Single Silver Nanoparticle Instillation Induced Early and Persisting Moderate Cortical Damage in Rat Kidneys
Abstract
:1. Introduction
2. Results
2.1. Model Silver Nanoparticles (AgNPs): Physicochemical Characteristics
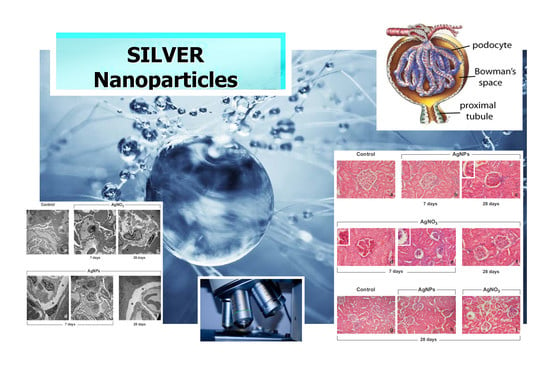
2.2. Histopathology: Haematoxylin & Eosin (H & E) Staining
2.3. Transmission Electron Microscopy (TEM) Observations: UA & LC Staining
2.4. Oxidative Stress Evaluation by Protein Carbonylation Assessment in Kidney Slice Homogenates and Plasma
3. Discussion
4. Materials and Methods
4.1. Chemicals
4.2. Animals and Experimental Design of Silver Exposure
4.3. Kidney Histology and Ultrastructural Morphology Evaluations
4.3.1. Rat Renal Specimen Preparation and Histology
4.3.2. Transmission Electron Microscopy (TEM): UA & LC Staining
4.3.3. Semiquantitative Kidney Lesion Analysis
4.3.4. Statistical Analysis
4.4. Oxidative Stress Evaluation: Detection of Protein Carbonylation in Kidneys and Plasma
Acknowledgments
Author Contributions
Conflicts of Interest
Abbreviations
| AgNPs | Silver nanoparticles |
| AgNO3 | Silver nitrate |
| i.t. | Intratracheal instillation |
| H&E | Haematoxylin/Eosin staining |
| TEM | Transmission electron microscopy |
| DLS | Dynamic light scattering |
| AGEs | Advanced glycation end products |
| ALEs | Advanced lipoxidation end products |
| DNP | 2,4-Dinitrophenylhydrazone |
| DNPH | 2,4-Dinitrophenylhydrazine |
| PCOs | Protein carbonyls |
| PVDF | Polyvinylidene fluoride membrane |
| ROS | Reactive oxygen species |
References
- Boonruksa, P.; Bello, D.; Zhang, J.; Isaacs, J.A.; Mead, J.L.; Woskie, S.R. Characterization of potential exposures to nanoparticles and fibers during manufacturing and recycling of carbon nanotube reinforced polypropylene composites. Ann. Occup. Hyg. 2016, 60, 40–55. [Google Scholar] [CrossRef] [PubMed]
- Debia, M.; Bakhiyi, B.; Ostiguy, C.; Verbeek, J.H.; Brouwer, D.H.; Murashov, V. A Systematic review of reported exposure to engineered nanomaterials. Ann. Occup. Hyg. 2016, 60, 916–935. [Google Scholar] [CrossRef] [PubMed]
- Iavicoli, I.; Leso, V.; Fontana, L.; Cottica, D.; Bergamaschi, A. Characterization of inhalable, thoracic, and respirable fractions and ultrafine particle exposure during grinding, brazing, and welding activities in a mechanical engineering factory. J. Occup. Environ. Med. 2013, 55, 430–445. [Google Scholar] [CrossRef] [PubMed]
- McGillicuddy, E.; Murray, I.; Kavanagh, S.; Morrison, L.; Fogarty, A.; Cormican, M.; Dockery, P.; Prendergast, M.; Rowan, N.; Morris, D. Silver nanoparticles in the environment: Sources, detection and ecotoxicology. Sci. Total Environ. 2017, 575, 231–246. [Google Scholar] [CrossRef] [PubMed]
