Magnetic Fields and Reactive Oxygen Species
Abstract
:1. Introduction
2. Reactive Oxygen Species Generation and Elimination
2.1. ROS Generation
2.2. ROS Elimination
3. The Types of Magnetic Fields (MFs)
4. The Effects of Magnetic Fields on ROS
4.1. Static Magnetic Field (SMF)
4.1.1. SMFs That Increase ROS Levels
4.1.2. Differential Effects of SMFs on ROS Levels
4.2. Extremely Low Frequency Magnetic Field (ELF-EMF)
4.2.1. ELF-EMFs That Increase ROS Levels
Human Leukemia Cell Line (K562), Human Neuroblastoma Cells (SH-SY5Y), Human Amniotic Epithelial Cells (FL) and Other Human Cells
Mice and Rat Studies at Cellular and Animal Levels
4.2.2. Differential Effects of ELF-EMFs on ROS Levels
4.3. Radio Frequency Electromagnetic Radiation (RF-EMR)
4.3.1. RF-EMRs That Increase ROS Levels
4.3.2. RF-EMRs That Have No Effects on ROS Levels
5. Underlying the ROS Changes Induced by MFs
6. Summary and Future Perspectives
Acknowledgments
Conflicts of Interest
References
- Zhang, X.; Yarema, K.; Xu, A. Biological Effects of Static Magnetic Fields; Springer: Singapore, 2017; ISBN 978-981-10-3579-1. [Google Scholar]
- Allen, R.G.; Tresini, M. Oxidative stress and gene regulation. Free Radic. Biol. Med. 2000, 28, 463–499. [Google Scholar] [CrossRef]
- Yin, C.; Luo, X.; Duan, Y.; Duan, W.; Zhang, H.; He, Y.; Sun, G.; Sun, X. Neuroprotective effects of lotus seedpod procyanidins on extremely low frequency electromagnetic field-induced neurotoxicity in primary cultured hippocampal neurons. Biomed. Pharmacother. 2016, 82, 628–639. [Google Scholar] [CrossRef] [PubMed]
- Staniek, K.; Gille, L.; Kozlov, A.V.; Nohl, H. Mitochondrial superoxide radical formation is controlled by electron bifurcation to the high and low potential pathways. Free Radic. Res. 2002, 36, 381–387. [Google Scholar] [CrossRef] [PubMed]
- Bagkos, G.; Koufopoulos, K.; Piperi, C. Mitochondrial emitted electromagnetic signals mediate retrograde signaling. Med. Hypotheses 2015, 85, 810–818. [Google Scholar] [CrossRef] [PubMed]
- Richter, C.; Gogvadze, V.; Laffranchi, R.; Schlapbach, R.; Schweizer, M.; Suter, M.; Walter, P.; Yaffee, M. Oxidants in mitochondria: From physiology to diseases. Biochim. Biophys. Acta 1995, 1271, 67–74. [Google Scholar] [CrossRef]
- Dansen, T.B.; Wirtz, K.W. The peroxisome in oxidative stress. IUBMB Life 2001, 51, 223–230. [Google Scholar] [PubMed]
- Bonekamp, N.A.; Volkl, A.; Fahimi, H.D.; Schrader, M. Reactive oxygen species and peroxisomes: Struggling for balance. BioFactors 2009, 35, 346–355. [Google Scholar] [CrossRef] [PubMed]
- Babior, B.M. The respiratory burst oxidase. Curr. Opin. Hematol. 1995, 2, 55–60. [Google Scholar] [CrossRef] [PubMed]
- Segal, A.W.; Shatwell, K.P. The NADPH oxidase of phagocytic leukocytes. Ann. N. Y. Acad. Sci. 1997, 832, 215–222. [Google Scholar] [CrossRef] [PubMed]
- Pelicano, H.; Carney, D.; Huang, P. ROS stress in cancer cells and therapeutic implications. Drug Resist. Updates 2004, 7, 97–110. [Google Scholar] [CrossRef] [PubMed]
- Liou, G.Y.; Storz, P. Reactive oxygen species in cancer. Free Radic. Res. 2010, 44, 479–496. [Google Scholar] [CrossRef] [PubMed]
- Storz, P. Reactive oxygen species in tumor progression. Front. Biosci. 2005, 10, 1881–1896. [Google Scholar] [CrossRef] [PubMed]
- Ongaro, A.; Pellati, A.; Setti, S.; Masieri, F.F.; Aquila, G.; Fini, M.; Caruso, A.; De Mattei, M. Electromagnetic fields counteract IL-1β activity during chondrogenesis of bovine mesenchymal stem cells. J. Tissue Eng. Regen. Med. 2015, 9, E229–E238. [Google Scholar] [CrossRef] [PubMed]
- Ongaro, A.; Varani, K.; Masieri, F.F.; Pellati, A.; Massari, L.; Cadossi, R.; Vincenzi, F.; Borea, P.A.; Fini, M.; Caruso, A.; et al. Electromagnetic fields (EMFs) and adenosine receptors modulate prostaglandin E(2) and cytokine release in human osteoarthritic synovial fibroblasts. J. Cell. Physiol. 2012, 227, 2461–2469. [Google Scholar] [CrossRef] [PubMed]
- De Mattei, M.; Varani, K.; Masieri, F.F.; Pellati, A.; Ongaro, A.; Fini, M.; Cadossi, R.; Vincenzi, F.; Borea, P.A.; Caruso, A. Adenosine analogs and electromagnetic fields inhibit prostaglandin E2 release in bovine synovial fibroblasts. Osteoarthr. Cartil. 2009, 17, 252–262. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.; Liao, Y.; Xie, H.; Liao, Y.; Liu, H.; Zeng, Y.; Li, N. Pulsed electromagnetic field ameliorates cartilage degeneration by inhibiting mitogen-activated protein kinases in a rat model of osteoarthritis. Phys. Ther. Sport 2017, 24, 32–38. [Google Scholar] [CrossRef] [PubMed]
- Fini, M.; Torricelli, P.; Giavaresi, G.; Aldini, N.N.; Cavani, F.; Setti, S.; Nicolini, A.; Carpi, A.; Giardino, R. Effect of pulsed electromagnetic field stimulation on knee cartilage, subchondral and epyphiseal trabecular bone of aged Dunkin Hartley guinea pigs. Biomed. Pharmacother. 2008, 62, 709–715. [Google Scholar] [CrossRef] [PubMed]
- Benazzo, F.; Cadossi, M.; Cavani, F.; Fini, M.; Giavaresi, G.; Setti, S.; Cadossi, R.; Giardino, R. Cartilage repair with osteochondral autografts in sheep: Effect of biophysical stimulation with pulsed electromagnetic fields. J. Orthop. Res. 2008, 26, 631–642. [Google Scholar] [CrossRef] [PubMed]
- Krath, A.; Klüter, T.; Stukenberg, M.; Zielhardt, P.; Gollwitzer, H.; Harrasser, N.; Hausdorf, J.; Ringeisen, M.; Gerdesmeyer, L. Electromagnetic transduction therapy in non-specific low back pain: A prospective randomised controlled trial. J. Orthop. 2017, 14, 410–415. [Google Scholar] [CrossRef] [PubMed]
