Licochalcone A Prevents the Loss of Dopaminergic Neurons by Inhibiting Microglial Activation in Lipopolysaccharide (LPS)-Induced Parkinson’s Disease Models
Abstract
:1. Introduction
2. Results
2.1. Lico.A Inhibits NO and PGE2 Production by Suppressing iNOS and COX-2 Expression in LPS-Stimulated Microglial BV-2 Cells
2.2. Lico.A Inhibits the Production of TNF-α, IL-6 and IL-1β and Down-Regulates the Expression of Cytokine mRNA in LPS-Stimulated BV-2 Cells
2.3. Lico.A Suppresses the Phosphorylation of the ERK 1/2 MAPK Signaling Pathway in LPS-Induced BV-2 Cells
2.4. Lico.A Attenuates the NF-κB p65 Inflammatory Signaling Pathway in LPS-Activated BV-2 Cells
2.5. Lico.A Prevents the Degeneration of Dopaminergic Neurons in LPS-Induced PD Models In Vitro
2.6. Lico.A Treatment Ameliorates the Behavioral Dysfunction of LPS-Induced PD Model Rats
2.7. Lico.A Treatment Prevents the Loss of TH-Positive Cells in the SN of LPS-Induced PD Model Rats
2.8. Lico.A Treatment Inhibits Microglial Activation and Down-Regulates mRNA Expression of Neuronal Toxic Factors in the SN of LPS-Induced PD Model Rats
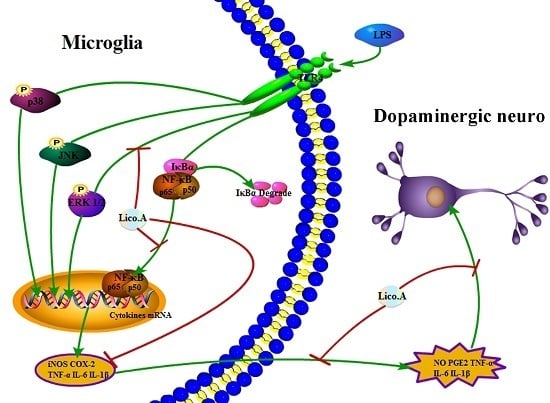
3. Discussion
4. Materials and Methods
4.1. Reagents
4.2. Cell Culture and Treatment
4.3. Animals and Treatment
4.4. NO Assay
4.5. Enzyme-Linked Immunosorbent Assay (ELISA)
4.6. RNA Isolation and Quantitative PCR
4.7. Western Blotting Analysis
4.8. Immunofluorescence Assay
4.9. Rat Mesencephalic Neuron-Glia Cultures
4.10. TH and Iba-1 Immunohistological Analysis
4.11. Motor Function Test with the Rotarod
4.12. [3H]DA Uptake Assay
4.13. Statistical Analysis
Acknowledgments
Author Contributions
Conflicts of Interest
Abbreviations
| Lico.A | Licochalcone A |
| PD | Parkinson’s disease |
| LPS | Lipopolysaccharide |
| SN | Substantia nigra |
| SNpc | Substantia nigra pars compacta |
| TH | Tyrosine hydroxylase |
| Iba-1 | Ionized calcium-binding adaptor molecule-1 |
References
- Schapira, A.H. Neurobiology and treatment of parkinson’s disease. Trends Pharmacol. Sci. 2009, 30, 41–47. [Google Scholar] [CrossRef] [PubMed]
- Obeso, J.A.; Rodriguez-Oroz, M.C.; Goetz, C.G.; Marin, C.; Kordower, J.H.; Rodriguez, M.; Hirsch, E.C.; Farrer, M.; Schapira, A.H.; Halliday, G. Missing pieces in the Parkinson’s disease puzzle. Nat. Med. 2010, 16, 653–661. [Google Scholar] [CrossRef] [PubMed]
- McGeer, P.L.; Itagaki, S.; Akiyama, H.; McGeer, E.G. Rate of cell death in parkinsonism indicates active neuropathological process. Ann. Neurol. 1988, 24, 574–576. [Google Scholar] [CrossRef] [PubMed]
- Block, M.L.; Zecca, L.; Hong, J.S. Microglia-mediated neurotoxicity: Uncovering the molecular mechanisms. Nat. Rev. Neurosci. 2007, 8, 57–69. [Google Scholar] [CrossRef] [PubMed]
- McGeer, P.L.; McGeer, E.G. Inflammation and neurodegeneration in Parkinson’s disease. Park. Relat. Disord. 2004, 10, S3–S7. [Google Scholar] [CrossRef] [PubMed]
- Graeber, M.B.; Streit, W.J. Microglia: Immune network in the CNS. Brain Pathol. 1990, 1, 2–5. [Google Scholar] [CrossRef] [PubMed]
- Whitton, P.S. Inflammation as a causative factor in the aetiology of Parkinson’s disease. Br. J. Pharmacol. 2007, 150, 963–976. [Google Scholar] [CrossRef] [PubMed]
