The Impact of Oxidative Stress on the Bone System in Response to the Space Special Environment
Abstract
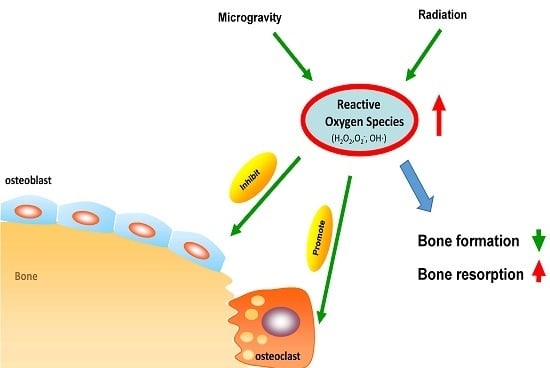
:1. Introduction
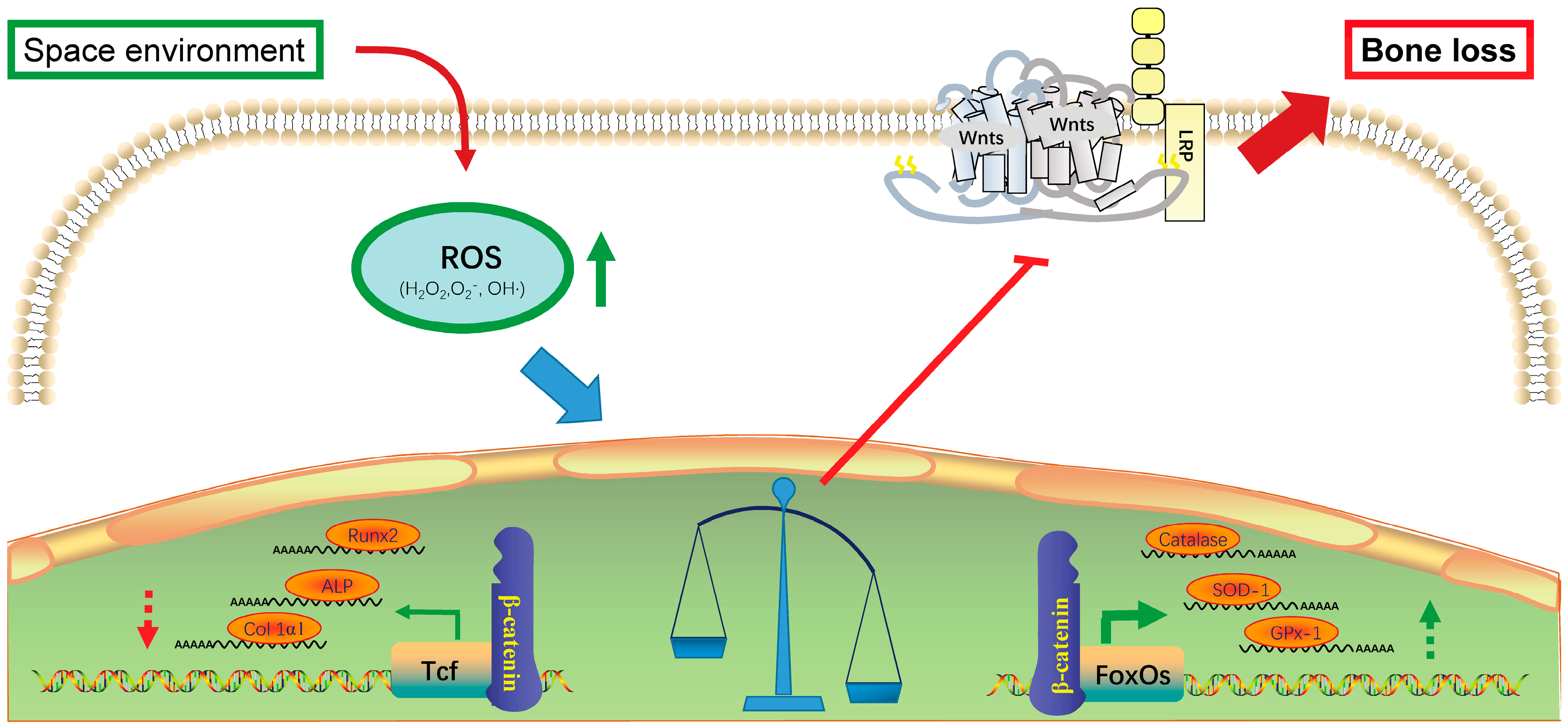
2. Effects of Oxidative Stress on Bone Formation
3. Microgravity Increases Oxidative Stress in Bone System
4. Radiation Induces Oxidative Stress in Bone System
5. Countermeasures against Bone Loss Caused by Oxidative Stress in Spaceflight
6. Conclusions and Perspectives
Acknowledgments
Author Contributions
Conflicts of Interest
Abbreviations
| ROS | Reactive Oxygen Species |
| HLU | Hind-limb Unloadings |
| ISS | International Space Station |
| FoxO | Forkhead box O |
| Mn-SOD | Manganese superoxide dismutase |
| GPx-1 | Glutathione peroxidase-1 |
| Tcf | T-cell factor |
| LRP | LDL receptor-related proteins |
| aBMD | Areal bone mineral density |
| DXA | Dual-energy X-ray absorptionmetry |
| RWVB | Rotary wall vessel bioreactor |
| SD | Sprague-Dawley |
| RANKL | Receptor activator of nuclear factor-kappa B ligand |
References
- Williams, D.R.; Turnock, M. Human space exploration the next fifty years. Mcgill. J. Med. 2011, 13, 76. [Google Scholar] [PubMed]
- Zhao, L.; Gao, Y.; Mi, D.; Sun, Y. Mining potential biomarkers associated with space flight in Caenorhabditis elegans experienced Shenzhou-8 mission with multiple feature selection techniques. Mutat. Res. 2016, 791–792, 27–34. [Google Scholar] [CrossRef] [PubMed]
- Fu, Y.; Li, L.; Xie, B.; Dong, C.; Wang, M.; Jia, B.; Shao, L.; Dong, Y.; Deng, S.; Liu, H. How to Establish a Bioregenerative Life Support System for Long-Term Crewed Missions to the Moon or Mars. Astrobiology 2016, 16, 925–936. [Google Scholar] [CrossRef] [PubMed]
- Byloos, B.; Coninx, I.; Van Hoey, O.; Cockell, C.; Nicholson, N.; Ilyin, V.; Van Houdt, R.; Boon, N.; Leys, N. The Impact of Space Flight on Survival and Interaction of Cupriavidus metallidurans CH34 with Basalt, a Volcanic Moon Analog Rock. Front. Microbiol. 2017, 8, 671. [Google Scholar] [CrossRef] [PubMed]
- Witze, A. NASA rethinks approach to Mars exploration. Nature 2016, 538, 149–150. [Google Scholar] [CrossRef] [PubMed]
- Caceres, M. Creating a space exploration industry. Aerosp. Am. 2005, 43, 10–12. [Google Scholar] [PubMed]
- Demontis, G.C.; Germani, M.M.; Caiani, E.G.; Barravecchia, I.; Passino, C.; Angeloni, D. Human Pathophysiological Adaptations to the Space Environment. Front. Physiol. 2017, 8, 547. [Google Scholar] [CrossRef] [PubMed]
- Otsuka, K.; Cornelissen, G.; Furukawa, S.; Kubo, Y.; Hayashi, M.; Shibata, K.; Mizuno, K.; Aiba, T.; Ohshima, H.; Mukai, C. Long-term exposure to space’s microgravity alters the time structure of heart rate variability of astronauts. Heliyon 2016, 2, e00211. [Google Scholar] [CrossRef] [PubMed]