- Deutschlander, M.E.; Phillips, J.B.; Borland, S.C. The case for light-dependent magnetic orientation in animals. J. Exp. Biol. 1999, 202, 891–908. [Google Scholar] [PubMed]
- Bekhite, M.M.; Finkensieper, A.; Abou-Zaid, F.A.; El-Shourbagy, I.K.; El-Fiky, N.K.; Omar, K.M.; Sauer, H.; Wartenberg, M. Differential effects of high and low strength magnetic fields on mouse embryonic development and vasculogenesis of embryonic stem cells. Reprod. Toxicol. 2016, 65, 46–58. [Google Scholar] [CrossRef] [PubMed]
- Repacholi, M.H. An overview of WHO’s EMF project and the health effects of EMF exposure. In Proceedings of the International Conference on Non-Ionizing Radiation at UNITEN (ICNIR 2003) Electromagnetic Fields and Our Health, Kuala Lumpur, Malaysia, 20–22 October 2003. [Google Scholar]
- Frahm, J.; Mattsson, M.O.; Simko, M. Exposure to ELF magnetic fields modulate redox related protein expression in mouse macrophages. Toxicol. Lett. 2010, 192, 330–336. [Google Scholar] [CrossRef] [PubMed]
- Okano, H. Effects of static magnetic fields in biology: Role of free radicals. Front. Biosci. 2008, 13, 6106–6125. [Google Scholar] [CrossRef] [PubMed]
- Martino, C.F.; Castello, P.R. Modulation of hydrogen peroxide production in cellular systems by low level magnetic fields. PLoS ONE 2011, 6, e22753. [Google Scholar] [CrossRef] [PubMed]
- Calabro, E.; Condello, S.; Curro, M.; Ferlazzo, N.; Caccamo, D.; Magazu, S.; Ientile, R. Effects of low intensity static magnetic field on FTIR spectra and ROS production in SH-SY5Y neuronal-like cells. Bioelectromagnetics 2013, 34, 618–629. [Google Scholar] [CrossRef] [PubMed]
- Vergallo, C.; Ahmadi, M.; Mobasheri, H.; Dini, L. Impact of inhomogeneous static magnetic field (31.7–232.0 mT) exposure on human neuroblastoma SH-SY5Y cells during cisplatin administration. PLoS ONE 2014, 9, e113530. [Google Scholar] [CrossRef] [PubMed]
- De Nicola, M.; Cordisco, S.; Cerella, C.; Albertini, M.C.; D’Alessio, M.; Accorsi, A.; Bergamaschi, A.; Magrini, A.; Ghibelli, L. Magnetic fields protect from apoptosis via redox alteration. Ann. N. Y. Acad. Sci. 2006, 1090, 59–68. [Google Scholar] [CrossRef] [PubMed]
- Poniedzialek, B.; Rzymski, P.; Karczewski, J.; Jaroszyk, F.; Wiktorowicz, K. Reactive oxygen species (ROS) production in human peripheral blood neutrophils exposed In Vitro to static magnetic field. Electromagn. Biol. Med. 2013, 32, 560–568. [Google Scholar] [CrossRef] [PubMed]
- Sullivan, K.; Balin, A.K.; Allen, R.G. Effects of static magnetic fields on the growth of various types of human cells. Bioelectromagnetics 2011, 32, 140–147. [Google Scholar] [CrossRef] [PubMed]
- Zablotskii, V.; Syrovets, T.; Schmidt, Z.W.; Dejneka, A.; Simmet, T. Modulation of monocytic leukemia cell function and survival by high gradient magnetic fields and mathematical modeling studies. Biomaterials 2014, 35, 3164–3171. [Google Scholar] [CrossRef] [PubMed]
- Zhao, G.; Chen, S.; Wang, L.; Zhao, Y.; Wang, J.; Wang, X.; Zhang, W.; Wu, R.; Wu, L.; Wu, Y.; et al. Cellular ATP content was decreased by a homogeneous 8.5 T static magnetic field exposure: Role of reactive oxygen species. Bioelectromagnetics 2011, 32, 94–101. [Google Scholar] [CrossRef] [PubMed]
- Bekhite, M.M.; Figulla, H.R.; Sauer, H.; Wartenberg, M. Static magnetic fields increase cardiomyocyte differentiation of Flk-1+ cells derived from mouse embryonic stem cells via Ca2+ influx and ROS production. Int. J. Cardiol. 2013, 167, 798–808. [Google Scholar] [CrossRef] [PubMed]
- Bae, J.E.; Huh, M.I.; Ryu, B.K.; Do, J.Y.; Jin, S.U.; Moon, M.J.; Jung, J.C.; Chang, Y.; Kim, E.; Chi, S.G.; et al. The effect of static magnetic fields on the aggregation and cytotoxicity of magnetic nanoparticles. Biomaterials 2011, 32, 9401–9414. [Google Scholar] [CrossRef] [PubMed]
- Bekhite, M.M.; Finkensieper, A.; Abou-Zaid, F.A.; El-Shourbagy, I.K.; Omar, K.M.; Figulla, H.R.; Sauer, H.; Wartenberg, M. Static electromagnetic fields induce vasculogenesis and chondro-osteogenesis of mouse embryonic stem cells by reactive oxygen species-mediated up-regulation of vascular endothelial growth factor. Stem Cells Dev. 2010, 19, 731–743. [Google Scholar] [CrossRef] [PubMed]
- Shine, M.B.; Guruprasad, K.N.; Anand, A. Effect of stationary magnetic field strengths of 150 and 200 mT on reactive oxygen species production in soybean. Bioelectromagnetics 2012, 33, 428–437. [Google Scholar] [CrossRef] [PubMed]
- Csillag, A.; Kumar, B.V.; Szabo, K.; Szilasi, M.; Papp, Z.; Szilasi, M.E.; Pazmandi, K.; Boldogh, I.; Rajnavolgyi, E.; Bacsi, A.; et al. Exposure to inhomogeneous static magnetic field beneficially affects allergic inflammation in a murine model. J. R. Soc. Interface 2014, 11, 20140097. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fu, J.P.; Mo, W.C.; Liu, Y.; He, R.Q. Decline of cell viability and mitochondrial activity in mouse skeletal muscle cell in a hypomagnetic field. Bioelectromagnetics 2016, 37, 212–222. [Google Scholar] [CrossRef] [PubMed]
- Romeo, S.; Sannino, A.; Scarfi, M.R.; Massa, R.; d’Angelo, R.; Zeni, O. Lack of effects on key cellular parameters of MRC-5 human lung fibroblasts exposed to 370 mT static magnetic field. Sci. Rep. 2016, 6, 19398. [Google Scholar] [CrossRef] [PubMed]
- Bajpai, I.; Balani, K.; Basu, B. Synergistic effect of static magnetic field and HA-Fe3O4 magnetic composites on viability of S. aureus and E. coli bacteria. J. Biomed. Mater. Res. B 2014, 102, 524–532. [Google Scholar] [CrossRef] [PubMed]
- Sauer, H.; Rahimi, G.; Hescheler, J.; Wartenberg, M. Effects of electrical fields on cardiomyocyte differentiation of embryonic stem cells. J. Cell. Biochem. 1999, 75, 710–723. [Google Scholar] [CrossRef]
