Anti-Photoaging Effect of Jeju Putgyul (Unripe Citrus) Extracts on Human Dermal Fibroblasts and Ultraviolet B-induced Hairless Mouse Skin
Abstract
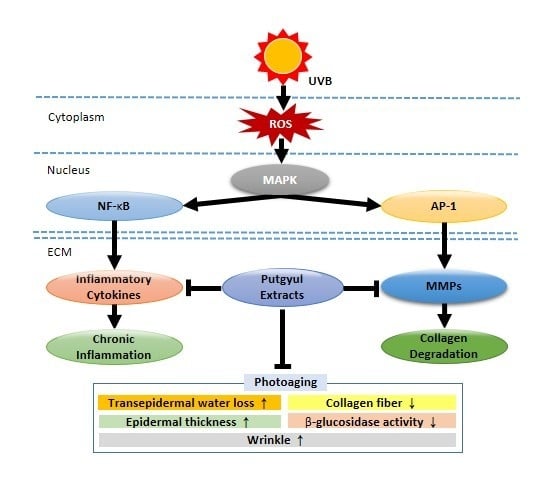
:1. Introduction
2. Results
2.1. Antioxidant Activity of Putgyul Extracts
2.2. Putgyul Extracts Improve Intracellular Collagen and Matrix Metalloproteinase (MMP)-1 in Human Dermal Fibroblasts (HDFs)
2.3. Putgyul Extract Inhibits Inflammatory Cytokines in UVB-Induced Hairless Mice
2.4. Putgyul Extract Inhibits mRNA and Protein Expression of MMPs In Vivo
2.5. Putgyul Extract Inhibits UVB-Induced Photoaging In Vivo
2.6. Putgyul Extract Inhibits Increased Epidermal Thickness and Collagen Degradation by UVB-Induced Photoaging In Vivo
2.7. Putgyul Extract Improves Transepidermal Water Loss (TEWL) and β-Glucosidase Activity In Vivo
3. Discussion
4. Materials and Methods
4.1. Materials
4.2. Sample Preparation
4.3. Determination of Antioxidant Activity
4.4. Cell Culture and Treatments
4.5. Cell Viability
4.6. Measurement of Intracellular Collagen
4.7. Measurement of Intracellular Collagenase
4.8. Animal Experiment
4.9. Wrinkle Measurement
4.10. Measurement of Transepidermal Water Loss (TEWL)
4.11. Measurement of β-Glucosidase Activity
4.12. Real-Time Quantitative Reverse Transcription-Polymerase Chain Reaction (RT-PCR)
4.13. Enzyme-Linked Immunosorbent Assay (ELISA)
4.14. Histological Analysis
4.15. Statistical Analysis
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
Abbreviations
| UV | Ultraviolet |
| PIP | Procollagen type I c-peptide |
| MMP | Matrix metalloproteinase |
| RA | Retinoic acid |
| EGCG | Epigallocatechin gallate |
References
- Hu, L.; Tan, J.; Yang, X.; Tan, H.; Xu, X.; You, M.; Qin, W.; Huang, L.; Li, S.; Mo, M.; et al. Polysaccharide extracted from Laminaria japonica delays intrinsic skin aging in mice. Evid. Based Complement Altern. Med. 2016, 2016, 1–8. [Google Scholar]
- Elias, P. The skin barrier as an innate immune element. Semin. Immunopathol. 2007, 29, 3–14. [Google Scholar] [CrossRef] [PubMed]
- Chung, J.H. Photoaging in Asians. Photodermatol. Photoimmunol. Photomed. 2003, 19, 109–121. [Google Scholar] [CrossRef] [PubMed]
- Naylor, E.C.; Watson, R.E.; Sherratt, M.J. Molecular aspects of skin ageing. Maturitas. 2011, 69, 249–256. [Google Scholar] [CrossRef] [PubMed]
- Fisher, G.J.; Wang, Z.; Datta, S.C.; Varani, J.; Kang, S.; Voorhees, J.J. Pathophysiology of premature skin aging induced by ultraviolet light. N. Engl. J. Med. 1997, 337, 1419–1429. [Google Scholar] [CrossRef] [PubMed]
- Afaq, F.; Mukhtar, H. Botanical antioxidants in the prevention of photocarcinogenesis and photoaging. Exp. Dermatol. 2006, 15, 678–684. [Google Scholar] [CrossRef] [PubMed]
- Fisher, G.J.; Kang, S.; Varani, J.; Bata-Csorgo, Z.; Wan, Y.; Datta, S.; Voorhees, J.J. Mechanisms of photoaging and chronological skin aging. Arch. Dermatol. 2002, 138, 1462–1470. [Google Scholar] [CrossRef] [PubMed]
- Ganceviciene, R.; Liakou, A.I.; Theodoridis, A.; Makrantonaki, E.; Zouboulis, C.C. Skin anti-aging strategies. Dermatoendocrinology 2012, 4, 308–319. [Google Scholar] [CrossRef] [PubMed]