- Mao, X.W.; Nishiyama, N.C.; Pecaut, M.J.; Campbell-Beachler, M.; Gifford, P.; Haynes, K.E.; Becronis, C.; Gridley, D.S. Simulated Microgravity and Low-Dose/Low-Dose-Rate Radiation Induces Oxidative Damage in the Mouse Brain. Radiat. Res. 2016, 185, 647–657. [Google Scholar] [CrossRef] [PubMed]
- Luo, H.; Wang, C.; Feng, M.; Zhao, Y. Microgravity inhibits resting T cell immunity in an exposure time-dependent manner. Int. J. Med. Sci. 2014, 11, 87–96. [Google Scholar] [CrossRef] [PubMed]
- Sanzari, J.K.; Romeroweaver, A.L.; James, G.; Krigsfeld, G.; Lin, L.; Diffenderfer, E.S.; Kennedy, A.R. Leukocyte Activity Is Altered in a Ground Based Murine Model of Microgravity and Proton Radiation Exposure. PLoS ONE 2013, 8, e71757. [Google Scholar] [CrossRef] [PubMed]
- Cazzaniga, A.; Maier, J.A.M.; Castiglioni, S. Impact of simulated microgravity on human bone stem cells: New hints for space medicine. Biochem. Biophys. Res. Commun. 2016, 473, 181–186. [Google Scholar] [CrossRef] [PubMed]
- Grimm, D.; Grosse, J.; Wehland, M.; Mann, V.; Reseland, J.E.; Sundaresan, A.; Corydon, T.J. The impact of microgravity on bone in humans. Bone 2016, 87, 44–56. [Google Scholar] [CrossRef] [PubMed]
- Cappellesso, R.; Nicole, L.; Guido, A.; Pizzol, D. Spaceflight osteoporosis: Current state and future perspective. Endocr. Regul. 2015, 49, 231–239. [Google Scholar] [CrossRef] [PubMed]
- Sibonga, J.D. Spaceflight-induced bone loss: Is there an osteoporosis risk? Curr. Osteoporos. Rep. 2013, 11, 92–98. [Google Scholar] [CrossRef] [PubMed]
- Lang, T.; Van Loon, J.; Bloomfield, S.; Vico, L.; Chopard, A.; Rittweger, J.; Kyparos, A.; Blottner, D.; Vuori, I.; Gerzer, R.; et al. Towards human exploration of space: The THESEUS review series on muscle and bone research priorities. NPJ Microgravity 2017, 3, 8. [Google Scholar] [CrossRef] [PubMed]
- Markin, A.A.; Zhuravlëva, O.A. Lipid peroxidation and antioxidant defense system in rats after a 14-day space flight in the “Space-2044” spacecraft. Aviakosm. Ekolog. Med. 1993, 27, 47–50. [Google Scholar] [PubMed]
- Markin, A.A.; Popova, I.A.; Vetrova, E.G.; Zhuravleva, O.A.; Balashov, O.I. Lipid peroxidation and activity of diagnostically significant enzymes in cosmonauts after flights of various durations. Aviakosm. Ekolog. Med. 1997, 31, 14–18. [Google Scholar] [PubMed]
- Stein, T.P.; Leskiw, M.J. Oxidant damage during and after spaceflight. Am. J. Physiol. Endocrinol. Metab. 2000, 278, E375–E382. [Google Scholar] [PubMed]