- Sauer, H.; Neukirchen, W.; Rahimi, G.; Grunheck, F.; Hescheler, J.; Wartenberg, M. Involvement of reactive oxygen species in cardiotrophin-1-induced proliferation of cardiomyocytes differentiated from murine embryonic stem cells. Exp. Cell Res. 2004, 294, 313–324. [Google Scholar] [CrossRef] [PubMed]
- Politanski, P.; Rajkowska, E.; Pawlaczyk-Luszczynska, M.; Dudarewicz, A.; Wiktorek-Smagur, A.; Sliwinska-Kowalska, M.; Zmyslony, M. Static magnetic field affects oxidative stress in mouse cochlea. Int. J. Occup. Med. Environ. Health 2010, 23, 377–384. [Google Scholar] [CrossRef] [PubMed]
- Limoli, C.L.; Rola, R.; Giedzinski, E.; Mantha, S.; Huang, T.T.; Fike, J.R. Cell-density-dependent regulation of neural precursor cell function. Proc. Natl. Acad. Sci. USA 2004, 101, 16052–16057. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Ji, X.; Yang, X.; Zhang, X. Cell type- and density-dependent effect of 1 T static magnetic field on cell proliferation. Oncotarget 2017, 8, 13126–13141. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Wang, J.; Wang, H.; Wang, W.; Li, Z.; Liu, J.; Yang, X.; Ji, X.; Luo, Y.; Hu, C.; et al. Moderate and strong static magnetic fields directly affect EGFR kinase domain orientation to inhibit cancer cell proliferation. Oncotarget 2016, 7, 41527–41539. [Google Scholar] [CrossRef] [PubMed]
- Tang, R.; Xu, Y.; Ma, F.; Ren, J.; Shen, S.; Du, Y.; Hou, Y.; Wang, T. Extremely low frequency magnetic fields regulate differentiation of regulatory T cells: Potential role for ROS-mediated inhibition on AKT. Bioelectromagnetics 2016, 37, 89–98. [Google Scholar] [CrossRef] [PubMed]
- Mannerling, A.C.; Simko, M.; Mild, K.H.; Mattsson, M.O. Effects of 50-Hz magnetic field exposure on superoxide radical anion formation and HSP70 induction in human K562 cells. Radiat. Environ. Biophys. 2010, 49, 731–741. [Google Scholar] [CrossRef] [PubMed]
- Garip, A.I.; Akan, Z. Effect of ELF-EMF on number of apoptotic cells; correlation with reactive oxygen species and HSP. Acta Biol. Hung. 2010, 61, 158–167. [Google Scholar] [CrossRef] [PubMed]
- Patruno, A.; Tabrez, S.; Pesce, M.; Shakil, S.; Kamal, M.A.; Reale, M. Effects of extremely low frequency electromagnetic field (ELF-EMF) on catalase, cytochrome P450 and nitric oxide synthase in erythro-leukemic cells. Life Sci. 2015, 121, 117–123. [Google Scholar] [CrossRef] [PubMed]
- Ayse, I.G.; Zafer, A.; Sule, O.; Isil, I.T.; Kalkan, T. Differentiation of K562 cells under ELF-EMF applied at different time courses. Electromagn. Biol. Med. 2010, 29, 122–130. [Google Scholar] [CrossRef] [PubMed]
- Luukkonen, J.; Liimatainen, A.; Juutilainen, J.; Naarala, J. Induction of genomic instability, oxidative processes, and mitochondrial activity by 50 Hz magnetic fields in human SH-SY5Y neuroblastoma cells. Mutat. Res. 2014, 760, 33–41. [Google Scholar] [CrossRef] [PubMed]
- Kesari, K.K.; Luukkonen, J.; Juutilainen, J.; Naarala, J. Genomic instability induced by 50 Hz magnetic fields is a dynamically evolving process not blocked by antioxidant treatment. Mutat. Res. 2015, 794, 46–51. [Google Scholar] [CrossRef] [PubMed]
- Benassi, B.; Filomeni, G.; Montagna, C.; Merla, C.; Lopresto, V.; Pinto, R.; Marino, C.; Consales, C. Extremely low frequency magnetic field (ELF-MF) exposure sensitizes SH-SY5Y cells to the Pro-Parkinson’s disease toxin MPP+. Mol. Neurobiol. 2016, 53, 4247–4260. [Google Scholar] [CrossRef] [PubMed]
- Feng, B.; Dai, A.; Chen, L.; Qiu, L.; Fu, Y.; Sun, W. NADPH oxidase-produced superoxide mediated a 50-Hz magnetic field-induced epidermal growth factor receptor clustering. Int. J. Radiat. Biol. 2016, 92, 596–602. [Google Scholar] [CrossRef] [PubMed]
- Feng, B.; Ye, C.; Qiu, L.; Chen, L.; Fu, Y.; Sun, W. Mitochondrial ROS release and subsequent akt activation potentially mediated the anti-apoptotic effect of a 50-Hz magnetic field on FL cells. Cell. Physiol. Biochem. 2016, 38, 2489–2499. [Google Scholar] [CrossRef] [PubMed]
- Feng, B.; Qiu, L.; Ye, C.; Chen, L.; Fu, Y.; Sun, W. Exposure to a 50-Hz magnetic field induced mitochondrial permeability transition through the ROS/GSK-3beta signaling pathway. Int. J. Radiat. Biol. 2016, 92, 148–155. [Google Scholar] [CrossRef] [PubMed]
- Calcabrini, C.; Mancini, U.; De Bellis, R.; Diaz, A.R.; Martinelli, M.; Cucchiarini, L.; Sestili, P.; Stocchi, V.; Potenza, L. Effect of extremely low-frequency electromagnetic fields on antioxidant activity in the human keratinocyte cell line NCTC 2544. Biotechnol. Appl. Biochem. 2017, 64, 415–422. [Google Scholar] [CrossRef] [PubMed]
- Lupke, M.; Frahm, J.; Lantow, M.; Maercker, C.; Remondini, D.; Bersani, F.; Simko, M. Gene expression analysis of ELF-MF exposed human monocytes indicating the involvement of the alternative activation pathway. Biochim. Biophys. Acta 2006, 1763, 402–412. [Google Scholar] [CrossRef] [PubMed]
- Lupke, M.; Rollwitz, J.; Simko, M. Cell activating capacity of 50 Hz magnetic fields to release reactive oxygen intermediates in human umbilical cord blood-derived monocytes and in Mono Mac 6 cells. Free Radic. Res. 2004, 38, 985–993. [Google Scholar] [CrossRef] [PubMed]
- Park, J.E.; Seo, Y.K.; Yoon, H.H.; Kim, C.W.; Park, J.K.; Jeon, S. Electromagnetic fields induce neural differentiation of human bone marrow derived mesenchymal stem cells via ROS mediated EGFR activation. Neurochem. Int. 2013, 62, 418–424. [Google Scholar] [CrossRef] [PubMed]