- Mackevica, A.; Foss, H.S. Release of nanomaterials from solid nanocomposites and consumer exposure assessment–A forward-looking review. Nanotoxicology 2016, 10, 641–653. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Song, K.S.; Sung, J.H.; Ji, J.H.; Lee, J.H.; Lee, J.S.; Ryu, H.R.; Lee, J.K.; Chung, Y.H.; Park, H.M.; Shin, B.S.; et al. Recovery from silver-nanoparticle-exposure-induced lung inflammation and lung function changes in Sprague Dawley rats. Nanotoxicology 2013, 7, 169–180. [Google Scholar] [CrossRef] [PubMed]
- Simeonova, P.P.; Erdely, A. Engineered nanoparticle respiratory exposure and potential risks for cardiovascular toxicity: Predictive tests and biomarkers. Inhal. Toxicol. 2009, 21, 68–73. [Google Scholar] [CrossRef] [PubMed]
- Gaillet, S.; Rouanet, J.-M. Silver nanoparticles: Their potential toxic effects after oral exposure and underlying mechanisms—A review. Food Chem. Toxicol. 2015, 77, 58–63. [Google Scholar] [CrossRef] [PubMed]
- Wijnhoven, S.W.P.; Peijnenbourg, W.J.G.M.; Herberts, C.A.; Hagens, W.I.; Oomen, A.G.; Heugens, E.H.W.; Roszek, B.; Bisschops, J.; Gosens, I.; van de Meent, D.; et al. Nano-silver–A review of available data and knowledge gaps in human and environmental risk assessment. Nanotoxicology 2009, 3, 109–138. [Google Scholar] [CrossRef]
- Loeschner, K.; Hadrup, N.; Qvortrup, K.; Larsen, A.; Gao, X.; Vogel, U.; Mortensen, A.; Lam, H.R.; Larsen, E.H. Distribution of silver in rats following 28 days of repeated oral exposure to silver nanoparticles or silver acetate. Part. Fibre Toxicol. 2011, 8, 18. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schäfer, B.; vom Brocke, J.; Epp, A.; Götz, M.; Herzberg, F.; Kneuer, C.; Sommer, Y.; Tentschert, J.; Noll, M.; Günther, I.; et al. State of the art in human risk assessment of silver compounds in consumer products: A conference report on silver and nanosilver held at the BfR in 2012. Arch. Toxicol. 2013, 87, 2249–2262. [Google Scholar] [CrossRef] [PubMed]
- Recordati, C.; de Maglie, M.; Bianchissi, S.; Argentiere, S.; Cella, C.; Mattiello, S.; Cubadda, F.; Aureli, F.; D’Amato, M.; Raggi, A.; et al. Tissue distribution and acute toxicity of silver after single intravenous administration in mice: Nano-specific and size-dependent effects. Part. Fibre Toxicol. 2016, 13, 12. [Google Scholar] [CrossRef] [PubMed]
- Gliga, A.R.; Skoglund, S.; Wallinder, I.O.; Fadeel, B.; Karlsson, H.L. Size-dependent cytotoxicity of silver nanoparticles in human lung cells: The role of cellular uptake, agglomeration and Ag release. Part. Fibre Toxicol. 2014, 11, 11. [Google Scholar] [CrossRef] [PubMed]
- Park, E.J.; Bae, E.; Yi, J.; Kim, Y.; Choi, K.; Lee, S.H.; Yoon, J.; Lee, B.C.; Park, K. Repeated-dose toxicity and inflammatory responses in mice by oral administration of silver nanoparticles. Environ. Toxicol. Pharmacol. 2010, 30, 162–168. [Google Scholar] [CrossRef] [PubMed]
- Piao, M.J.; Kang, K.A.; Lee, I.K.; Kim, H.S.; Kim, S.; Choi, J.Y.; Choi, J.; Hyun, J.W. Silver nanoparticles induce oxidative cell damage in human liver cells through inhibition of reduced glutathione and induction of mitochondria-involved apoptosis. Toxicol. Lett. 2011, 201, 92–100. [Google Scholar] [CrossRef] [PubMed]