- Rojo, A.I.; McBean, G.; Cindric, M.; Egea, J.; Lopez, M.G.; Rada, P.; Zarkovic, N.; Cuadrado, A. Redox control of microglial function: Molecular mechanisms and functional significance. Antioxid. Redox Signal. 2014, 21, 1766–1801. [Google Scholar] [CrossRef] [PubMed]
- Castano, A.; Herrera, A.J.; Cano, J.; Machado, A. Lipopolysaccharide intranigral injection induces inflammatory reaction and damage in nigrostriatal dopaminergic system. J. Neurochem. 1998, 70, 1584–1592. [Google Scholar] [CrossRef] [PubMed]
- Qin, L.; Wu, X.; Block, M.L.; Liu, Y.; Breese, G.R.; Hong, J.S.; Knapp, D.J.; Crews, F.T. Systemic LPS causes chronic neuroinflammation and progressive neurodegeneration. Glia 2007, 55, 453–462. [Google Scholar] [CrossRef] [PubMed]
- Fu, S.P.; Wang, J.F.; Xue, W.J.; Liu, H.M.; Liu, B.R.; Zeng, Y.L.; Li, S.N.; Huang, B.X.; Lv, Q.K.; Wang, W.; et al. Anti-inflammatory effects of BHBA in both in vivo and in vitro Parkinson’s disease models are mediated by GPR109A-dependent mechanisms. J. Neuroinflamm. 2015, 12, 9. [Google Scholar] [CrossRef] [PubMed]
- Machado, A.; Herrera, A.J.; Venero, J.L.; Santiago, M.; de Pablos, R.M.; Villaran, R.F.; Espinosa-Oliva, A.M.; Arguelles, S.; Sarmiento, M.; Delgado-Cortes, M.J.; et al. Inflammatory animal model for Parkinson’s disease: The intranigral injection of lps induced the inflammatory process along with the selective degeneration of nigrostriatal dopaminergic neurons. ISRN Neurol. 2011, 2011, 476158. [Google Scholar] [CrossRef] [PubMed]
- Mastroeni, D.; Grover, A.; Leonard, B.; Joyce, J.N.; Coleman, P.D.; Kozik, B.; Bellinger, D.L.; Rogers, J. Microglial responses to dopamine in a cell culture model of Parkinson’s disease. Neurobiol. Aging 2009, 30, 1805–1817. [Google Scholar] [CrossRef] [PubMed]
- Iczkiewicz, J.; Broom, L.; Cooper, J.D.; Wong, A.M.; Rose, S.; Jenner, P. The RGD-containing peptide fragment of osteopontin protects tyrosine hydroxylase positive cells against toxic insult in primary ventral mesencephalic cultures and in the rat substantia nigra. J. Neurochem. 2010, 114, 1792–1804. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Wang, C.; Wang, J.; Zhao, S.; Zhang, K.; Wang, J.; Zhang, W.; Wu, C.; Yang, J. Pseudoginsenoside-F11 (PF11) exerts anti-neuroinflammatory effects on LPS-activated microglial cells by inhibiting TLR4-mediated TAK1/IKK/NF-κB, mapks and akt signaling pathways. Neuropharmacology 2014, 79, 642–656. [Google Scholar] [CrossRef] [PubMed]
- Kim, I.S.; Ko, H.M.; Koppula, S.; Kim, B.W.; Choi, D.K. Protective effect of chrysanthemum indicum linne against 1-methyl-4-phenylpridinium ion and lipopolysaccharide-induced cytotoxicity in cellular model of parkinson’s disease. Food Chem. Toxicol. 2011, 49, 963–973. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Huang, D.; Xu, J.; Tong, J.; Wang, Z.; Huang, L.; Yang, Y.; Bai, X.; Wang, P.; Suo, H.; et al. Tiagabine protects dopaminergic neurons against neurotoxins by inhibiting microglial activation. Sci. Rep. 2015, 5, 15720. [Google Scholar] [CrossRef] [PubMed]