- Jeong, W.Y.; Kim, J.B.; Kim, H.J.; Kim, C.W. Extremely low-frequency electromagnetic field promotes astrocytic differentiation of human bone marrow mesenchymal stem cells by modulating SIRT1 expression. Biosci. Biotechnol. Biochem. 2017, 81, 1356–1362. [Google Scholar] [CrossRef] [PubMed]
- Yang, M.L.; Ye, Z.M. Extremely low frequency electromagnetic field induces apoptosis of osteosarcoma cells via oxidative stress. Zhejian. Da Xue Xue Bao 2015, 44, 323–328. [Google Scholar]
- Koh, E.K.; Ryu, B.K.; Jeong, D.Y.; Bang, I.S.; Nam, M.H.; Chae, K.S. A 60-Hz sinusoidal magnetic field induces apoptosis of prostate cancer cells through reactive oxygen species. Int. J. Radiat. Biol. 2008, 84, 945–955. [Google Scholar] [CrossRef] [PubMed]
- Sadeghipour, R.; Ahmadian, S.; Bolouri, B.; Pazhang, Y.; Shafiezadeh, M. Effects of extremely low-frequency pulsed electromagnetic fields on morphological and biochemical properties of human breast carcinoma cells (T47D). Electromagn. Biol. Med. 2012, 31, 425–435. [Google Scholar] [CrossRef] [PubMed]
- Lim, S.; Kim, S.C.; Kim, J.Y. Protective effect of 10-Hz, 1-mT electromagnetic field exposure against hypoxia/reoxygenation injury in HK-2 cells. Biomed. Environ. Sci. 2015, 28, 231–234. [Google Scholar] [PubMed]
- Duong, C.N.; Kim, J.Y. Exposure to electromagnetic field attenuates oxygen-glucose deprivation-induced microglial cell death by reducing intracellular Ca(2+) and ROS. Int. J. Radiat. Biol. 2016, 92, 195–201. [Google Scholar] [CrossRef] [PubMed]
- Falone, S.; Marchesi, N.; Osera, C.; Fassina, L.; Comincini, S.; Amadio, M.; Pascale, A. Pulsed electromagnetic field (PEMF) prevents pro-oxidant effects of H2O2 in SK-N-BE(2) human neuroblastoma cells. Int. J. Radiat. Biol. 2016, 92, 281–286. [Google Scholar] [CrossRef] [PubMed]
- Vincenzi, F.; Ravani, A.; Pasquini, S.; Merighi, S.; Gessi, S.; Setti, S.; Cadossi, R.; Borea, P.A.; Varani, K. Pulsed electromagnetic field exposure reduces hypoxia and inflammation damage in neuron-like and microglial cells. J. Cell. Physiol. 2017, 232, 1200–1208. [Google Scholar] [CrossRef] [PubMed]
- Osera, C.; Amadio, M.; Falone, S.; Fassina, L.; Magenes, G.; Amicarelli, F.; Ricevuti, G.; Govoni, S.; Pascale, A. Pre-exposure of neuroblastoma cell line to pulsed electromagnetic field prevents H2O2-induced ROS production by increasing MnSOD activity. Bioelectromagnetics 2015, 36, 219–232. [Google Scholar] [CrossRef] [PubMed]
- Ferroni, L.; Bellin, G.; Emer, V.; Rizzuto, R.; Isola, M.; Gardin, C.; Zavan, B. Treatment by Therapeutic Magnetic Resonance (TMR) increases fibroblastic activity and keratinocyte differentiation in an In Vitro model of 3D artificial skin. J. Tissue Eng. Regen. Med. 2017, 11, 1332–1342. [Google Scholar] [CrossRef] [PubMed]
- Palumbo, R.; Capasso, D.; Brescia, F.; Mita, P.; Sarti, M.; Bersani, F.; Scarfi, M.R. Effects on apoptosis and reactive oxygen species formation by Jurkat cells exposed to 50 Hz electromagnetic fields. Bioelectromagnetics 2006, 27, 159–162. [Google Scholar] [CrossRef] [PubMed]
- Hong, M.N.; Han, N.K.; Lee, H.C.; Ko, Y.K.; Chi, S.G.; Lee, Y.S.; Gimm, Y.M.; Myung, S.H.; Lee, J.S. Extremely low frequency magnetic fields do not elicit oxidative stress in MCF10A cells. J. Radiat. Res. 2012, 53, 79–86. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Yoon, Y.; Yun, S.; Park, G.S.; Lee, H.J.; Song, K. Time-varying magnetic fields of 60 Hz at 7 mT induce DNA double-strand breaks and activate DNA damage checkpoints without apoptosis. Bioelectromagnetics 2012, 33, 383–393. [Google Scholar] [CrossRef] [PubMed]
- Poniedzialek, B.; Rzymski, P.; Nawrocka-Bogusz, H.; Jaroszyk, F.; Wiktorowicz, K. The effect of electromagnetic field on reactive oxygen species production in human neutrophils In Vitro. Electromagn. Biol. Med. 2013, 32, 333–341. [Google Scholar] [CrossRef] [PubMed]
- Morabito, C.; Rovetta, F.; Bizzarri, M.; Mazzoleni, G.; Fano, G.; Mariggio, M.A. Modulation of redox status and calcium handling by extremely low frequency electromagnetic fields in C2C12 muscle cells: A real-time, single-cell approach. Free Radic. Biol. Med. 2010, 48, 579–589. [Google Scholar] [CrossRef] [PubMed]
- Buldak, R.J.; Polaniak, R.; Buldak, L.; Zwirska-Korczala, K.; Skonieczna, M.; Monsiol, A.; Kukla, M.; Dulawa-Buldak, A.; Birkner, E. Short-term exposure to 50 Hz ELF-EMF alters the cisplatin-induced oxidative response in AT478 murine squamous cell carcinoma cells. Bioelectromagnetics 2012, 33, 641–651. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Hong, L.; Zeng, Y.; Shen, Y.; Zeng, Q. Power frequency magnetic fields induced reactive oxygen species-related autophagy in mouse embryonic fibroblasts. Int. J. Biochem. Cell Biol. 2014, 57, 108–114. [Google Scholar] [CrossRef] [PubMed]
- Morabito, C.; Guarnieri, S.; Fano, G.; Mariggio, M.A. Effects of acute and chronic low frequency electromagnetic field exposure on PC12 cells during neuronal differentiation. Cell. Physiol. Biochem. 2010, 26, 947–958. [Google Scholar] [CrossRef] [PubMed]
- Wolf, F.I.; Torsello, A.; Tedesco, B.; Fasanella, S.; Boninsegna, A.; D’Ascenzo, M.; Grassi, C.; Azzena, G.B.; Cittadini, A. 50-Hz extremely low frequency electromagnetic fields enhance cell proliferation and DNA damage: Possible involvement of a redox mechanism. Biochim. Biophys. Acta 2005, 1743, 120–129. [Google Scholar] [CrossRef] [PubMed]
- Roy, S.; Noda, Y.; Eckert, V.; Traber, M.G.; Mori, A.; Liburdy, R.; Packer, L. The phorbol 12-myristate 13-acetate (PMA)-induced oxidative burst in rat peritoneal neutrophils is increased by a 0.1 mT (60 Hz) magnetic field. FEBS Lett. 1995, 376, 164–166. [Google Scholar] [CrossRef]