- Bachi, A.; Dalle-Donne, I.; Scaloni, A. Redox proteomics: Chemical principles, methodological approaches and biological/biomedical promises. Chem. Rev. 2013, 113, 596–698. [Google Scholar] [CrossRef] [PubMed]
- Colombo, G.; Clerici, M.; Giustarini, D.; Portinaro, N.M.; Aldini, G.; Rossi, R.; Milzani, A.; Dalle-Donne, I. Pathophysiology of tobacco smoke exposure: Recent insights from comparative and redox proteomics. Mass Spectrom. Rev. 2014, 33, 183–218. [Google Scholar] [CrossRef] [PubMed]
- Sung, J.H.; Ji, J.H.; Park, J.D.; Yoon, J.U.; Kim, D.S.; Jeon, K.S.; Song, M.Y.; Jeong, J.; Han, B.S.; Han, J.H.; et al. Subchronic inhalation toxicity of silver nanoparticles. Toxicol. Sci. 2009, 108, 452–461. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.S.; Song, M.Y.; Park, J.D.; Song, K.S.; Ryu, H.R.; Chung, Y.H.; Chang, H.K.; Lee, J.H.; Oh, K.H.; Kelman, B.J.; et al. Subchronic oral toxicity of silver nanoparticles. Part. Fibre Toxicol. 2010, 7, 20. [Google Scholar] [CrossRef] [PubMed]
- Hadrup, N.; Loeschner, K.; Bergström, A.; Wilcks, A.; Gao, X.; Vogel, U.; Frandsen, H.L.; Larsen, E.H.; Lam, H.R.; Mortensen, A. Subacute oral toxicity investigation of nanoparticulate and ionic silver in rats. Arch. Toxicol. 2012, 86, 543–551. [Google Scholar] [CrossRef] [PubMed]
- Davenport, L.L.; Hsieh, H.; Eppert, B.L.; Carreira, V.S.; Krishan, M.; Ingle, T.; Howard, P.C.; Williams, M.T.; Genter, M.B. Systemic and behavioral effects of intranasal administration of silver nanoparticles. Neurotoxicol. Teratol. 2015, 51, 68–76. [Google Scholar] [CrossRef] [PubMed]
- De Jong, W.H.; van der Ven, L.T.; Sleijffers, A.; Park, M.V.; Jansen, E.H.; van Loveren, H.; Vandebriel, R.J. Systemic and immunotoxicity of silver nanoparticles in an intravenous 28 days repeated dose toxicity study in rats. Biomaterials 2013, 34, 8333–8343. [Google Scholar] [CrossRef] [PubMed]
- Shahare, B.; Yashpal, M. Toxic effects of repeated oral exposure of silver nanoparticles on small intestine mucosa of mice. Toxicol. Mech. Methods 2013, 23, 161–167. [Google Scholar] [CrossRef] [PubMed]
- Van der Zande, M.; Vandebriel, R.J.; van Doren, E.; Kramer, E.; Herrera Rivera, Z.; Serrano-Rojero, C.S.; Gremmer, E.R.; Mast, J.; Peters, R.J.; Hollman, P.C.; et al. Distribution, elimination, and toxicity of silver nanoparticles and silver ions in rats after 28-day oral exposure. ACS Nano 2012, 6, 7427–7442. [Google Scholar] [CrossRef] [PubMed]
- Gaiser, B.K.; Fernandes, T.F.; Jepson, M.A.; Lead, J.R.; Tyler, C.R.; Baalousha, M.; Biswas, A.; Britton, G.J.; Cole, P.A.; Johnston, B.D.; et al. Interspecies comparisons on the uptake and toxicity of silver and cerium dioxide nanoparticles. Environ. Toxicol. Chem. 2012, 31, 144–154. [Google Scholar] [CrossRef] [PubMed]
- Iavicoli, I.; Fontana, L.; Nordberg, G. The effects of nanoparticles on the renal system. Crit. Rev. Toxicol. 2016, 46, 490–560. [Google Scholar] [CrossRef] [PubMed]