- Saijo, K.; Winner, B.; Carson, C.T.; Collier, J.G.; Boyer, L.; Rosenfeld, M.G.; Gage, F.H.; Glass, C.K. A Nurr1/corest pathway in microglia and astrocytes protects dopaminergic neurons from inflammation-induced death. Cell 2009, 137, 47–59. [Google Scholar] [CrossRef] [PubMed]
- Daher, J.P.; Volpicelli-Daley, L.A.; Blackburn, J.P.; Moehle, M.S.; West, A.B. Abrogation of α-synuclein-mediated dopaminergic neurodegeneration in LRRK2-deficient rats. Proc. Natl. Acad. Sci. USA 2014, 111, 9289–9294. [Google Scholar] [CrossRef] [PubMed]
- Gao, H.M.; Zhang, F.; Zhou, H.; Kam, W.; Wilson, B.; Hong, J.S. Neuroinflammation and α-synuclein dysfunction potentiate each other, driving chronic progression of neurodegeneration in a mouse model of parkinson’s disease. Environ. Health Perspect. 2011, 119, 807–814. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Zhang, S.M.; Hernan, M.A.; Schwarzschild, M.A.; Willett, W.C.; Colditz, G.A.; Speizer, F.E.; Ascherio, A. Nonsteroidal anti-inflammatory drugs and the risk of parkinson disease. Arch. Neurol. 2003, 60, 1059–1064. [Google Scholar] [CrossRef] [PubMed]
- Esposito, E.; di Matteo, V.; Benigno, A.; Pierucci, M.; Crescimanno, G.; di Giovanni, G. Non-steroidal anti-inflammatory drugs in Parkinson’s disease. Exp. Neurol. 2007, 205, 295–312. [Google Scholar] [CrossRef] [PubMed]
- Moore, A.H.; Bigbee, M.J.; Boynton, G.E.; Wakeham, C.M.; Rosenheim, H.M.; Staral, C.J.; Morrissey, J.L.; Hund, A.K. Non-steroidal anti-inflammatory drugs in Alzheimer’s disease and Parkinson’s disease: Reconsidering the role of neuroinflammation. Pharmaceuticals 2010, 3, 1812–1841. [Google Scholar] [CrossRef] [PubMed]
- Shibata, S. A drug over the millennia: Pharmacognosy, chemistry, and pharmacology of licorice. Yakugaku Zasshi 2000, 120, 849–862. [Google Scholar] [CrossRef] [PubMed]
- Kolbe, L.; Immeyer, J.; Batzer, J.; Wensorra, U.; tom Dieck, K.; Mundt, C.; Wolber, R.; Stab, F.; Schonrock, U.; Ceilley, R.I.; et al. Anti-inflammatory efficacy of licochalcone A: Correlation of clinical potency and in vitro effects. Arch. Dermatol. Res. 2006, 298, 23–30. [Google Scholar] [CrossRef] [PubMed]
- Mi-Ichi, F.; Miyadera, H.; Kobayashi, T.; Takamiya, S.; Waki, S.; Iwata, S.; Shibata, S.; Kita, K. Parasite mitochondria as a target of chemotherapy: Inhibitory effect of licochalcone a on the plasmodium falciparum respiratory chain. Ann. N. Y. Acad. Sci. 2005, 1056, 46–54. [Google Scholar] [CrossRef] [PubMed]
- Lv, H.; Ren, H.; Wang, L.; Chen, W.; Ci, X. Lico A enhances NRF2-mediated defense mechanisms against t-BHP-induced oxidative stress and cell death via Akt and ERK activation in raw 264.7 cells. Oxid. Med. Cell. Longev. 2015, 2015, 709845. [Google Scholar] [CrossRef] [PubMed]
- Rafi, M.M.; Rosen, R.T.; Vassil, A.; Ho, C.T.; Zhang, H.; Ghai, G.; Lambert, G.; DiPaola, R.S. Modulation of Bcl-2 and cytotoxicity by licochalcone-a, a novel estrogenic flavonoid. Anticancer Res. 2000, 20, 2653–2658. [Google Scholar] [PubMed]
- Fu, Y.; Hsieh, T.C.; Guo, J.; Kunicki, J.; Lee, M.Y.; Darzynkiewicz, Z.; Wu, J.M. Licochalcone-A, a novel flavonoid isolated from licorice root (Glycyrrhiza glabra), causes G2 and late-G1 arrests in androgen-independent PC-3 prostate cancer cells. Biochem. Biophys. Res. Commun. 2004, 322, 263–270. [Google Scholar] [CrossRef] [PubMed]