- Balaban, R.S.; Nemoto, S.; Finkel, T. Mitochondria, oxidants, and aging. Cell 2005, 120, 483–495. [Google Scholar] [CrossRef] [PubMed]
- Giorgio, M.; Migliaccio, E.; Orsini, F.; Paolucci, D.; Moroni, M.; Contursi, C.; Pelliccia, G.; Luzi, L.; Minucci, S.; Marcaccio, M.; et al. Electron transfer between cytochrome c and p66Shc generates reactive oxygen species that trigger mitochondrial apoptosis. Cell 2005, 122, 221–233. [Google Scholar] [CrossRef] [PubMed]
- Quarrie, J.K.; Riabowol, K.T. Murine models of life span extension. Sci. Aging Knowl. Environ. 2004, 2004, re5. [Google Scholar] [CrossRef] [PubMed]
- Finkel, T.; Holbrook, N.J. Oxidants, oxidative stress and the biology of ageing. Nature 2000, 408, 239–247. [Google Scholar] [CrossRef] [PubMed]
- Glasauer, A.; Chandel, N.S. Ros. Curr. Biol. 2013, 23, R100–R102. [Google Scholar] [CrossRef] [PubMed]
- Valko, M.; Leibfritz, D.; Moncol, J.; Cronin, M.T.; Mazur, M.; Telser, J. Free radicals and antioxidants in normal physiological functions and human disease. Int. J. Biochem. Cell Biol. 2007, 39, 44–84. [Google Scholar] [CrossRef] [PubMed]
- De Boer, J.; Andressoo, J.O.; de Wit, J.; Huijmans, J.; Beems, R.B.; van Steeg, H.; Weeda, G.; van der Horst, G.T.; van Leeuwen, W.; Themmen, A.P.; et al. Premature aging in mice deficient in DNA repair and transcription. Science 2002, 296, 1276–1279. [Google Scholar] [CrossRef] [PubMed]
- Bai, X.C.; Lu, D.; Bai, J.; Zheng, H.; Ke, Z.Y.; Li, X.M.; Luo, S.Q. Oxidative stress inhibits osteoblastic differentiation of bone cells by ERK and NF-kappaB. Biochem. Biophys. Res. Commun. 2004, 314, 197–207. [Google Scholar] [CrossRef] [PubMed]
- Lean, J.M.; Davies, J.T.; Fuller, K.; Jagger, C.J.; Kirstein, B.; Partington, G.A.; Urry, Z.L.; Chambers, T.J. A crucial role for thiol antioxidants in estrogen-deficiency bone loss. J. Clin. Investig. 2003, 112, 915–923. [Google Scholar] [CrossRef] [PubMed]
- Almeida, M.; Han, L.; Martin-Millan, M.; Plotkin, L.I.; Stewart, S.A.; Roberson, P.K.; Kousteni, S.; O’Brien, C.A.; Bellido, T.; Parfitt, A.M.; et al. Skeletal involution by age-associated oxidative stress and its acceleration by loss of sex steroids. J. Biol. Chem. 2007, 282, 27285–27297. [Google Scholar] [CrossRef] [PubMed]
- Manolagas, S.C. From estrogen-centric to aging and oxidative stress: A revised perspective of the pathogenesis of osteoporosis. Endocr. Rev. 2010, 31, 266–300. [Google Scholar] [CrossRef] [PubMed]
- Almeida, M.; Han, L.; Martin-Millan, M.; O’Brien, C.A.; Manolagas, S.C. Oxidative stress antagonizes Wnt signaling in osteoblast precursors by diverting beta-catenin from T cell factor- to forkhead box O-mediated transcription. J. Biol. Chem. 2007, 282, 27298–27305. [Google Scholar] [CrossRef] [PubMed]