- Duan, Y.; Wang, Z.; Zhang, H.; He, Y.; Lu, R.; Zhang, R.; Sun, G.; Sun, X. The preventive effect of lotus seedpod procyanidins on cognitive impairment and oxidative damage induced by extremely low frequency electromagnetic field exposure. Food Funct. 2013, 4, 1252–1262. [Google Scholar] [CrossRef] [PubMed]
- Goraca, A.; Ciejka, E.; Piechota, A. Effects of extremely low frequency magnetic field on the parameters of oxidative stress in heart. J. Physiol. Pharmacol. 2010, 61, 333–338. [Google Scholar] [PubMed]
- Manikonda, P.K.; Rajendra, P.; Devendranath, D.; Gunasekaran, B.; Channakeshava; Aradhya, S.R.; Sashidhar, R.B.; Subramanyam, C. Extremely low frequency magnetic fields induce oxidative stress in rat brain. Gen. Physiol. Biophys. 2014, 33, 81–90. [Google Scholar] [CrossRef] [PubMed]
- Ma, S.; Zhang, Z.; Yi, F.; Wang, Y.; Zhang, X.; Li, X.; Yuan, Y.; Cao, F. Protective effects of low-frequency magnetic fields on cardiomyocytes from ischemia reperfusion injury via ROS and NO/ONOO. Oxid. Med. Cell. Longev. 2013, 2013, 529173. [Google Scholar] [CrossRef] [PubMed]
- Di Loreto, S.; Falone, S.; Caracciolo, V.; Sebastiani, P.; D’Alessandro, A.; Mirabilio, A.; Zimmitti, V.; Amicarelli, F. Fifty hertz extremely low-frequency magnetic field exposure elicits redox and trophic response in rat-cortical neurons. J. Cell. Physiol. 2009, 219, 334–343. [Google Scholar] [CrossRef] [PubMed]
- De Groot, M.W.; Kock, M.D.; Westerink, R.H. Assessment of the neurotoxic potential of exposure to 50 Hz extremely low frequency electromagnetic fields (ELF-EMF) in naive and chemically stressed PC12 cells. Neurotoxicology 2014, 44, 358–364. [Google Scholar] [CrossRef] [PubMed]
- Ciejka, E.; Kleniewska, P.; Skibska, B.; Goraca, A. Effects of extremely low frequency magnetic field on oxidative balance in brain of rats. J. Physiol. Pharmacol. 2011, 62, 657–661. [Google Scholar] [PubMed]
- Foster, K.R.; Glaser, R. Thermal mechanisms of interaction of radiofrequency energy with biological systems with relevance to exposure guidelines. Health Phys. 2007, 92, 609–620. [Google Scholar] [CrossRef] [PubMed]
- Agarwal, A.; Desai, N.R.; Makker, K.; Varghese, A.; Mouradi, R.; Sabanegh, E.; Sharma, R. Effects of radiofrequency electromagnetic waves (RF-EMW) from cellular phones on human ejaculated semen: An In Vitro pilot study. Fertil. Steril. 2009, 92, 1318–1325. [Google Scholar] [CrossRef] [PubMed]
- De Iuliis, G.N.; Newey, R.J.; King, B.V.; Aitken, R.J. Mobile phone radiation induces reactive oxygen species production and DNA damage in human spermatozoa In Vitro. PLoS ONE 2009, 4, e6446. [Google Scholar] [CrossRef] [PubMed]
- Luukkonen, J.; Hakulinen, P.; Maki-Paakkanen, J.; Juutilainen, J.; Naarala, J. Enhancement of chemically induced reactive oxygen species production and DNA damage in human SH-SY5Y neuroblastoma cells by 872 MHz radiofrequency radiation. Mutat. Res. 2009, 662, 54–58. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.S.; Huang, B.T.; Huang, Y.X. Reactive oxygen species formation and apoptosis in human peripheral blood mononuclear cell induced by 900 MHz mobile phone radiation. Oxid. Med. Cell. Longev. 2012, 2012, 740280. [Google Scholar] [CrossRef] [PubMed]
- Sefidbakht, Y.; Moosavi-Movahedi, A.A.; Hosseinkhani, S.; Khodagholi, F.; Torkzadeh-Mahani, M.; Foolad, F.; Faraji-Dana, R. Effects of 940 MHz EMF on bioluminescence and oxidative response of stable luciferase producing HEK cells. Photochem. Photobiol. Sci. 2014, 13, 1082–1092. [Google Scholar] [CrossRef] [PubMed]
- Yao, K.; Wu, W.; Yu, Y.; Zeng, Q.; He, J.; Lu, D.; Wang, K. Effect of superposed electromagnetic noise on DNA damage of lens epithelial cells induced by microwave radiation. Investig. Ophthalmol. Vis. Sci. 2008, 49, 2009–2015. [Google Scholar] [CrossRef] [PubMed]
- Usselman, R.J.; Hill, I.; Singel, D.J.; Martino, C.F. Spin biochemistry modulates reactive oxygen species (ROS) production by radio frequency magnetic fields. PLoS ONE 2014, 9, e93065. [Google Scholar] [CrossRef] [PubMed]
- Campisi, A.; Gulino, M.; Acquaviva, R.; Bellia, P.; Raciti, G.; Grasso, R.; Musumeci, F.; Vanella, A.; Triglia, A. Reactive oxygen species levels and DNA fragmentation on astrocytes in primary culture after acute exposure to low intensity microwave electromagnetic field. Neurosci. Lett. 2010, 473, 52–55. [Google Scholar] [CrossRef] [PubMed]
- Kesari, K.K.; Kumar, S.; Behari, J. Effects of radiofrequency electromagnetic wave exposure from cellular phones on the reproductive pattern in male Wistar rats. Appl. Biochem. Biotechnol. 2011, 164, 546–559. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Kesari, K.K.; Behari, J. Influence of microwave exposure on fertility of male rats. Fertil. Steril. 2011, 95, 1500–1502. [Google Scholar] [CrossRef] [PubMed]
- Kesari, K.K.; Kumar, S.; Behari, J. 900-MHz microwave radiation promotes oxidation in rat brain. Electromagn. Biol. Med. 2011, 30, 219–234. [Google Scholar] [CrossRef] [PubMed]
- Manta, A.K.; Stravopodis, D.J.; Papassideri, I.S.; Margaritis, L.H. Reactive oxygen species elevation and recovery in Drosophila bodies and ovaries following short-term and long-term exposure to DECT base EMF. Electromagn. Biol. Med. 2014, 33, 118–131. [Google Scholar] [CrossRef] [PubMed]
- Luukkonen, J.; Juutilainen, J.; Naarala, J. Combined effects of 872 MHz radiofrequency radiation and ferrous chloride on reactive oxygen species production and DNA damage in human SH-SY5Y neuroblastoma cells. Bioelectromagnetics 2010, 31, 417–424. [Google Scholar] [CrossRef] [PubMed]