- Hao, W.; Yuan, X.; Yu, L.; Gao, C.; Sun, X.; Wang, D.; Zheng, Q. Licochalcone A-induced human gastric cancer BGC-823 cells apoptosis by regulating ROS-mediated MAPKs and PI3K/AKT signaling pathways. Sci. Rep. 2015, 5, 10336. [Google Scholar] [CrossRef] [PubMed]
- Chu, X.; Jiang, L.; Wei, M.; Yang, X.; Guan, M.; Xie, X.; Wei, J.; Liu, D.; Wang, D. Attenuation of allergic airway inflammation in a murine model of asthma by licochalcone A. Immunopharmacol. Immunotoxicol. 2013, 35, 653–661. [Google Scholar] [CrossRef] [PubMed]
- Chu, X.; Ci, X.; Wei, M.; Yang, X.; Cao, Q.; Guan, M.; Li, H.; Deng, Y.; Feng, H.; Deng, X. Licochalcone A inhibits lipopolysaccharide-induced inflammatory response in vitro and in vivo. J. Agric. Food Chem. 2012, 60, 3947–3954. [Google Scholar] [CrossRef] [PubMed]
- Hu, J.; Liu, J. Licochalcone A attenuates lipopolysaccharide-induced acute kidney injury by inhibiting NF-κb activation. Inflammation 2016, 39, 569–574. [Google Scholar] [CrossRef] [PubMed]
- Park, H.Y.; Han, M.H.; Park, C.; Jin, C.Y.; Kim, G.Y.; Choi, I.W.; Kim, N.D.; Nam, T.J.; Kwon, T.K.; Choi, Y.H. Anti-inflammatory effects of fucoidan through inhibition of NF-κB, MAPK and Akt activation in lipopolysaccharide-induced BV2 microglia cells. Food Chem. Toxicol. 2011, 49, 1745–1752. [Google Scholar] [CrossRef] [PubMed]
- Murakami, A.; Ohigashi, H. Targeting NOX, INOS and COX-2 in inflammatory cells: Chemoprevention using food phytochemicals. Int. J. Cancer 2007, 121, 2357–2363. [Google Scholar] [CrossRef] [PubMed]
- Collins, L.M.; Toulouse, A.; Connor, T.J.; Nolan, Y.M. Contributions of central and systemic inflammation to the pathophysiology of parkinson’s disease. Neuropharmacology 2012, 62, 2154–2168. [Google Scholar] [CrossRef] [PubMed]
- Kaminska, B. Mapk signalling pathways as molecular targets for anti-inflammatory therapy—From molecular mechanisms to therapeutic benefits. Biochim. Biophys. Acta 2005, 1754, 253–262. [Google Scholar] [CrossRef] [PubMed]
- Fu, S.P.; Li, S.N.; Wang, J.F.; Li, Y.; Xie, S.S.; Xue, W.J.; Liu, H.M.; Huang, B.X.; Lv, Q.K.; Lei, L.C.; et al. Bhba suppresses LPS-induced inflammation in BV-2 cells by inhibiting NF-κB activation. Mediat. Inflamm. 2014, 2014, 983401. [Google Scholar] [CrossRef] [PubMed]
- Iancu, R.; Mohapel, P.; Brundin, P.; Paul, G. Behavioral characterization of a unilateral 6-OHDA-lesion model of Parkinson’s disease in mice. Behav. Brain Res. 2005, 162, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Castano, A.; Herrera, A.J.; Cano, J.; Machado, A. The degenerative effect of a single intranigral injection of LPS on the dopaminergic system is prevented by dexamethasone, and not mimicked by rh-TNF-α, IL-1β and IFN-γ. J. Neurochem. 2002, 81, 150–157. [Google Scholar] [CrossRef] [PubMed]
- Herrera, A.J.; Castano, A.; Venero, J.L.; Cano, J.; Machado, A. The single intranigral injection of LPS as a new model for studying the selective effects of inflammatory reactions on dopaminergic system. Neurobiol. Dis. 2000, 7, 429–447. [Google Scholar] [CrossRef] [PubMed]