- Berneburg, M.; Plettenberg, H.; Medve-König, K.; Pfahlberg, A.; Gers-Barlag, H.; Gefeller, O.; Krutmann, J. Induction of the photoaging-associated mitochondrial common deletion in vivo in normal human skin. J. Investig. Dermatol. 2004, 122, 1277–1283. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.S.; Oh, G.H.; Kim, M.J.; Hwang, J.K. Fucosterol inhibits matrix metalloproteinase expression and promotes type-1 procollagen production in UVB-induced HaCaT cells. Photochem. Photobiol. 2013, 89, 911–918. [Google Scholar] [CrossRef] [PubMed]
- Liu, D.; Ji, L.; Wang, Y.; Zheng, L. Cyclooxygenase-2 expression, prostacyclin production and endothelial protection of high-density lipoprotein. Cardiovasc. Hematol. Disord. Drug Targets 2012, 12, 98–105. [Google Scholar] [CrossRef] [PubMed]
- Indra, M.R.; Karyono, S.; Ratnawati, R.; Malik, S.G. Quercetin suppresses inflammation by reducing ERK1/2 phosphorylation and NFκB activation in leptin-induced human umbilical vein endothelial cells (HUVECs). BMC Res. Notes 2013, 6, 275–282. [Google Scholar] [CrossRef] [PubMed]
- Cooper, S.J.; Bowden, G.T. Ultraviolet B regulation of transcription factor families: Roles of nuclear factor-kappa B (NF-κB) and activator protein-1 (AP-1) in UVB-induced skin carcinogenesis. Curr. Cancer Drug Targets 2007, 7, 325–334. [Google Scholar] [CrossRef] [PubMed]
- Kawaii, S.; Tomono, Y.; Katase, E.; Ogawa, K.; Yano, M. Quantitation of flavonoid constituents in citrus fruits. J. Agric. Food Chem. 1999, 47, 3565–3571. [Google Scholar] [CrossRef] [PubMed]
- Tanizawa, H.; Ohkawa, Y.; Takino, Y.; Miyase, T.; Ueno, A.; Kageyama, T.; Hara, S. Studies on natural antioxidants in citrus species. I. Determination of antioxidative activities of citrus fruits. Chem. Pharm. Bull. 1992, 40, 1940–1942. [Google Scholar] [CrossRef] [PubMed]
- Kang, Y.J.; Yang, M.H.; Ko, W.J.; Park, S.R.; Lee, B.G. Studies on the major components and antioxidative properties of whole fruit powder and juice prepared from premature mandarin orange. Korean J. Food Sci. Technol. 2005, 37, 783–788. [Google Scholar]
- Chen, C.C.; Chiang, A.N.; Liu, H.N.; Chang, Y.T. EGb-761 prevents ultraviolet B-induced photoaging via inactivation of mitogen-activated protein kinases and proinflammatory cytokine expression. J. Dermatol. Sci. 2014, 75, 55–62. [Google Scholar] [CrossRef] [PubMed]
- Chakraborti, S.; Mandal, M.; Das, S.; Mandal, A.; Chakraborti, T. Regulation of matrix metalloproteinases: An overview. Mol. Cell. Biochem. 2003, 253, 269–285. [Google Scholar] [CrossRef] [PubMed]
- Im, A.R.; Song, J.H.; Lee, M.Y.; Chae, S. Magnolol reduces UVB-induced photodamage by regulating matrix metalloproteinase activity. Environ. Toxicol. Pharmacol. 2015, 39, 417–423. [Google Scholar] [CrossRef] [PubMed]
- Kitatani, K.; Sheldon, K.; Rajagopalan, V.; Anelli, V.; Jenkins, R.W.; Sun, Y.; Grabowski, G.A.; Obeid, L.M.; Hannun, Y.A. Involvement of acid β-glucosidase 1 in the salvage pathway of ceramide formation. J. Biol. Chem. 2009, 284, 12972–12978. [Google Scholar] [CrossRef] [PubMed]
- Rittié, L.; Fisher, G.J. UV-light-induced signal cascades and skin aging. Ageing Res. Rev. 2002, 1, 705–720. [Google Scholar] [CrossRef]
- El-Domyati, M.; Attia, S.; Saleh, F.; Brown, D.; Birk, D.E.; Gasparro, F.; Ahmad, H.; Uitto, J. Intrinsic aging vs. photoaging: A comparative histopathological, immunohistochemical, and ultrastructural study of skin. Exp. Dermatol. 2002, 11, 398–405. [Google Scholar] [CrossRef] [PubMed]
- Helfrich, Y.R.; Sachs, D.L.; Voorhees, J.J. Overview of skin aging and photoaging. Dermatol Nurs. 2008, 20, 177–183. [Google Scholar] [PubMed]