- Silva, V.; Hilly, O.; Strenov, Y.; Tzabari, C.; Hauptman, Y.; Feinmesser, R. Effect of cell phone-like electromagnetic radiation on primary human thyroid cells. Int. J. Radiat. Biol. 2016, 92, 107–115. [Google Scholar] [CrossRef] [PubMed]
- Lantow, M.; Lupke, M.; Frahm, J.; Mattsson, M.O.; Kuster, N.; Simko, M. ROS release and Hsp70 expression after exposure to 1800 MHz radiofrequency electromagnetic fields in primary human monocytes and lymphocytes. Radiat. Environ. Biophys. 2006, 45, 55–62. [Google Scholar] [CrossRef] [PubMed]
- Zeni, O.; Di Pietro, R.; d’Ambrosio, G.; Massa, R.; Capri, M.; Naarala, J.; Juutilainen, J.; Scarfi, M.R. Formation of reactive oxygen species in L929 cells after exposure to 900 MHz RF radiation with and without co-exposure to 3-chloro-4-(dichloromethyl)-5-hydroxy-2(5H)-furanone. Radiat. Res. 2007, 167, 306–311. [Google Scholar] [CrossRef] [PubMed]
- Zmyslony, M.; Politanski, P.; Rajkowska, E.; Szymczak, W.; Jajte, J. Acute exposure to 930 MHz CW electromagnetic radiation a In Vitro ffects reactive oxygen species level in rat lymphocytes treated by iron ions. Bioelectromagnetics 2004, 25, 324–328. [Google Scholar] [CrossRef] [PubMed]
- Fasseas, M.K.; Fragopoulou, A.F.; Manta, A.K.; Skouroliakou, A.; Vekrellis, K.; Margaritis, L.H.; Syntichaki, P. Response of Caenorhabditis elegans to wireless devices radiation exposure. Int. J. Radiat. Biol. 2015, 91, 286–293. [Google Scholar] [CrossRef] [PubMed]
- Friedman, J.; Kraus, S.; Hauptman, Y.; Schiff, Y.; Seger, R. Mechanism of short-term ERK activation by electromagnetic fields at mobile phone frequencies. Biochem. J. 2007, 405, 559–568. [Google Scholar] [CrossRef] [PubMed]
- Wolf-Goldberg, T.; Barbul, A.; Ben-Dov, N.; Korenstein, R. Low electric fields induce ligand-independent activation of EGF receptor and ERK via electrochemical elevation of H(+) and ROS concentrations. Biochim. Biophys. Acta 2013, 1833, 1396–1408. [Google Scholar] [CrossRef] [PubMed]
- Jouni, F.J.; Abdolmaleki, P.; Ghanati, F. Oxidative stress in broad bean (Vicia faba L.) induced by static magnetic field under natural radioactivity. Mutat. Res. 2012, 741, 116–121. [Google Scholar] [CrossRef] [PubMed]
- Zwirska-Korczala, K.; Jochem, J.; Adamczyk-Sowa, M.; Sowa, P.; Polaniak, R.; Birkner, E.; Latocha, M.; Pilc, K.; Suchanek, R. Influence of melatonin on cell proliferation, antioxidative enzyme activities and lipid peroxidation in 3T3-L1 preadipocytes—An In Vitro study. J. Physiol. Pharmacol. 2005, 56, 91–99. [Google Scholar] [PubMed]
- Lee, B.C.; Johng, H.M.; Lim, J.K.; Jeong, J.H.; Baik, K.Y.; Nam, T.J.; Lee, J.H.; Kim, J.; Sohn, U.D.; Yoon, G.; et al. Effects of extremely low frequency magnetic field on the antioxidant defense system in mouse brain: A chemiluminescence study. J. Photochem. Photobiol. B 2004, 73, 43–48. [Google Scholar] [CrossRef] [PubMed]
- Kesari, K.K.; Behari, J. Microwave exposure affecting reproductive system in male rats. Appl. Biochem. Biotechnol. 2010, 162, 416–428. [Google Scholar] [CrossRef] [PubMed]
- Ilhan, A.; Gurel, A.; Armutcu, F.; Kamisli, S.; Iraz, M.; Akyol, O.; Ozen, S. Ginkgo biloba prevents mobile phone-induced oxidative stress in rat brain. Clin. Chim. Acta 2004, 340, 153–162. [Google Scholar] [CrossRef] [PubMed]
- Ozguner, F.; Oktem, F.; Ayata, A.; Koyu, A.; Yilmaz, H.R. A novel antioxidant agent caffeic acid phenethyl ester prevents long-term mobile phone exposure-induced renal impairment in rat. Prognostic value of malondialdehyde, N-acetyl-beta-d-glucosaminidase and nitric oxide determination. Mol. Cell. Biochem. 2005, 277, 73–80. [Google Scholar] [CrossRef] [PubMed]
- Irmak, M.K.; Fadillioglu, E.; Gulec, M.; Erdogan, H.; Yagmurca, M.; Akyol, O. Effects of electromagnetic radiation from a cellular telephone on the oxidant and antioxidant levels in rabbits. Cell Biochem. Funct. 2002, 20, 279–283. [Google Scholar] [CrossRef] [PubMed]
| Species | Cell Lines/Organisms | SMF | Exposure Time | ROS Levels | Specific ROS | Refs. |
|---|---|---|---|---|---|---|
| Human cells | Human fibrosarcoma cancer cell line (HT1080) | Low level MF (0.2–2 μT, GMF as control, 45–60 μT) | 6/12/24 h | Increased * | H2O2 | [26] |
| Neuroblastoma cells (SH-SY5Y) | 2.2 mT | 24 h | Increased | [27] | ||
| 31.7–232.0 mT | •O2− | [28] | ||||
| Monocyte tumor cells (U937) | 6 mT | 2 h | H2O2 | [29] | ||
| Peripheral blood neutrophils | 60 mT (S pole) | 45 min | H2O2/HOCl | [30] | ||
| Diploid embryonic lung fibroblast cell (WI-38) | 230–250 mT | 18 h | H2O2 | [31] | ||
| Leukemia cells (THP-1) | 1.2 T | 24 h | [32] | |||
| Human-hamster hybrid A(L) cells, mitochondria-deficient rho(0) A(L) cells, and double-strand break (DSB) repair-deficient XRS-5 cells | 8.5 T | 3 h | [33] | |||
| Mouse cells | Embryonic stem (ES) cell (CGR8)-derived embryoid bodies and ES cell-derived Flk-1+ cardiovascular progenitor cells | 0.2–5 mT | 1 h/day, 10 days | [34] | ||
| Normal liver cell line (NCTC 1469) | 0.4 T | 1/24/48/72 h | [35] | |||
| Embryonic Stem Cells | 1/10 mT | 8 h/day, 17 days | [36] | |||
| Bovine cells | Bovine pulmonary artery endothelial cells (PAEC) | Low level MF (0.2–2 μT, GMF as control, 45–60 μT) | 8/24 h | Increased * | [26] | |
| Plant | Soybean seeds | 150–200 mT | 1 h | Increased | •O2−/•OH/H2O2 | [37] |
| Human cells | Peripheral blood neutrophils | 60 mT | 15 min | Decreased | H2O2/HOCl | [30] |
| Bronchial epithelial cells (A549) | 389 mT | 30 min | Decreased RWPE-induced ROS | H2O2 | [38] | |
| Mouse cells | Primary mouse skeletal muscle cell | <3 μT (GMF as control, ~50 μT) | 3 days | Decreased * | [39] | |