- Harada; Wu, J.; Haycock, J.W.; Goldstein, M. Regulation of l-DOPA biosynthesis by site-specific phosphorylation of tyrosine hydroxylase in AtT-20 cells expressing wild-type and serine 40-substituted enzyme. J. Neurochem. 1996, 67, 629–635. [Google Scholar]
- Hirsch, E.C.; Hunot, S.; Hartmann, A. Neuroinflammatory processes in Parkinson’s disease. Park. Relat. Disord. 2005, 11, S9–S15. [Google Scholar] [CrossRef] [PubMed]
- Liu, B.; Jiang, J.W.; Wilson, B.C.; Du, L.; Yang, S.N.; Wang, J.Y.; Wu, G.C.; Cao, X.D.; Hong, J.S. Systemic infusion of naloxone reduces degeneration of rat substantia nigral dopaminergic neurons induced by intranigral injection of lipopolysaccharide. J. Pharm. Exp. Ther. 2000, 295, 125–132. [Google Scholar]
- Candore, G.; Balistreri, C.R.; Grimaldi, M.P.; Vasto, S.; Listi, F.; Chiappelli, M.; Licastro, F.; Lio, D.; Caruso, C. Age-related inflammatory diseases: Role of genetics and gender in the pathophysiology of Alzheimer’s disease. Ann. N. Y. Acad. Sci. 2006, 1089, 472–486. [Google Scholar] [CrossRef] [PubMed]
- Sanchez-Guajardo, V.; Barnum, C.J.; Tansey, M.G.; Romero-Ramos, M. Neuroimmunological processes in parkinson’s disease and their relation to α-synuclein: Microglia as the referee between neuronal processes and peripheral immunity. ASN Neuro 2013, 5, 113–139. [Google Scholar] [CrossRef] [PubMed]
- Song, Y.; Qu, R.; Zhu, S.; Zhang, R.; Ma, S. Rhynchophylline attenuates LPS-induced pro-inflammatory responses through down-regulation of MAPK/NF-κB signaling pathways in primary microglia. Phytother. Res. PTR 2012, 26, 1528–1533. [Google Scholar] [CrossRef] [PubMed]
- De Oliveira, A.C.; Candelario-Jalil, E.; Bhatia, H.S.; Lieb, K.; Hull, M.; Fiebich, B.L. Regulation of prostaglandin E2 synthase expression in activated primary rat microglia: Evidence for uncoupled regulation of mPGES-1 and COX-2. Glia 2008, 56, 844–855. [Google Scholar] [CrossRef] [PubMed]
- McGeer, P.L.; McGeer, E.G. The inflammatory response system of brain: Implications for therapy of alzheimer and other neurodegenerative diseases. Brain Res. Rev. 1995, 21, 195–218. [Google Scholar] [CrossRef]
- Levy, O.A.; Malagelada, C.; Greene, L.A. Cell death pathways in Parkinson’s disease: Proximal triggers, distal effectors, and final steps. Apoptosis 2009, 14, 478–500. [Google Scholar] [CrossRef] [PubMed]
- Peng, G.S.; Li, G.; Tzeng, N.S.; Chen, P.S.; Chuang, D.M.; Hsu, Y.D.; Yang, S.; Hong, J.S. Valproate pretreatment protects dopaminergic neurons from LPS-induced neurotoxicity in rat primary midbrain cultures: Role of microglia. Mol. Brain Res. 2005, 134, 162–169. [Google Scholar] [CrossRef] [PubMed]
- Li, F.Q.; Lu, X.Z.; Liang, X.B.; Zhou, H.F.; Xue, B.; Liu, X.Y.; Niu, D.B.; Han, J.S.; Wang, X.M. Triptolide, a chinese herbal extract, protects dopaminergic neurons from inflammation-mediated damage through inhibition of microglial activation. J. Neuroimmunol. 2004, 148, 24–31. [Google Scholar] [CrossRef] [PubMed]
- Hoban, D.B.; Connaughton, E.; Connaughton, C.; Hogan, G.; Thornton, C.; Mulcahy, P.; Moloney, T.C.; Dowd, E. Further characterisation of the LPS model of Parkinson’s disease: A comparison of intra-nigral and intra-striatal lipopolysaccharide administration on motor function, microgliosis and nigrostriatal neurodegeneration in the rat. Brain Behav. Immun. 2013, 27, 91–100. [Google Scholar] [CrossRef] [PubMed]