- Almeida, M.; Ambrogini, E.; Han, L.; Manolagas, S.C.; Jilka, R.L. Increased lipid oxidation causes oxidative stress, increased peroxisome proliferator-activated receptor-gamma expression, and diminished pro-osteogenic Wnt signaling in the skeleton. J. Biol. Chem. 2009, 284, 27438–27448. [Google Scholar] [CrossRef] [PubMed]
- Almeida, M.; Martin-Millan, M.; Ambrogini, E.; Bradsher, R., 3rd; Han, L.; Chen, X.D.; Roberson, P.K.; Weinstein, R.S.; O’Brien, C.A.; Jilka, R.L.; et al. Estrogens attenuate oxidative stress and the differentiation and apoptosis of osteoblasts by DNA-binding-independent actions of the ERalpha. J. Bone. Miner. Res. 2010, 25, 769–781. [Google Scholar] [PubMed]
- Katoh, M.; Katoh, M. Human FOX gene family (Review). Int. J. Oncol. 2004, 25, 1495–1500. [Google Scholar] [CrossRef] [PubMed]
- Klotz, L.O.; Sanchez-Ramos, C.; Prieto-Arroyo, I.; Urbanek, P.; Steinbrenner, H.; Monsalve, M. Redox regulation of FoxO transcription factors. Redox. Biol. 2015, 6, 51–72. [Google Scholar] [CrossRef] [PubMed]
- Essers, M.A.; de Vries-Smits, L.M.; Barker, N.; Polderman, P.E.; Burgering, B.M.; Korswagen, H.C. Functional interaction between beta-catenin and FOXO in oxidative stress signaling. Science 2005, 308, 1181–1184. [Google Scholar] [CrossRef] [PubMed]
- Staal, F.J.; Clevers, H. Tcf/Lef transcription factors during T-cell development: Unique and overlapping functions. Hematol. J. 2000, 1, 3–6. [Google Scholar] [CrossRef] [PubMed]
- Moon, R.T.; Bowerman, B.; Boutros, M.; Perrimon, N. The promise and perils of Wnt signaling through beta-catenin. Science 2002, 296, 1644–1646. [Google Scholar] [CrossRef] [PubMed]
- Iyer, S.; Ambrogini, E.; Bartell, S.M.; Han, L.; Roberson, P.K.; de Cabo, R.; Jilka, R.L.; Weinstein, R.S.; O’Brien, C.A.; Manolagas, S.C.; et al. FOXOs attenuate bone formation by suppressing Wnt signaling. J. Clin. Investig. 2013, 123, 3409–3419. [Google Scholar] [CrossRef] [PubMed]
- Manolagas, S.C.; Almeida, M. Gone with the Wnts: Beta-catenin, T-cell factor, forkhead box O, and oxidative stress in age-dependent diseases of bone, lipid, and glucose metabolism. Mol. Endocrinol. 2007, 21, 2605–2614. [Google Scholar] [CrossRef] [PubMed]
- Rached, M.T.; Kode, A.; Xu, L.; Yoshikawa, Y.; Paik, J.H.; Depinho, R.A.; Kousteni, S. FoxO1 is a positive regulator of bone formation by favoring protein synthesis and resistance to oxidative stress in osteoblasts. Cell Metab. 2010, 11, 147–160. [Google Scholar] [CrossRef] [PubMed]