| Human cells | Pancreatic cancer cell line (AsPC-1) | Low level MF (0.2–2 μT, GMF as control, 45–0 μT) | 12/24 h | No change | H2O2 | [26] |
| Peripheral blood neutrophils | 60 mT | 30 min | H2O2/HOCl | [30] | ||
| 45 min (N pole) | ||||||
| Diploid embryonic lung fibroblast cell (WI-38) | 230–250 mT | 5 days | H2O2 | [31] | ||
| Lung fibroblasts (MRC-5) | 370 mT | 1 h/day, for 4 days | [40] | |||
| Bronchial epithelial cells (A549) | 389 mT | 30 min | [38] | |||
| Bacteria | E. coli and S. aureus | 100 mT | [41] |
| Cell Lines/Animal | ELF-EMF | Exposure Time | ROS Levels | Specific ROS | Refs. | |
|---|---|---|---|---|---|---|
| Frequency | Intensity | |||||
| Jurkat cells | 7.5 Hz | 0.4 T | 2 h | Increased | H2O2 | [48] |
| Leukemia cells (K562) | 50 Hz | 0.025–0.1 mT | 1 h | •O2− | [49] | |
| 1 mT | 3 h | [50] | ||||
| 24 h | [51] | |||||
| 5 mT | 1 h | [52] | ||||
| Neuroblastoma cells (SH-SY5Y) | 100 μT | 24 h (measured at 15 days) | H2O2 | [53] | ||
| 24 h (measured at 45 days) | [54] | |||||
| 1 mT | 24/48/72 h | •O2−/H2O2 | [55] | |||
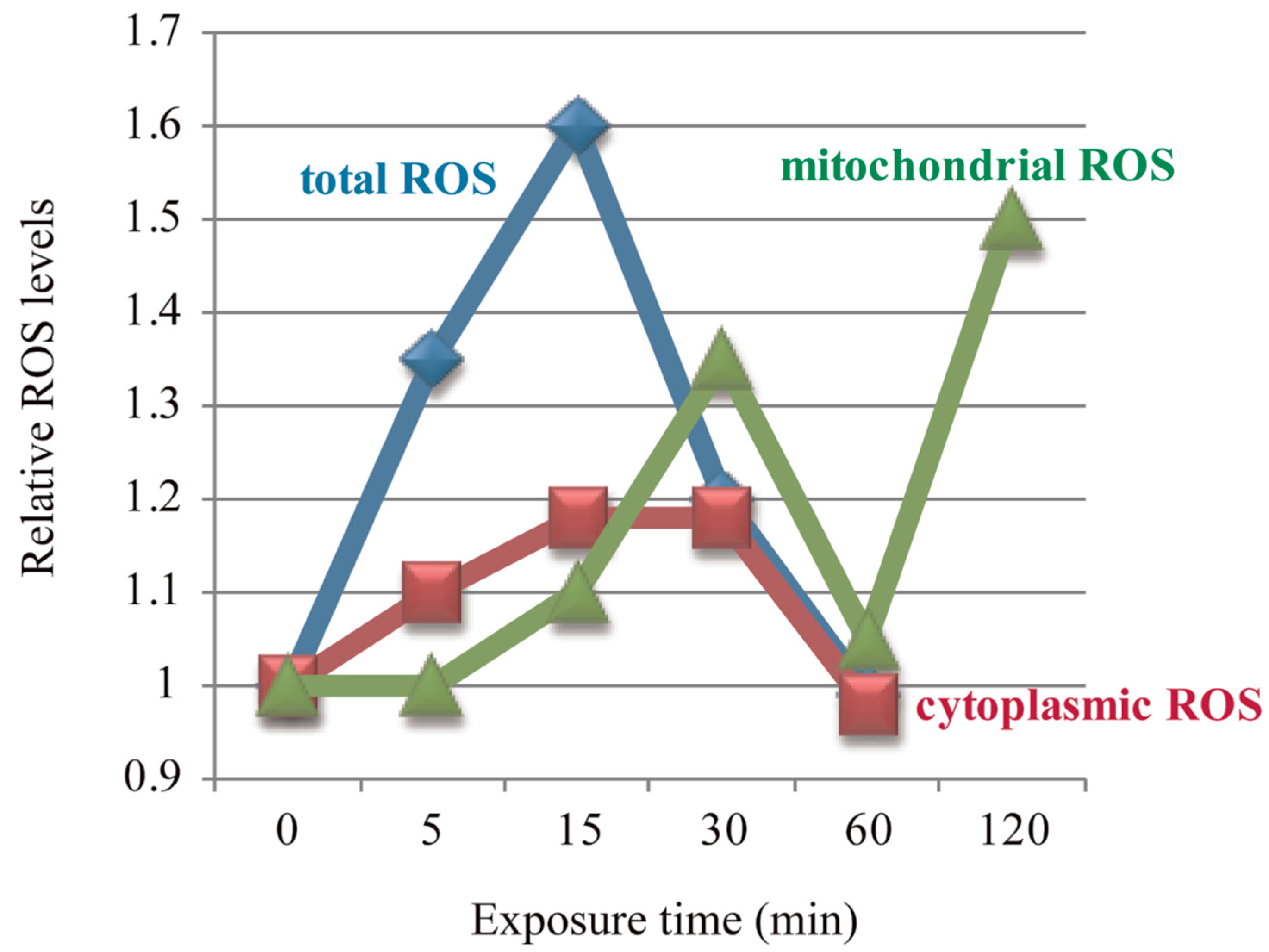
| Amniotic epithelial cells (FL) | 0.2 mT | 15 min | H2O2 | [56] | ||
| 0.4 mT | 5/15/30 min | •O2−/H2O2 | ||||
| 15/30 min | •O2− in mitochondria | |||||
| 30/120 min | [57] | |||||
| 5/15/30 min | H2O2 | [58] | ||||
| Keratinocyte cells (NCTC 2544) | 0.05/0.1 mT | 1/2 h | H2O2/HOCl | [59] | ||
| Umbilical cord blood monocytes | 1 mT | 5/15/30/45 min | [60] | |||
| Umbilical cord blood monocytes and acute monocytic leukaemia cell (Mono Mac 6) | 45 min | •O2− | [61] | |||
| Bone marrow mesenchymal stem cells (hBM-MSCs) | 90 min | H2O2 | [62] | |||
| 12 days | [63] | |||||
| Osteosarcoma cells (MG-63, MNNG-GOS C1) | 1/2/3 h | [64] | ||||
| Prostate carcinoma cells (DU145, PC3, and LNCaP) | 60 Hz | 6/24/48/72 h | [65] | |||
| Breast carcinoma cells (T47D) | 217 Hz | 0.1 mT | 72 h | [66] | ||
| Renal proximal tubular cells (HK-2) | 10 Hz | 1 mT | N/A | Decreased | H2O2 | [67] |
| Microglial cells (HMO6) | 50 Hz | 4 h | Decreased oxygen-glucose deprivation-induced ROS | [68] | ||
| Neuroblastoma cells (SK-N-BE(2)) | 75 Hz | 2 mT | 15 min/day, 3 days | Decreased H2O2-induced ROS | [69] | |
| Neuroblastoma cells (SH-SY5Y) | 1.5 ± 0.2 mT | 24/48 h | Decreased hypoxia-induced ROS | [70] | ||
| 75 ± 2 Hz | 2 ± 0.2 mT | 10 min, 4 times/week | Decreased H2O2-induced ROS | [71] | ||
| Keratinocytes and dermal fibroblasts | 100 Hz | <40 μT | 24 min twice daily, 30 days | Decreased | [72] | |
| Cell Lines/Animal | ELF-EMF | Exposure Time | ROS Levels | Specific ROS | Refs. | |
|---|---|---|---|---|---|---|
| Frequency | Intensity | |||||
| Jurkat cells | 7.5 Hz | 0.4 T | 1/3 h | No change | H2O2 | [48] |
| Renal proximal tubular cell | 10 Hz | 0.01/0.1 mT | N/A | [67] | ||
| Keratinocyte cells (NCTC 2544) | 50 Hz | 0.025/0.15/0.2 mT | 1 h | H2O2/HOCl | [59] | |
| 0.05/0.1 mT | 4 h | |||||
| Neuroblastoma cells (SH-SY5Y) | 100 μT | 24 h (measured at 8/15/30 days) | H2O2 | [53] | ||
| [54] | ||||||
| Amniotic epithelial cells (FL) | 0.1 mT | 15 min | [56] | |||
| 0.4 mT | 5 min | •O2− in mitochondria | ||||
| 60 min | •O2−/ H2O2/•O2− in mitochondria | |||||
| •O2− in mitochondria | [57] | |||||
| H2O2 | [58] | |||||
| Jurkat cells | 1 mT | 1 h (5 min on/10 min off) | [73] | |||
| Prostate carcinoma cells (DU145, PC3, and LNCaP) | 60 Hz | 1 mT | 3 h | [65] | ||
| Normal breast epithelial cells (MCF10A) | 4 h | [74] | ||||
| Lung fibroblast (IMR90) and cervical carcinoma (HeLa) cells | 7 mT | 30/60 min | [75] | |||
| Neuroblastoma cells (SK-N-BE(2)) | 75 Hz | 2 mT | 15 min/day, 3 d | No change in cells without H2O2 | [69] | |
| Neuroblastoma cells (SH-SY5Y) | 75 ± 2 Hz | 2 ± 0.2 mT | 10 min, 4 times/week | [71] | ||
| Renal proximal tubular cells (HK-2) | 50/100 Hz | 1 mT | N/A | No change | [67] | |
| Neutrophils | 180–195 Hz | 10/40/60 μT | N/A | H2O2/HOCl | [76] | |
| Breast carcinoma cells (T47D) | 100 Hz | 0.1 mT | 24/48/72 h | H2O2 | [66] | |
| 217 Hz | 24/48 h | |||||
| Species | Cell Lines/Animal | ELF-EMF | Exposure Time | ROS Levels | Specific ROS | Refs. | |
|---|---|---|---|---|---|---|---|
| Frequency | Intensity | ||||||
| Mouse cells | Primary mouse T cells from female C57BL/6 mice | 7.5 Hz | 0.4 T | 2 h | Increased | H2O2 | [48] |
| Undifferentiated C2C12 cells (myoblasts) and terminally differentiated myotubes | 50 Hz | 1 mT | 5/30 min | [77] | |||
| Squamous cell carcinoma cells (AT478) | 16 min | [78] | |||||
| Bone marrow-derived (MBM) macrophages | 45 min | H2O2/HOCl | [24] | ||||
| Embryonic fibroblasts | 2 mT | 2/6 h | H2O2 | [79] | |||
| Embryonic stem (ES) cell-derived embryoid bodies | 1/10 mT | 8 h/day, 7 days | [22] | ||||
| Rat cells | Undifferentiated pheochromocytoma-derived cells (PC12) | 1 mT | 30 min | [80] | |||
| Rat immortalized fibroblasts (Rat1) | 3/24 h | [81] | |||||
| Primary hippocampal neurons | 8 mT | 90 min | [3] | ||||
| Rat peritoneal neutrophils | 60 Hz | 0.1 mT | 5 days | [82] | |||
| Mouse | Hippocampus mitochondria of male ICR mice | 50 Hz | 8 mT | 4 h/day, 28 days | [83] | ||
| Rat | Male Wistar rats | 40 Hz | 7 mT | 60 min/day, 14 days | [84] | ||
| Hippocampus/cerebellum of male Wistar rats | 50 Hz | 50 μT | 90 days | [85] | |||
| Hippocampus/cerebellum/cortex of male Wistar rats | 100 μT | ||||||
| Mouse cells | Squamous cell carcinoma cells (AT478) | 50 Hz | 1 mT | 16 min | Decreased cisplatin-induced ROS | H2O2 | [78] |