- Ming, L.; Jin, F.; Huang, P.; Luo, H.; Liu, W.; Zhang, L.; Yuan, W.; Zhang, Y.; Jin, Y. Licochalcone a up-regulates of Fasl in mesenchymal stem cells to strengthen bone formation and increase bone mass. Sci. Rep. 2014, 4, 7209. [Google Scholar] [CrossRef] [PubMed]
- Kwon, H.S.; Park, J.H.; Kim, D.H.; Kim, Y.H.; Park, J.H.; Shin, H.K.; Kim, J.K. Licochalcone A isolated from licorice suppresses lipopolysaccharide-stimulated inflammatory reactions in raw264.7 cells and endotoxin shock in mice. J. Mol. Med. 2008, 86, 1287–1295. [Google Scholar] [CrossRef] [PubMed]
- Funakoshi-Tago, M.; Tago, K.; Nishizawa, C.; Takahashi, K.; Mashino, T.; Iwata, S.; Inoue, H.; Sonoda, Y.; Kasahara, T. Licochalcone A is a potent inhibitor of TEL-Jak2-mediated transformation through the specific inhibition of Stat3 activation. Biochem. Pharmacol. 2008, 76, 1681–1693. [Google Scholar] [CrossRef] [PubMed]
- Tsai, J.P.; Lee, C.H.; Ying, T.H.; Lin, C.L.; Lin, C.L.; Hsueh, J.T.; Hsieh, Y.H. Licochalcone A induces autophagy through PI3K/Akt/mTOR inactivation and autophagy suppression enhances licochalcone A-induced apoptosis of human cervical cancer cells. Oncotarget 2015, 6, 28851–28866. [Google Scholar] [CrossRef] [PubMed]
- Boje, K.M. Nitric oxide neurotoxicity in neurodegenerative diseases. Front. Biosci. 2004, 9, 763–776. [Google Scholar] [CrossRef] [PubMed]
- Heneka, M.T.; O’Banion, M.K. Inflammatory processes in Alzheimer’s disease. J. Neuroimmunol. 2007, 184, 69–91. [Google Scholar] [CrossRef] [PubMed]
- Liu, B.; Gao, H.M.; Wang, J.Y.; Jeohn, G.H.; Cooper, C.L.; Hong, J.S. Role of nitric oxide in inflammation-mediated neurodegeneration. Ann. N. Y. Acad. Sci. 2002, 962, 318–331. [Google Scholar] [CrossRef] [PubMed]
- Saha, R.N.; Pahan, K. Regulation of inducible nitric oxide synthase gene in glial cells. Antioxid. Redox Signal. 2006, 8, 929–947. [Google Scholar] [CrossRef] [PubMed]
- Nakanishi, M.; Rosenberg, D.W. Multifaceted roles of PGE2 in inflammation and cancer. Semin. Immunopathol. 2013, 35, 123–137. [Google Scholar] [CrossRef] [PubMed]
- Dewapriya, P.; Li, Y.X.; Himaya, S.W.; Pangestuti, R.; Kim, S.K. Neoechinulin a suppresses amyloid- oligomer-induced microglia activation and thereby protects PC-12 cells from inflammation-mediated toxicity. Neurotoxicology 2013, 35, 30–40. [Google Scholar] [CrossRef] [PubMed]
- Rubio-Perez, J.M.; Morillas-Ruiz, J.M. A review: Inflammatory process in alzheimer’s disease, role of cytokines. Sci. World J. 2012, 2012, 756357. [Google Scholar] [CrossRef] [PubMed]
- Rothwell, N. Interleukin-1 and neuronal injury: Mechanisms, modification, and therapeutic potential. Brain Behav. Immun. 2003, 17, 152–157. [Google Scholar] [CrossRef]
- Guadagno, J.; Swan, P.; Shaikh, R.; Cregan, S.P. Microglia-derived IL-1β triggers p53-mediated cell cycle arrest and apoptosis in neural precursor cells. Cell Death Dis. 2015, 6, e1779. [Google Scholar] [CrossRef] [PubMed]