- Coccini, T.; Gornati, R.; Rossi, F.; Signoretto, E.; Vanetti, I.; Bernardini, G.; Manzo, L. Gene Expression changes in rat liver and testes after lung instillation of a low dose of silver nanoparticles. J. Nanomed. Nanotechnol. 2014, 5, 5. [Google Scholar] [CrossRef]
- Oberdörster, G. Nanotoxicology: In vitro-in vivo dosimetry. Environ. Health Perspect. 2012, 120, A13. [Google Scholar] [CrossRef] [PubMed]
- Stefaniak, A.B.; Hackley, V.A.; Roebben, G.; Ehara, K.; Hankin, S.; Michael, T.; Postek, M.T.; Lynch, I.; Fu, W.-E.; Linsinger, T.P.J.; et al. Nanoscale reference materials for environmental, health and safety measurements: Needs, gaps and opportunities. Nanotoxicology 2013, 7, 1325–1337. [Google Scholar] [CrossRef] [PubMed]
- Leso, V.; Fontana, L.; Mauriello, M.C.; Iavicoli, I. Occupational risk assessment of engineered nanomaterials: Limits, challenges and opportunities. Curr. Nanosci. 2017, 13, 55–78. [Google Scholar] [CrossRef]
- Coccini, T.; Manzo, L.; Roda, E. Safety evaluation of engineered nanomaterials for health risk assessment: An experimental tiered testing approach using pristine and functionalized carbon nanotubes. ISRN Toxicol. 2013, 2013, 13. [Google Scholar] [CrossRef] [PubMed]
- Ulm, L.; Krivohlavek, A.; Jurašin, D.; Ljubojević, M.; Šinko, G.; Crnković, T.; Žuntar, I.; Šikić, S.; Vinković Vrček, I. Response of biochemical biomarkers in the aquatic crustacean Daphnia magna exposed to silver nanoparticles. Environ. Sci. Pollut. Res. Int. 2015, 22, 19990–19999. [Google Scholar] [CrossRef] [PubMed]
- Gomes, T.; Pereira, C.G.; Cardoso, C.; Bebianno, M.J. Differential protein expression in mussels Mytilus galloprovincialis exposed to nano and ionic Ag. Aquat. Toxicol. 2013, 136–137, 79–90. [Google Scholar] [CrossRef] [PubMed]
- McCarthy, M.P.; Carroll, D.L.; Ringwood, A.H. Tissue specific responses of oysters, Crassostrea virginica, to silver nanoparticles. Aquat. Toxicol. 2013, 138–139, 123–128. [Google Scholar] [CrossRef] [PubMed]
- Sharma, H.S.; Ali, S.F.; Hussain, S.M.; Schlager, J.J.; Sharma, A. Influence of engineered nanoparticles from metals on the blood-brain barrier permeability, cerebral blood flow, brain edema and neurotoxicity. An experimental study in the rat and mice using biochemical and morphological approaches. J. Nanosci. Nanotechnol. 2009, 9, 5055–5072. [Google Scholar] [CrossRef] [PubMed]
- Struzynski, W.; Dabrowska-Bouta, B.; Grygorowicz, T.; Zieminska, E.; Struzynska, L. Markers of oxidative stress in hepatopancreas of crayfish (Orconectes limosus, raf.) experimentally exposed to nanosilver. Environ. Toxicol. 2014, 29, 1283–1291. [Google Scholar] [CrossRef] [PubMed]
- Takenaka, S.; Erwin Karg, E.; Roth, C.; Schulz, H.; Ziesenis, A.; Heinzmann, U.; Schramel, P.; Heyder, J. Pulmonary and systemic distribution of inhaled ultrafine silver particles in rats. Environ. Health Perspect. 2001, 109, 547–551. [Google Scholar] [CrossRef] [PubMed]