- Ambrogini, E.; Almeida, M.; Martin, M. FoxO-Mediated Defense against Oxidative Stress in Osteoblasts Is Indispensable for Skeletal Homeostasis in Mice. Cell Metab. 2010, 11, 136. [Google Scholar] [CrossRef] [PubMed]
- Bartell, S.M.; Kim, H.N.; Ambrogini, E.; Han, L.; Iyer, S.; Serra Ucer, S.; Rabinovitch, P.; Jilka, R.L.; Weinstein, R.S.; Zhao, H.; et al. FoxO proteins restrain osteoclastogenesis and bone resorption by attenuating H2O2 accumulation. Nat. Commun. 2014, 5, 3773. [Google Scholar] [CrossRef] [PubMed]
- Zhu, H.; Zhang, L.; Itoh, K.; Yamamoto, M.; Ross, D.; Trush, M.A.; Zweier, J.L.; Li, Y. Nrf2 controls bone marrow stromal cell susceptibility to oxidative and electrophilic stress. Free. Radic. Biol. Med. 2006, 41, 132–143. [Google Scholar] [CrossRef] [PubMed]
- Gan, X.; Huang, S.; Yu, Q.; Yu, H.; Yan, S.S. Blockade of Drp1 rescues oxidative stress-induced osteoblast dysfunction. Biochem. Biophys. Res. Commun. 2015, 468, 719–725. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.H.; Li, B.; Zheng, X.F.; Chen, J.W.; Chen, K.; Jiang, S.D.; Jiang, L.S. Oxidative damage to osteoblasts can be alleviated by early autophagy through the endoplasmic reticulum stress pathway--implications for the treatment of osteoporosis. Free Radic. Biol. Med. 2014, 77, 10–20. [Google Scholar] [CrossRef] [PubMed]
- Almeida, M.; O’Brien, C.A. Basic biology of skeletal aging: Role of stress response pathways. J. Gerontol. A Biol. Sci. Med. Sci. 2013, 68, 1197–1208. [Google Scholar] [CrossRef] [PubMed]
- Smith, S.M.; Heer, M. Calcium and bone metabolism during space flight. Nutrition 2002, 18, 849–852. [Google Scholar] [CrossRef]
- Smith, S.M.; Heer, M.A.; Shackelford, L.C.; Sibonga, J.D.; Ploutz-Snyder, L.; Zwart, S.R. Benefits for bone from resistance exercise and nutrition in long-duration spaceflight: Evidence from biochemistry and densitometry. J. Bone Miner. Res. 2012, 27, 1896–1906. [Google Scholar] [CrossRef] [PubMed]
- Smith, S.M.; Wastney, M.E.; O’Brien, K.O.; Morukov, B.V.; Larina, I.M.; Abrams, S.A.; Davis-Street, J.E.; Oganov, V.; Shackelford, L.C. Bone Markers, Calcium Metabolism, and Calcium Kinetics During Extended-Duration Space Flight on the Mir Space Station. J. Bone Miner. Res. Off. J. Am. Soc. Bone Miner. Res. 2005, 20, 208–218. [Google Scholar] [CrossRef] [PubMed]
- Morgan, J.L.; Zwart, S.R.; Heer, M.; Ploutz-Snyder, R.; Ericson, K.; Smith, S.M. Bone metabolism and nutritional status during 30-day head-down-tilt bed rest. J. Appl. Physiol. (1985) 2012, 113, 1519–1529. [Google Scholar] [CrossRef] [PubMed]
- LeBlanc, A.; Schneider, V.; Shackelford, L.; West, S.; Oganov, V.; Bakulin, A.; Voronin, L. Bone mineral and lean tissue loss after long duration space flight. J. Musculoskelet. Neuronal Interact. 2000, 1, 157–160. [Google Scholar] [PubMed]