- Burton, M.D.; Rytych, J.L.; Freund, G.G.; Johnson, R.W. Central inhibition of interleukin-6 trans-signaling during peripheral infection reduced neuroinflammation and sickness in aged mice. Brain Behav. Immun. 2013, 30, 66–72. [Google Scholar] [CrossRef] [PubMed]
- Neher, J.J.; Brown, G.C. Neurodegeneration in models of gram-positive bacterial infections of the central nervous system. Biochem. Soc. Trans. 2007, 35, 1166–1167. [Google Scholar] [CrossRef] [PubMed]
- Liu, T.Y.; Yang, X.Y.; Zheng, L.T.; Wang, G.H.; Zhen, X.C. Activation of Nur77 in microglia attenuates proinflammatory mediators production and protects dopaminergic neurons from inflammation-induced cell death. J. Neurochem. 2016, 140, 589–604. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Shao, S.Y.; Song, X.Y.; Xia, C.Y.; Yang, Y.N.; Zhang, P.C.; Chen, N.H. Protective effects of forsythia suspense extract with antioxidant and anti-inflammatory properties in a model of rotenone induced neurotoxicity. Neurotoxicology 2016, 52, 72–83. [Google Scholar] [CrossRef] [PubMed]
- Soos, J.; Engelhardt, J.I.; Siklos, L.; Havas, L.; Majtenyi, K. The expression of PARP, NF-κB and parvalbumin is increased in parkinson disease. Neuroreport 2004, 15, 1715–1718. [Google Scholar] [CrossRef] [PubMed]
- Gayle, D.A.; Ling, Z.; Tong, C.; Landers, T.; Lipton, J.W.; Carvey, P.M. Lipopolysaccharide (LPS)-induced dopamine cell loss in culture: Roles of tumor necrosis factor-α, interleukin-1, and nitric oxide. Dev. Brain Res. 2002, 133, 27–35. [Google Scholar] [CrossRef]
- Chen, S.H.; Oyarzabal, E.A.; Sung, Y.F.; Chu, C.H.; Wang, Q.; Chen, S.L.; Lu, R.B.; Hong, J.S. Microglial regulation of immunological and neuroprotective functions of astroglia. Glia 2015, 63, 118–131. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Chen, X.; Liu, Z.; Peng, Y.P.; Qiu, Y.H. Interleukin-10 protection against lipopolysaccharide-induced neuro-inflammation and neurotoxicity in ventral mesencephalic cultures. Int. J. Mol. Sci. 2016, 17, 25. [Google Scholar] [CrossRef] [PubMed]
- Zhou, H.F.; Liu, X.Y.; Niu, D.B.; Li, F.Q.; He, Q.H.; Wang, X.M. Triptolide protects dopaminergic neurons from inflammation-mediated damage induced by lipopolysaccharide intranigral injection. Neurobiol. Dis. 2005, 18, 441–449. [Google Scholar] [CrossRef] [PubMed]
- Hernandez-Romero, M.C.; Arguelles, S.; Villaran, R.F.; de Pablos, R.M.; Delgado-Cortes, M.J.; Santiago, M.; Herrera, A.J.; Cano, J.; Machado, A. Simvastatin prevents the inflammatory process and the dopaminergic degeneration induced by the intranigral injection of lipopolysaccharide. J. Neurochem. 2008, 105, 445–459. [Google Scholar] [CrossRef] [PubMed]
- Lu, X.; Bing, G.; Hagg, T. Naloxone prevents microglia-induced degeneration of dopaminergic substantia nigra neurons in adult rats. Neuroscience 2000, 97, 285–291. [Google Scholar] [CrossRef]
- Bollimpelli, V.S.; Kondapi, A.K. Enriched rat primary ventral mesencephalic neurons as an in-vitro culture model. Neuroreport 2015, 26, 728–734. [Google Scholar] [CrossRef] [PubMed]
- Tolosa, A.; Zhou, X.; Spittau, B.; Krieglstein, K. Establishment of a survival and toxic cellular model for Parkinson's disease from chicken mesencephalon. Neurotox. Res. 2013, 24, 119–129. [Google Scholar] [CrossRef] [PubMed]