- Silva, R.M.; Anderson, D.S.; Franzi, L.M.; Peake, J.L.; Edwards, P.C.; van Winkle, L.S.; Pinkerton, K.E. Pulmonary effects of silver nanoparticle size, coating, and dose over time upon intratracheal instillation. Toxicol. Sci. 2015, 144, 151–162. [Google Scholar] [CrossRef] [PubMed]
- Smulders, S.; Luyts, K.; Brabants, G.; van Landuyt, K.; Kirschhock, C.; Smolders, E.; Golanski, L.; Vanoirbeek, J.; Hoet, P.H.M. Toxicity of nanoparticles embedded in paints compared with pristine nanoparticles in mice. Toxicol. Sci. 2014, 141, 132–140. [Google Scholar] [CrossRef] [PubMed]
- Arai, Y.; Miyayama, T.; Hirano, S. Difference in the toxicity mechanism between ion and nanoparticle forms of silver in the mouse lung and in macrophages. Toxicology 2015, 328, 84–92. [Google Scholar] [CrossRef] [PubMed]
- Keller, A.A.; McFerran, S.; Lazareva, A.; Suh, S. Global life cycle releases of engineered nanomaterials. J. Nanopart. Res. 2013, 15, 1692. [Google Scholar] [CrossRef]
- Oberdörster, G.; Oberdörster, E.; Oberdörster, J. Nanotoxicology: An emerging discipline evolving from studies of ultrafine particles. Environ. Health Perspect. 2005, 113, 823–839. [Google Scholar] [CrossRef] [PubMed]
- Dziendzikowska, K.; Gromadzka-Ostrowska, J.; Lankoff, A.; Oczkowski, M.; Krawczyńska, A.; Chwastowska, J.; Sadowska-Bratek, M.; Chajduk, E.; Wojewódzka, M.; Dusinská, M.; et al. Time-dependent biodistribution and excretion of silver nanoparticles in male Wistar rats. J. Appl. Toxicol. 2012, 32, 920–928. [Google Scholar] [CrossRef] [PubMed]
- Garza-Ocanas, L.; Ferrer, D.A.; Burt, J.; Diaz-Torres, L.A.; Ramírez Cabrera, M.; Rodríguez, V.T.; Luján Rangel, R.; Romanovicz, D.; Jose-Yacaman, M. Biodistribution and long-term fate of silver nanoparticles functionalized with bovine serum albumin in rats. Met. Integr. Biometal Sci. 2010, 2, 204–210. [Google Scholar] [CrossRef] [PubMed]
- Katsnelson, B.A.; Privalova, L.I.; Gurvich, V.B.; Makeyev, O.H.; Shur, V.Y.; Beikin, Y.B.; Sutunkova, M.P.; Kireyeva, E.P.; Minigalieva, I.A.; Loginova, N.V.; et al. Comparative in vivo assessment of some adverse bioeffects of equidimensional gold and silver nanoparticles and the attenuation of nanosilver’s effects with a complex of innocuous bioprotectors. Int. J. Mol. Sci. 2013, 14, 2449–2483. [Google Scholar] [CrossRef] [PubMed]
- Boudreau, M.D.; Imam, M.S.; Paredes, A.M.; Bryant, M.S.; Cunningham, C.K.; Felton, R.P.; Jones, M.Y.; Davis, K.J.; Olson, G.R. Differential effects of silver nanoparticles and silver ions on tissue accumulation, distribution, and toxicity in the sprague dawley rat following daily oral gavage administration for 13-weeks. Toxicol. Sci. 2016, 150, 131–160. [Google Scholar] [CrossRef] [PubMed]
- Juling, S.; Bachler, G.; von Goetz, N.; Lichtenstein, D.; Böhmert, L.; Niedzwiecka, A.; Selve, S.; Braeuning, A.; Lampen, A. In vivo distribution of nanosilver in the rat: The role of ions and denovo-formed secondary particles. Food Chem. Toxicol. 2016, 97, 327–335. [Google Scholar] [CrossRef] [PubMed]