- Huang, C.C.; Hsu, B.Y.; Wu, N.L.; Tsui, W.H.; Lin, T.J.; Su, C.C.; Hung, C.F. Anti-photoaging effects of soy isoflavone extract (aglycone and acetylglucoside form) from soybean cake. Int. J. Mol. Sci. 2010, 11, 4782–4795. [Google Scholar] [CrossRef] [PubMed]
- Seo, Y.K.; Jung, S.H.; Song, K.Y.; Park, J.K.; Park, C.S. Anti-photoaging effect of fermented rice bran extract on UV-induced normal skin fibroblasts. Eur. Food Res. Technol. 2010, 231, 163–169. [Google Scholar] [CrossRef]
- Darr, D.; Fridovich, I. Free radicals in cutaneous biology. J. Investig. Dermatol. 1994, 102, 671–675. [Google Scholar] [CrossRef] [PubMed]
- Hanawalt, P.C.; Cooper, P.K.; Ganesan, A.K.; Smith, C.A. DNA repair in bacteria and mammalian cells. Ann. Rev. Biochem. 1979, 48, 783–836. [Google Scholar] [CrossRef] [PubMed]
- Ghasemi, K.; Ghasemi, Y.; Ebrahimzadeh, M.A. Antioxidant activity, phenol and flavonoid contents of 13 citrus species peels and tissues. Pak. J. Pharm. Sci. 2009, 22, 277–281. [Google Scholar] [PubMed]
- Yi, M.R.; Hwang, J.H.; Oh, Y.S.; Oh, H.J.; Lim, S.B. Quality characteristics and antioxidant activity of immature Citrus unshiu vinegar. J. Korean Soc. Food Sci. Nutr. 2014, 43, 250–257. [Google Scholar] [CrossRef]
- Park, C.H.; Lee, M.J.; Kim, J.P.; Yoo, I.D.; Chung, J.H. Prevention of UV radiation-induced premature skin aging in hairless mice by the novel compound melanocin A. Photochem. Photobiol. 2006, 82, 574–578. [Google Scholar] [CrossRef] [PubMed]
- Kong, C.S.; Kim, J.A.; Ahn, B.; Byun, H.G.; Kim, S.K. Carboxymethylations of chitosan and chitin inhibit MMP expression and ROS scavenging in human fibrosarcoma cells. Process Biochem. 2010, 45, 179–186. [Google Scholar] [CrossRef]
- Hwang, I.S.; Kim, J.E.; Choi, S.I.; Lee, H.R.; Lee, Y.J.; Jang, M.J.; Son, H.J.; Lee, H.S.; Oh, C.H.; Kim, B.H.; et al. UV radiation-induced skin aging in hairless mice is effectively prevented by oral intake of sea buckthorn (Hippophae rhamnoides L.) fruit blend for 6 weeks through MMP suppression and increase of SOD activity. Int. J. Mol. Sci. 2012, 30, 392–400. [Google Scholar] [CrossRef] [PubMed]
- Brennan, M.; Bhatti, H.; Nerusu, K.C.; Bhagavathula, N.; Kang, S.; Fisher, G.J.; Varani, J.; Voorhees, J.J. Matrix metalloproteinase-1 is the major collagenolytic enzyme responsible for collagen damage in UV-irradiated human skin. Photochem. Photobiol. 2003, 78, 43–48. [Google Scholar] [CrossRef]
- Hong, K.B.; Park, Y.; Kim, J.H.; Kim, J.M.; Suh, H.J. Effects of porcine placenta extract ingestion on ultraviolet B-induced skin damage in hairless mice. Korean J. Food Sci. Ani. Resour. 2015, 35, 413. [Google Scholar] [CrossRef] [PubMed]
- Park, J.E.; Pyun, H.B.; Woo, S.W.; Jeong, J.H.; Hwang, J.K. The protective effect of Kaempferia parviflora extract on UVB-induced skin photoaging in hairless mice. Photodermatol. Photoimmunol. Photomed. 2014, 30, 237–245. [Google Scholar] [CrossRef] [PubMed]
- Afaq, F.; Adhami, V.M.; Mukhtar, H. Photochemoprevention of ultraviolet B signaling and photocarcinogenesis. Mutat. Res. 2005, 571, 153–173. [Google Scholar] [CrossRef] [PubMed]
- Halliday, G.M. Inflammation, gene mutation and photoimmunosuppression in response to UVR-induced oxidative damage contributes to photocarcinogenesis. Mutat. Res. 2005, 571, 107–120. [Google Scholar] [CrossRef] [PubMed]