- Ha, S.K.; Moon, E.; Ju, M.S.; Kim, D.H.; Ryu, J.H.; Oh, M.S.; Kim, S.Y. 6-Shogaol, a ginger product, modulates neuroinflammation: A new approach to neuroprotection. Neuropharmacology 2012, 63, 211–223. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Liu, W.; Li, L.; Holscher, C. Neuroprotective effects of a GIP analogue in the MPTP Parkinson's disease mouse model. Neuropharmacology 2016, 101, 255–263. [Google Scholar] [CrossRef] [PubMed]







| Gene | Sequences | Length (bp) |
|---|---|---|
| β-actin | (F) 5′-GTCAGGTCATCACTATCGGCAAT-3′ | 147 |
| (R) 5′-AGAGGTCTTTACGGATGTCAACGT-3′ | ||
| iNOS | (F) 5′-GAACTGTAGCACAGCACAGGAAAT-3′ | 158 |
| (R) 5′-CGTACCGGATGAGCTGTGAAT-3′ | ||
| COX-2 | (F) 5′-CAGTTTATGTTGTCTGTCCAGAGTTTC-3′ | 127 |
| (R) 5′-CCAGCACTTCACCCATCAGTT-3′ | ||
| TNF-α | (F) 5′-CCCCAAAGGGATGAGAAGTTC-3′ | 136 |
| (R) 5′-CCTCCACTTGGTGGTTTGCT-3′ | ||
| IL-1β | (F) 5′-GTTCCCATTAGACAACTGCACTACAG-3′ | 139 |
| (R) 5′-GTCGTTGCTTGGTTCTCCTTGTA-3′ | ||
| IL-6 | (F) 5′-CCAGAAACCGCTATGAAGTTCC-3′ | 138 |
| (R) 5′-GTTGGGAGTGGTATCCTCTGTGA-3′ |
| Gene | Sequences | Length (bp) |
|---|---|---|
| β-actin | (F) 5′-GTCAGGTCATCACTATCGGCAAT-3′ | 147 |
| (R) 5′-AGAGGTCTTTACGGATGTCAACGT-3′ | ||
| iNOS | (F) 5′-CACCCAGAAGAGTTACAGC-3′ | 186 |
| (R) 5′-GGAGGGAAGGGAGAATAG-3′ | ||
| COX-2 | (F) 5′-AGAGTCAGTTAGTGGGTAGT-3′ | 170 |
| (R) 5′-CTTGTAGTAGGCTTAAACATAG-3′ | ||
| TNF-α | (F) 5′-CCACGCTCTTCTGTCTACTG-3′ | 145 |
| (R) 5′-GCTACGGGCTTGTCACTC-3′ | ||
| IL-1β | (F) 5′-TGTGATGTTCCCATTAGAC-3′ | 131 |
| (R) 5′-AATACCACTTGTTGGCTTA-3′ | ||
| IL-6 | (F) 5′-AGCCACTGCCTTCCCTAC-3′ | 156 |
| (R) 5′-TTGCCATTGCACAACTCTT-3′ |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huang, B.; Liu, J.; Ju, C.; Yang, D.; Chen, G.; Xu, S.; Zeng, Y.; Yan, X.; Wang, W.; Liu, D.; et al. Licochalcone A Prevents the Loss of Dopaminergic Neurons by Inhibiting Microglial Activation in Lipopolysaccharide (LPS)-Induced Parkinson’s Disease Models. Int. J. Mol. Sci. 2017, 18, 2043. https://doi.org/10.3390/ijms18102043
Huang B, Liu J, Ju C, Yang D, Chen G, Xu S, Zeng Y, Yan X, Wang W, Liu D, et al. Licochalcone A Prevents the Loss of Dopaminergic Neurons by Inhibiting Microglial Activation in Lipopolysaccharide (LPS)-Induced Parkinson’s Disease Models. International Journal of Molecular Sciences. 2017; 18(10):2043. https://doi.org/10.3390/ijms18102043
Chicago/Turabian StyleHuang, Bingxu, Juxiong Liu, Chen Ju, Dongxue Yang, Guangxin Chen, Shiyao Xu, Yalong Zeng, Xuan Yan, Wei Wang, Dianfeng Liu, and et al. 2017. "Licochalcone A Prevents the Loss of Dopaminergic Neurons by Inhibiting Microglial Activation in Lipopolysaccharide (LPS)-Induced Parkinson’s Disease Models" International Journal of Molecular Sciences 18, no. 10: 2043. https://doi.org/10.3390/ijms18102043
APA StyleHuang, B., Liu, J., Ju, C., Yang, D., Chen, G., Xu, S., Zeng, Y., Yan, X., Wang, W., Liu, D., & Fu, S. (2017). Licochalcone A Prevents the Loss of Dopaminergic Neurons by Inhibiting Microglial Activation in Lipopolysaccharide (LPS)-Induced Parkinson’s Disease Models. International Journal of Molecular Sciences, 18(10), 2043. https://doi.org/10.3390/ijms18102043