- Coccini, T.; Barni, S.; Manzo, L.; Roda, E. Apoptosis induction and histological changes in rat kidney following Cd-doped silica nanoparticle exposure: Evidence of persisting effects. Toxicol. Mech. Methods. 2013, 23, 566–575. [Google Scholar] [CrossRef] [PubMed]
- Bachler, G.; von Goetz, N.; Hungerbühler, K. A physiologically based pharmacokinetic model for ionic silver and silver nanoparticles. Int. J. Nanomed. 2013, 8, 3365–3382. [Google Scholar]
- Kim, Y.S.; Kim, J.S.; Cho, H.S.; Rha, D.S.; Kim, J.M.; Park, J.D.; Choi, B.S.; Lim, R.; Chang, H.K.; Chung, Y.H.; et al. Twenty-eight-day oral toxicity, genotoxicity, and gender-related tissue distribution of silver nanoparticles in Sprague dawley rats. Inhal. Toxicol. 2008, 20, 575–583. [Google Scholar] [CrossRef] [PubMed]
- Park, K.; Park, E.-J.; Chun, I.K.; Choi, K.; Lee, S.H.; Yoon, J.; Lee, B.C. Bioavailability and toxicokinetics of citrate-coated silver nanoparticles in rats. Arch. Pharmacal Res. 2011, 34, 153–158. [Google Scholar] [CrossRef] [PubMed]
- Patri, A.; Umbreit, T.; Zheng, J.; Nagashima, K.; Goering, P.; Francke-Carroll, S.; Gordon, E.; Weaver, J.; Miller, T.; Sadrieh, N.; et al. Energy dispersive X-ray analysis of titanium dioxide nanoparticle distribution after intravenous and subcutaneous injection in mice. J. Appl. Toxicol. 2009, 29, 662–672. [Google Scholar] [CrossRef] [PubMed]
- Fontana, L.; Leso, V.; Marinaccio, A.; Cenacchi, G.; Papa, V.; Leopold, K.; Schindl, R.; Bocca, B.; Alimonti, A.; Iavicoli, I. The effects of palladium nanoparticles on the renal function of female Wistar rats. Nanotoxicology 2015, 9, 843–851. [Google Scholar] [CrossRef] [PubMed]
- Driscoll, K.E.; Costa, D.L.; Hatch, G.; Henderson, R.; Oberdörster, G.; Salem, H.; Schlesinger, R.B. Intratracheal instillation as an exposure technique for the evaluation of respiratory tract toxicity: Uses and limitations. Toxicol. Sci. 2000, 55, 24–35. [Google Scholar] [CrossRef] [PubMed]
- Warheit, D.B.; Laurence, B.R.; Reed, K.L.; Roach, D.H.; Reynolds, G.A.M.; Webb, T.R. Comparative pulmonary toxicity assessment of single-wall carbon Nanotubes in rats. Toxicol. Sci. 2004, 77, 117–125. [Google Scholar] [CrossRef] [PubMed]
- Muller, J.; Huaux, F.; Moreau, N.; Misson, P.; Heilier, J.F.; Delos, M.; Arras, M.; Fonseca, A.; Nagy, J.; Lison, D. Respiratory toxicity of multiwalled carbon nanotubes. Toxicol. Appl. Pharmacol. 2005, 207, 221–231. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Hulderman, T.; Salmen, R.; Chapman, R.; Leonard, S.S.; Young, S.H.; Shvedova, A.; Luster, M.I.; Simeonova, P.P. Cardiovascular effects of pulmonary exposure to single-wall carbon Nanotubes. Environ. Health Perspect. 2007, 115, 377–382. [Google Scholar] [CrossRef] [PubMed]