- Zwart, S.R.; Oliver, S.A.; Fesperman, J.V.; Kala, G.; Krauhs, J.; Ericson, K.; Smith, S.M. Nutritional status assessment before, during, and after long-duration head-down bed rest. Aviat. Space Environ. Med. 2009, 80, A15–A22. [Google Scholar] [CrossRef] [PubMed]
- Smith, S.M.; Zwart, S.R.; Block, G.; Rice, B.L.; Davis-Street, J.E. The nutritional status of astronauts is altered after long-term space flight aboard the International Space Station. J. Nutr. 2005, 135, 437–443. [Google Scholar] [PubMed]
- Zwart, S.R.; Morgan, J.L.; Smith, S.M. Iron status and its relations with oxidative damage and bone loss during long-duration space flight on the International Space Station. Am. J. Clin. Nutr. 2013, 98, 217–223. [Google Scholar] [CrossRef] [PubMed]
- Rizzo, A.M.; Corsetto, P.A.; Montorfano, G.; Milani, S.; Zava, S.; Tavella, S.; Cancedda, R.; Berra, B. Effects of long-term space flight on erythrocytes and oxidative stress of rodents. PLoS ONE 2012, 7, e32361. [Google Scholar] [CrossRef] [PubMed]
- Lawler, J.M.; Song, W.; Demaree, S.R. Hindlimb unloading increases oxidative stress and disrupts antioxidant capacity in skeletal muscle. Free Radic. Biol. Med. 2003, 35, 9–16. [Google Scholar] [CrossRef]
- Chowdhury, P.; Soulsby, M.; Kim, K. L-carnitine influence on oxidative stress induced by hind limb unloading in adult rats. Aviat. Space Environ. Med. 2007, 78, 554–556. [Google Scholar] [PubMed]
- Xin, M.; Yang, Y.; Zhang, D.; Wang, J.; Chen, S.; Zhou, D. Attenuation of hind-limb suspension-induced bone loss by curcumin is associated with reduced oxidative stress and increased vitamin D receptor expression. Osteoporos. Int. 2015, 26, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Shuang, F.; Chen, D.M.; Zhou, R.B. Treatment of hydrogen molecule abates oxidative stress and alleviates bone loss induced by modeled microgravity in rats. Osteoporos. Int. 2013, 24, 969–978. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, K.; Okumura, H.; Guo, R.; Naruse, K. Effect of Oxidative Stress on Cardiovascular System in Response to Gravity. Int. J. Mol. Sci. 2017, 18, 1426. [Google Scholar] [CrossRef] [PubMed]
- Smith, S.M.; Davis-Street, J.E.; Fesperman, J.V.; Smith, M.D.; Rice, B.L.; Zwart, S.R. Nutritional status changes in humans during a 14-day saturation dive: The NASA Extreme Environment Mission Operations V project. J. Nutr. 2004, 134, 1765–1771. [Google Scholar] [PubMed]
- Hollander, J.; Gore, M.; Fiebig, R.; Mazzeo, R.; Ohishi, S.; Ohno, H.; Ji, L. Spaceflight downregulates antioxidant defense systems in rat liver. Free Radic. Biol. Med. 1998, 24, 385–390. [Google Scholar] [CrossRef]