- Inomata, S.; Takada, K.; Tsunenaga, M.; Fukuda, M.; Matsunaga, Y.; Amano, S.; Takada, K.; Kobayashi, K.; Tsunenaga, M.; Nishiyama, T.; et al. Possible involvement of gelatinases in basement membrane damage and wrinkle formation in chronically ultraviolet B-exposed hairless mouse. J. Investig. Dermatol. 2003, 120, 128–134. [Google Scholar] [CrossRef] [PubMed]
- Fujimura, T.; Moriwaki, S.; Takema, Y.; Imokawa, G. Epidermal change can alter mechanical properties of hairless mouse skin topically treated with 1α, 25-dihydroxyvitamin D3. J. Dermatol. Sci. 2000, 24, 105–111. [Google Scholar] [CrossRef]
- Im, A.R.; Song, J.H.; Lee, M.Y.; Yeon, S.H.; Um, K.A.; Chae, S. Anti-wrinkle effects of fermented and non-fermented cyclopia intermedia in hairless mice. BMC Complement. Altern. Med. 2014, 14, 424–429. [Google Scholar] [CrossRef] [PubMed]
- Yaar, M.; Gilchrest, B.A. Photoageing: Mechanism, prevention and therapy. Br. J. Dermatol. 2007, 157, 874–887. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.O.; Kim, S.N.; Kim, Y.C. Anti-wrinkle effects of water extracts of teas in hairless mouse. Toxicol. Res. 2014, 30, 283–289. [Google Scholar] [CrossRef] [PubMed]
- Adnan Nasir. Diseases associated with cutaneous barrier dysfunction: Basic science aspects and clinical perspectives. In Toxicology of the Skin, 1st ed.; Monteiro-Riviere, N.A., Ed.; Informa Healthcare: New York, NY, USA, 2010; Volume 29, pp. 203–279. [Google Scholar]
- Blois, M.S. Antioxidant determinations by the use of a stable free radical. Nature 1958, 181, 1199–1200. [Google Scholar] [CrossRef]
- Kim, D.B.; Shin, G.H.; Kim, J.M.; Kim, Y.H.; Lee, J.H.; Lee, J.S.; Hong, H.J.; Choe, S.Y.; Park, I.J.; Cho, J.H.; et al. Antioxidant and anti-ageing activities of citrus-based juice mixture. Food Chem. 2016, 194, 920–927. [Google Scholar] [CrossRef] [PubMed]





© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Choi, S.-H.; Choi, S.-I.; Jung, T.-D.; Cho, B.-Y.; Lee, J.-H.; Kim, S.-H.; Yoon, S.-A.; Ham, Y.-M.; Yoon, W.-J.; Cho, J.-H.; et al. Anti-Photoaging Effect of Jeju Putgyul (Unripe Citrus) Extracts on Human Dermal Fibroblasts and Ultraviolet B-induced Hairless Mouse Skin. Int. J. Mol. Sci. 2017, 18, 2052. https://doi.org/10.3390/ijms18102052
Choi S-H, Choi S-I, Jung T-D, Cho B-Y, Lee J-H, Kim S-H, Yoon S-A, Ham Y-M, Yoon W-J, Cho J-H, et al. Anti-Photoaging Effect of Jeju Putgyul (Unripe Citrus) Extracts on Human Dermal Fibroblasts and Ultraviolet B-induced Hairless Mouse Skin. International Journal of Molecular Sciences. 2017; 18(10):2052. https://doi.org/10.3390/ijms18102052
Chicago/Turabian StyleChoi, Seung-Hyun, Sun-Il Choi, Tae-Dong Jung, Bong-Yeon Cho, Jin-Ha Lee, Seung-Hyung Kim, Seon-A Yoon, Young-Min Ham, Weon-Jong Yoon, Ju-Hyun Cho, and et al. 2017. "Anti-Photoaging Effect of Jeju Putgyul (Unripe Citrus) Extracts on Human Dermal Fibroblasts and Ultraviolet B-induced Hairless Mouse Skin" International Journal of Molecular Sciences 18, no. 10: 2052. https://doi.org/10.3390/ijms18102052
APA StyleChoi, S.-H., Choi, S.-I., Jung, T.-D., Cho, B.-Y., Lee, J.-H., Kim, S.-H., Yoon, S.-A., Ham, Y.-M., Yoon, W.-J., Cho, J.-H., & Lee, O.-H. (2017). Anti-Photoaging Effect of Jeju Putgyul (Unripe Citrus) Extracts on Human Dermal Fibroblasts and Ultraviolet B-induced Hairless Mouse Skin. International Journal of Molecular Sciences, 18(10), 2052. https://doi.org/10.3390/ijms18102052