- Elgrabli, D.; Abella-Gallart, S.; Robidel, F.; Rogerieux, F.; Boczkowski, J.; Lacroix, G. Induction of apoptosis and absence of inflammation in rat lung after intratracheal instillation of multiwalled carbon nanotubes. Toxicology 2008, 253, 131–136. [Google Scholar] [CrossRef] [PubMed]
- Dalle-Donne, I.; Carini, M.; Orioli, M.; Vistoli, G.; Regazzoni, L.; Colombo, G.; Rossi, R.; Milzani, A.; Aldini, G. Protein carbonylation: 2,4-dinitrophenylhydrazine reacts with both aldehydes/ketones and sulfenic acids. Free Radic. Biol. Med. 2009, 46, 1411–1419. [Google Scholar] [CrossRef] [PubMed]
- Colombo, G.; Aldini, G.; Orioli, M.; Giustarini, D.; Gornati, R.; Rossi, R.; Colombo, R.; Carini, M.; Milzani, A.; Dalle-Donne, I. Water-Soluble alpha,beta-unsaturated aldehydes of cigarette smoke induce carbonylation of human serum albumin. Antioxid. Redox Signal. 2010, 12, 349–364. [Google Scholar] [CrossRef] [PubMed]
- Colombo, G.; Clerici, M.; Garavaglia, M.E.; Giustarini, D.; Rossi, R.; Milzani, A.; Dalle-Donne, I. A step-by-step protocol for assaying protein carbonylation in biological samples. J. Chromatogr. B 2016, 1019, 178–190. [Google Scholar] [CrossRef] [PubMed]




| 7 Days | p Value | |||
| Control | AgNPs | AgNO3 | ||
| Bowman’s capsule enlargement | − | ++ | +++ | * |
| Mesangial cells and glomerular capillaries shrinkage | − | ++ | ++ | * |
| Edema and interstitial micro-hemorrhages | − | ++ | +++ | * |
| Podocyte foot processes alteration | − | + | + | ns |
| Apoptosis | − | − | ++ | * |
| 28 Days | p Value | |||
| Control | AgNPs | AgNO3 | ||
| Bowman’s capsule enlargement | − | ++ | +++ | * |
| Mesangial cells and glomerular capillaries shrinkage | − | ++ | ++ | * |
| Edema and interstitial micro-hemorrhages | − | ++ | +++ | * |
| Podocyte foot processes alteration | − | + | + | ns |
| Apoptosis | − | − | ++ | * |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Roda, E.; Barni, S.; Milzani, A.; Dalle-Donne, I.; Colombo, G.; Coccini, T. Single Silver Nanoparticle Instillation Induced Early and Persisting Moderate Cortical Damage in Rat Kidneys. Int. J. Mol. Sci. 2017, 18, 2115. https://doi.org/10.3390/ijms18102115
Roda E, Barni S, Milzani A, Dalle-Donne I, Colombo G, Coccini T. Single Silver Nanoparticle Instillation Induced Early and Persisting Moderate Cortical Damage in Rat Kidneys. International Journal of Molecular Sciences. 2017; 18(10):2115. https://doi.org/10.3390/ijms18102115
Chicago/Turabian StyleRoda, Elisa, Sergio Barni, Aldo Milzani, Isabella Dalle-Donne, Graziano Colombo, and Teresa Coccini. 2017. "Single Silver Nanoparticle Instillation Induced Early and Persisting Moderate Cortical Damage in Rat Kidneys" International Journal of Molecular Sciences 18, no. 10: 2115. https://doi.org/10.3390/ijms18102115
APA StyleRoda, E., Barni, S., Milzani, A., Dalle-Donne, I., Colombo, G., & Coccini, T. (2017). Single Silver Nanoparticle Instillation Induced Early and Persisting Moderate Cortical Damage in Rat Kidneys. International Journal of Molecular Sciences, 18(10), 2115. https://doi.org/10.3390/ijms18102115