- Nojiri, H.; Saita, Y.; Morikawa, D.; Kobayashi, K.; Tsuda, C.; Miyazaki, T.; Saito, M.; Marumo, K.; Yonezawa, I.; Kaneko, K.; Shirasawa, T.; Shimizu, T. Cytoplasmic superoxide causes bone fragility owing to low-turnover osteoporosis and impaired collagen cross-linking. J. Bone Miner. Res. 2011, 26, 2682–2694. [Google Scholar] [CrossRef] [PubMed]
- Morikawa, D.; Nojiri, H.; Saita, Y.; Kobayashi, K.; Watanabe, K.; Ozawa, Y.; Koike, M.; Asou, Y.; Takaku, T.; Kaneko, K.; Shimizu, T. Cytoplasmic reactive oxygen species and SOD1 regulate bone mass during mechanical unloading. J. Bone Miner. Res. 2013, 28, 2368–2380. [Google Scholar] [CrossRef] [PubMed]
- Beck, M.; Moreels, M.; Quintens, R.; Abou-El-Ardat, K.; El-Saghire, H.; Tabury, K.; Michaux, A.; Janssen, A.; Neefs, M.; Van Oostveldt, P.; et al. Chronic exposure to simulated space conditions predominantly affects cytoskeleton remodeling and oxidative stress response in mouse fetal fibroblasts. Int. J. Mol. Med. 2014, 34, 606–615. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Suman, S.; Rodriguez, O.C.; Winters, T.A.; Fornace, A.J., Jr.; Albanese, C.; Datta, K. Therapeutic and space radiation exposure of mouse brain causes impaired DNA repair response and premature senescence by chronic oxidant production. Aging 2013, 5, 607–622. [Google Scholar] [CrossRef] [PubMed]
- Christofidou-Solomidou, M.; Pietrofesa, R.A.; Arguiri, E.; Schweitzer, K.S.; Berdyshev, E.V.; McCarthy, M.; Corbitt, A.; Alwood, J.S.; Yu, Y.; Globus, R.K.; et al. Space radiation-associated lung injury in a murine model. Am. J. Physiol. Lung Cell. Mol. Physiol. 2015, 308, L416–L428. [Google Scholar] [CrossRef] [PubMed]
- Guan, J.; Stewart, J.; Ware, J.H.; Zhou, Z.; Donahue, J.J.; Kennedy, A.R. Effects of dietary supplements on the space radiation-induced reduction in total antioxidant status in CBA mice. Radiat. Res. 2006, 165, 373–378. [Google Scholar] [CrossRef] [PubMed]
- Kook, S.H.; Kim, K.A.; Ji, H.; Lee, D.; Lee, J.C. Irradiation inhibits the maturation and mineralization of osteoblasts via the activation of Nrf2/HO-1 pathway. Mol. Cell Biochem. 2015, 410, 255–266. [Google Scholar] [CrossRef] [PubMed]
- Kondo, H.; Limoli, C.; Searby, N.D.; Almeida, E.A.; Loftus, D.J.; Vercoutere, W.; Morey-Holton, E.; Giedzinski, E.; Mojarrab, R.; Hilton, D.; et al. Shared oxidative pathways in response to gravity-dependent loading and gamma-irradiation of bone marrow-derived skeletal cell progenitors. Radiatsionnaia Biol. Radioecol. 2007, 47, 281–285. [Google Scholar] [PubMed]