| Mouse microglial cells (N9) | 75 Hz | 1.5 ± 0.2 mT | 24/48 h | Decreased hypoxia-induced ROS | [70] | ||
| Rat cells | Primary cardiomyocytes from neonatal Sprague-Dawley (SD) rat hearts | 15 Hz | 4.5 mT | 3 h | Decreased hypoxia/reoxygenation (H/R)-induced ROS | •O2− | [86] |
| Pheochromocytoma cells (PC12) | 75 Hz | 1.5 ± 0.2 mT | 24/48 h | Decreased hypoxia-induced ROS | H2O2 | [70] | |
| Mouse cells | Primary mouse T cells from female C57BL/6 mice | 7.5 Hz | 0.4 T | 1/3 h | No change | H2O2 | [48] |
| Undifferentiated C2C12 cells (myoblasts) and terminally differentiated myotubes | 50 Hz | 0.1 mT | 5/30 min | [77] | |||
| Bone marrow-derived (MBM) macrophages | 1 mT | 5/15/30 min | H2O2/HOCl | [24] | |||
| Embryonic fibroblasts | 2 mT | 0.5/12/24 h | H2O2 | [79] | |||
| Undifferentiated PC12 cells | 0.1 mT | 30 min | [80] | ||||
| Differentiated PC12 cells | 0.1/1 mT | ||||||
| Rat cells | Rat-cortical neurons (from SD rat embryos) | 7 d | [87] | ||||
| Naive/chemically stressed PC12 | 1 mT | 30 min/48 h | [88] | ||||
| Rat | Male Wistar rats | 40 Hz | 7 mT | 30 min/day, 14 days | [84] | ||
| Male SD rats | 30/60 min/day, 10 days | [89] | |||||
| Cortex of male Wistar rats | 50 Hz | 50 μT | 90 days | [85] | |||
| Species | Cell Lines/Organisms | RF-EMR | ROS Levels | Specific ROS | Refs. | |
|---|---|---|---|---|---|---|
| Frequency | Time | |||||
| Human cells | Ejaculated semen | 870 MHz | 60 min | Increased | H2O2 | [91] |
| Spermatozoa | 1.8 GHz | 16 h | •O2− | [92] | ||
| Neuroblastoma cells (SH-SY5Y) | 872 MHz | 1 h | Increased menadione-induced ROS | H2O2 | [93] | |
| Peripheral blood mononuclear cell | 900 MHz | 1/2/4/6/8 h | Increased | [94] | ||
| HEK293T-harbouring the firefly luciferase gene | 940 MHz | 5/15/30/45 min | [95] | |||
| Lens epithelial cells | 1.8 GHz (3/4 W/kg) | 24 h | [96] | |||
| Rat cells | Pulmonary arterial smooth muscle cells (rPASMC) | 7 MHz | 2/3 days | [97] | ||
| Primary neocortical astroglial cell | 900 MHz CW modulated in 50 Hz AM | 20 min | [98] | |||
| Male Wistar rat semen | 900 MHz | 2 h/day, 35 days | Total ROS | [99] | ||
| 10 GHz | 2 h/day, 45 days | [100] | ||||
| Rat | Serum of male Wistar rats | 900 MHz | [101] | |||
| Drosophila | Male/female drosophila bodies | 1.88–1.90 GHz | 6/24/96 h | H2O2 | [102] | |
| Ovaries of female drosophila | 0.5/1/6/24/96 h | |||||
| Rat cells | Pulmonary arterial smooth muscle cells (rPASMC) | 7 MHz | 3 days | Decreased | •O2− | [97] |
| Human cells | Neuroblastoma cells (SH-SY5Y) | 872 MHz | 1 h | No change | H2O2 | [93,103] |
| Primary human thyroid cells | 900/895 MHz | 3/16 h (900 MHz)/65 h (895 MHz) | Total ROS | [104] | ||
| Primary monocytes and lymphocytes | 1800 MHz (CW/intermittent) | 30/45 min | H2O2/HOCl | [105] | ||
| Lens epithelial cells | 1.8 GHz (1/2 W/kg) | 24 h | H2O2 | [96] | ||
| Mouse cells | Murine fibrosarcoma cells (L929) | 900 MHz (CW or GSM) | 10/30 min | [106] | ||
| Rat cells | Primary neocortical astroglial celll | 900 MHz CW modulated in 50 Hz AM | 5/10 min | [98] | ||
| 900 MHz CW | 5/10/20 min | |||||
| Lymphocytes (male albino Wistar rats) | 930 MHz | 5/15 min | [107] | |||
| Drosophila | Male/female drosophila bodies | 1.88–1.90 GHz | 0.5/1 h | [102] | ||
| C. elegans | Caenorhabditis elegans | DECT/WI-FI/GSM | Dependent on strains and devices | [108] | ||
| MF Types | Species | Cell Lines/Organisms | MF Exposure | Antioxidant Enzymes | Refs. | |
|---|---|---|---|---|---|---|
| Conditions | Time | |||||
| SMFs | Mouse | Cochlea tissue of C57BL/6 mice | 5 mT | 1/3/5/7/14 days (8 h first day, 2 h/day for the rest) | CAT and SOD activities significantly increased only after 3 days exposure, but not others | [44] |
| Plant | Soybean seeds | 150–200 mT | 1 h | SOD activity was reduced | [37] | |
| Broad bean (Vicia faba L.) | 15 mT | 8 h/day, 8 days | SOD increased, CAT decreased, indirectly suggest ROS accumulation | [111] | ||
| ELF-EMFs | Mouse cells | Preadipocyte cell (3T3-L1) | 180–195 Hz, 120 μT | 36 min/day, 2 days | SOD decreased, CAT increased, GSH-Px and GSSG-Rd with no change after 24 h; but SOD, CAT, and GSH-Px significantly decreased, GSSG-Rd with no change after 48 h | [112] |
| Mouse | Balb/c mouse brain | 60 Hz, 1.2 mT | 3 h | SOD increased | [113] | |
| RF-EMRs | Rat cells | Male Wistar rats sperm | 50 GHz | 2 h/day, 45 days | CAT significantly increased, SOD and GSH-Px significantly decreased | [114] |
| Rat | Female Wistar rats | 900 MHz | 1 h/day, 7 days | No change (SOD and GSH-Px decreased non-significantly) | [115] | |
| Male SD rats | 30 min/day, 3 months | No change (SOD, CAT and GSH-Px decreased marginally) | [116] | |||
| Brain of male Wistar rats | 2 h/day, 45 days | SOD and GSH-Px decreased, CAT increased | [101] | |||
| Rabbit | Male albino rabbits | 30 min/day, 7 days | Serum SOD activity increased | [117] | ||
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, H.; Zhang, X. Magnetic Fields and Reactive Oxygen Species. Int. J. Mol. Sci. 2017, 18, 2175. https://doi.org/10.3390/ijms18102175
Wang H, Zhang X. Magnetic Fields and Reactive Oxygen Species. International Journal of Molecular Sciences. 2017; 18(10):2175. https://doi.org/10.3390/ijms18102175
Chicago/Turabian StyleWang, Huizhen, and Xin Zhang. 2017. "Magnetic Fields and Reactive Oxygen Species" International Journal of Molecular Sciences 18, no. 10: 2175. https://doi.org/10.3390/ijms18102175
APA StyleWang, H., & Zhang, X. (2017). Magnetic Fields and Reactive Oxygen Species. International Journal of Molecular Sciences, 18(10), 2175. https://doi.org/10.3390/ijms18102175