptor Inhibitor Erlotinib. Mol. Cancer Ther. 2013, 12, 2541–2558. [Google Scholar] [CrossRef] [PubMed]
- Linger, R.M.A.; Cohen, R.A.; Cummings, C.T.; Sather, S.; Migdall-Wilson, J.; Middleton, D.H.G.; Lu, X.; Barón, A.E.; Franklin, W.A.; Merrick, D.T.; et al. MER or AXL receptor tyrosine kinase inhibition promotes apoptosis, blocks growth and enhances chemosensitivity of human non-small cell lung cancer. Oncogene 2013, 32, 3420–3431. [Google Scholar] [CrossRef] [PubMed]
- Tworkoski, K.A.; Platt, J.T.; Bacchiocchi, A.; Bosenberg, M.; Boggon, T.J.; Stern, D.F. MERTK controls melanoma cell migration and survival and differentially regulates cell behavior relative to AXL. Pigment Cell Melanoma Res. 2013, 26, 527–541. [Google Scholar] [CrossRef] [PubMed]
- Holland, S.J.; Pan, A.; Franci, C.; Hu, Y.; Chang, B.; Li, W.; Duan, M.; Torneros, A.; Yu, J.; Heckrodt, T.J.; et al. R428, a Selective Small Molecule Inhibitor of AXL Kinase, Blocks Tumor Spread and Prolongs Survival in Models of Metastatic Breast Cancer. Cancer Res. 2010, 70, 1544–1554. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Wang, X.; Bi, S.; Zhao, K.; Yu, C. Inhibition of MER and AXL receptor tyrosine kinases leads to increased apoptosis and improved chemosensitivity in human neuroblastoma. Biochem. Biophys. Res. Commun. 2015, 457, 461–466. [Google Scholar] [CrossRef] [PubMed]
- Rankin, E.B.; Fuh, K.C.; Taylor, T.E.; Krieg, A.J.; Musser, M.; Yuan, J.; Wei, K.; Kuo, C.J.; Longacre, T.A.; Giaccia, A.J. AXL is an essential factor and therapeutic target for metastatic ovarian cancer. Cancer Res. 2010, 70, 7570–7579. [Google Scholar] [CrossRef] [PubMed]
- Göke, F.; Bode, M.; Franzen, A.; Kirsten, R.; Goltz, D.; Göke, A.; Sharma, R.; Boehm, D.; Vogel, W.; Wagner, P.; et al. Fibroblast growth factor receptor 1 amplification is a common event in squamous cell carcinoma of the head and neck. Mod. Pathol. 2013, 26, 1298–1306. [Google Scholar] [CrossRef] [PubMed]
- Wilbertz, T.; Wagner, P.; Petersen, K.; Stiedl, A.-C.; Scheble, V.J.; Maier, S.; Reischl, M.; Mikut, R.; Altorki, N.K.; Moch, H.; et al. SOX2 gene amplification and protein overexpression are associated with better outcome in squamous cell lung cancer. Mod. Pathol. 2011, 24, 944–953. [Google Scholar] [CrossRef] [PubMed]
- Braun, M.; Kirsten, R.; Rupp, N.J.; Moch, H.; Fend, F.; Wernert, N.; Kristiansen, G.; Perner, S. Quantification of protein expression in cells and cellular subcompartments on immunohistochemical sections using a computer supported image analysis system. Histol. Histopathol. 2013, 28, 605–610. [Google Scholar] [PubMed]
- Johannessen, C.M.; Boehm, J.S.; Kim, S.Y.; Thomas, S.R.; Wardwell, L.; Johnson, L.A.; Emery, C.M.; Stransky, N.; Cogdill, A.P.; Barretina, J.; et al. COT drives resistance to RAF inhibition through MAP kinase pathway reactivation. Nature 2010, 468, 968–972. [Google Scholar] [CrossRef] [PubMed]
- Von Mässenhausen, A.; Sanders, C.; Thewes, B.; Deng, M.; Queisser, A.; Vogel, W.; Kristiansen, G.; Duensing, S.; Schröck, A.; Bootz, F.; et al. MERTK as a novel therapeutic target in head and neck cancer. Oncotarget 2016, 7, 32678–32694. [Google Scholar] [CrossRef] [PubMed]
- Brägelmann, J.; Klümper, N.; Offermann, A.; von Mässenhausen, A.; Böhm, D.; Deng, M.; Queisser, A.; Sanders, C.; Syring, I.; Merseburger, A.S.; et al. Pan-cancer analysis of the Mediator complex transcriptome identifies CDK19 and CDK8 as therapeutic targets in advanced prostate cancer. Clin. Cancer Res. 2016. [Google Scholar] [CrossRef] [PubMed]