- Kondo, H.; Yumoto, K.; Alwood, J.S.; Mojarrab, R.; Wang, A.; Almeida, E.A.; Searby, N.D.; Limoli, C.L.; Globus, R.K. Oxidative stress and gamma radiation-induced cancellous bone loss with musculoskeletal disuse. J. Appl. Physiol. (1985) 2010, 108, 152–161. [Google Scholar] [CrossRef] [PubMed]
- Maillet, A.; Beaufrere, B.; Di, N.P.; Elia, M.; Pichard, C. Weightlessness as an accelerated model of nutritional disturbances. Curr. Opin. Clin. Nutr. Metab. Care 2001, 4, 301–306. [Google Scholar] [CrossRef] [PubMed]
- Bergouignan, A.; Stein, T.P.; Habold, C.; Coxam, V.; O’Gorman, D.; Blanc, S. Towards human exploration of space: The THESEUS review series on nutrition and metabolism research priorities. NPJ Microgravity 2016, 2, 16029. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Su, Y.; Wang, D.; Chen, Y.; Wu, T.; Li, G.; Sun, X.; Cui, L. Tanshinol attenuates the deleterious effects of oxidative stress on osteoblastic differentiation via Wnt/FoxO3a signaling. Oxid. Med. Cell. Longev. 2013, 2013, 351895. [Google Scholar] [CrossRef] [PubMed]
- Zeng, X.; Tian, J.; Cai, K.; Wu, X.; Wang, Y.; Zheng, Y.; Su, Y.; Cui, L. Promoting osteoblast differentiation by the flavanes from Huangshan Maofeng tea is linked to a reduction of oxidative stress. Phytomed. Int. J. Phytother. Phytopharmacol. 2014, 21, 217–224. [Google Scholar] [CrossRef] [PubMed]
- Vester, H.; Holzer, N.; Neumaier, M.; Lilianna, S.; Nussler, A.K.; Seeliger, C. Green Tea Extract (GTE) improves differentiation in human osteoblasts during oxidative stress. J. Inflamm. 2014, 11, 15. [Google Scholar] [CrossRef] [PubMed]
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tian, Y.; Ma, X.; Yang, C.; Su, P.; Yin, C.; Qian, A.-R. The Impact of Oxidative Stress on the Bone System in Response to the Space Special Environment. Int. J. Mol. Sci. 2017, 18, 2132. https://doi.org/10.3390/ijms18102132
Tian Y, Ma X, Yang C, Su P, Yin C, Qian A-R. The Impact of Oxidative Stress on the Bone System in Response to the Space Special Environment. International Journal of Molecular Sciences. 2017; 18(10):2132. https://doi.org/10.3390/ijms18102132
Chicago/Turabian StyleTian, Ye, Xiaoli Ma, Chaofei Yang, Peihong Su, Chong Yin, and Ai-Rong Qian. 2017. "The Impact of Oxidative Stress on the Bone System in Response to the Space Special Environment" International Journal of Molecular Sciences 18, no. 10: 2132. https://doi.org/10.3390/ijms18102132
APA StyleTian, Y., Ma, X., Yang, C., Su, P., Yin, C., & Qian, A.-R. (2017). The Impact of Oxidative Stress on the Bone System in Response to the Space Special Environment. International Journal of Molecular Sciences, 18(10), 2132. https://doi.org/10.3390/ijms18102132