- Kaplan, E.L.; Meier, P. Nonparametric estimation from incomplete observations. J. Am. Stat. Assoc. 1958, 53, 457–481. [Google Scholar] [CrossRef]
- Cox, D.R. Regression models and life-tables. J. R. Stat. Soc. Ser. B 1972, 34, 187–220. [Google Scholar]



| Clinical Parameter | Number | Median AXL Expression | Statistics |
|---|---|---|---|
| Available Tissues | |||
| Normal Mucosa | 24 (13 a) | 0.256 | p < 0.0001 b (normal mucosa vs. primary tumor, lymph node metastasis and recurrence) |
| Primary Tumor | 281 (17 a) | 0.304 | |
| Lymph node metastasis | 146 (14 a) | 0.401 | |
| Recurrence | 44 (5 a) | 0.551 | |
| Patients with Clinical Data (n = 321) | |||
| Gender | |||
| Male | 240 (74.8%) | 0.304 | p = 0.108 b |
| Female | 81 (25.2%) | 0.250 | |
| Age (Years, SD) | 61.7 (11.7) | ||
| Age | |||
| <54 | 86 (26.8) | 0.305 | p = 0.635 c |
| 54–62 | 84 (26.2) | 0.272 | |
| 62–70 | 80 (24.9) | 0.342 | |
| >70 | 71 (22.1) | 0.255 | |
| Anatomic Localization of Primary Tumor | |||
| Oral Cavity | 80 (24.9%) | 0.295 | p = 0.229 c |
| Oropharynx | 117 (36.5%) | 0.246 | |
| Hypopharynx/Larynx | 116 (36.1%) | 0.317 | |
| Unknown | 8 (2.5%) | ||
| Tobacco | |||
| Never-Smoker | 27 (8.4%) | 0.290 | p = 0.13 b |
| Ever-Smoker | 223 (69.5%) | 0.351 | |
| Unknown | 71 (22.1%) | ||
| Alcohol | |||
| Non-drinker | 89 (27.7%) | 0.312 | p = 0.112 c |
| Occasional | 58 (18.1%) | 0.351 | |
| Medium-Heavy | 85 (26.5%) | 0.244 | |
| Unknown | 89 (27.7%) | ||
| HPV Status | |||
| Positive | 30 (9.3%) | 0.295 | p = 0.429 b |
| Negative | 291 (90.7%) | 0.338 | |
| T-Stage of Primary | |||
| T1 | 76 (23.7%) | 0.299 | p = 0.324 c |
| T2 | 119 (37.1%) | 0.328 | |
| T3 | 72 (22.4%) | 0.274 | |
| T4 | 50 (15.6%) | 0.242 | |
| Unknown | 4 (1.2%) | ||
| N Stage of Primary | |||
| N0 | 137 (42.7%) | 0.288 | p = 0.495 c |
| N1 | 48 (15.0%) | 0.325 | |
| N2 | 124 (38.6%) | 0.260 | |
| N3 | 5 (1.5%) | 0.288 | |
| Unknown | 7 (2.2%) | ||
| M Stage of Primary | |||
| M0 | 305 (95.0%) | 0.290 | p = 0.32 b |
| M1 | 14 (4.4%) | 0.227 | |
| Unknown | 2 (0.6%) | ||
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Von Mässenhausen, A.; Brägelmann, J.; Billig, H.; Thewes, B.; Queisser, A.; Vogel, W.; Kristiansen, G.; Schröck, A.; Bootz, F.; Brossart, P.; et al. Implication of the Receptor Tyrosine Kinase AXL in Head and Neck Cancer Progression. Int. J. Mol. Sci. 2017, 18, 7. https://doi.org/10.3390/ijms18010007
Von Mässenhausen A, Brägelmann J, Billig H, Thewes B, Queisser A, Vogel W, Kristiansen G, Schröck A, Bootz F, Brossart P, et al. Implication of the Receptor Tyrosine Kinase AXL in Head and Neck Cancer Progression. International Journal of Molecular Sciences. 2017; 18(1):7. https://doi.org/10.3390/ijms18010007
Chicago/Turabian StyleVon Mässenhausen, Anne, Johannes Brägelmann, Hannah Billig, Britta Thewes, Angela Queisser, Wenzel Vogel, Glen Kristiansen, Andreas Schröck, Friedrich Bootz, Peter Brossart, and et al. 2017. "Implication of the Receptor Tyrosine Kinase AXL in Head and Neck Cancer Progression" International Journal of Molecular Sciences 18, no. 1: 7. https://doi.org/10.3390/ijms18010007
APA StyleVon Mässenhausen, A., Brägelmann, J., Billig, H., Thewes, B., Queisser, A., Vogel, W., Kristiansen, G., Schröck, A., Bootz, F., Brossart, P., Kirfel, J., & Perner, S. (2017). Implication of the Receptor Tyrosine Kinase AXL in Head and Neck Cancer Progression. International Journal of Molecular Sciences, 18(1), 7. https://doi.org/10.3390/ijms18010007