The Roles of Matricellular Proteins in Oncogenic Virus-Induced Cancers and Their Potential Utilities as Therapeutic Targets
Abstract
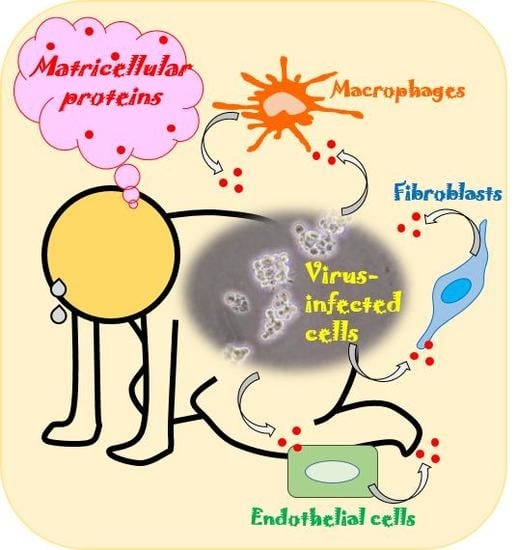
:1. Introduction
2. Current Knowledge Regarding the Roles of the Matricellular Proteins That Constitute the ECM in the Tumor Microenvironment
2.1. Osteopontin (OPN)
2.2. Tenascin-C (TNC)
2.3. Thrombospondin (TSP)
2.4. Periostin (POSTN)
2.5. Secreted Protein Acidic and Rich in Cysteine (SPARC)
3. The Involvement of Matricellular Proteins in the Tumorigenesis of Oncogenic Virus-Induced Cancers and Their Potential Utilities as Therapeutic Targets
3.1. HTLV-I-Induced ATL
3.2. HBV-Related HCC
3.3. HCV-Related HCC
3.4. HPV-Induced Cervical Cancer
3.5. EBV-Related Nasopharyngeal Carcinoma
3.6. KSHV-Related KS and PEL
3.7. MCPyV-Induced MCC
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
Abbreviations
| a.a. | Amino acid |
| Akt | Protein kinase B |
| ATL | Adult T-cell leukemia |
| EBER | Epstein–Barr virus-encoded small RNA |
| EBV | Epstein–Barr virus |
| ECM | Extracellular matrix |
| ELISA | Enzyme-linked immunosorbent assay |
| EMT | Epithelial-mesenchymal transition |
| ERK | Extracellular signal-regulated kinase |
| HBV | Hepatitis B virus |
| HBx | Hepatitis B virus x protein |
| HCC | Hepatocellular carcinoma |
| HCV | Hepatitis C virus |
| HHV | Human herpesvirus |
| HPV | Human papillomavirus |
| HTLV-I | Human T-cell leukemia virus type I |
| IHC | Immunohistochemical |
| KS | Kaposi’s sarcoma |
| KSHV | Kaposi’s sarcoma-associated herpesvirus |
| LMP1 | Latent membrane protein 1 |
| LOX | Lipoxygenase |
| mAb | Monoclonal antibody |
| MCPyV | Merkel cell polyomavirus |
| MCC | Merkel cell carcinoma |
| MEK | Mitogen-activated protein kinase kinase |
| miRNA | MicroRNA |
| MMP | Matrix metalloproteinase |
| NOG | NOD/Shi- scid,IL-2Rgnull |
| ODN | Oligodeoxynucleotide |
| OPN | Osteopontin |
| PEL | Primary effusion lymphoma |
| PI3K | Phosphoinositide 3-kinase |
| POSTN | Periostin |
| SPARC | Secreted protein acidic and rich in cysteine |
| sT | Small T |
| TGF-β | Transforming growth factor-beta |
| TNC | Tenascin-C |
| TSP | Thrombospondin |
| uPA | Urokinase-type plasminogen activator |
| VEGF | Vascular endothelial growth factor |
References
- Uede, T. Osteopontin, intrinsic tissue regulator of intractable inflammatory diseases. Pathol. Int. 2011, 61, 265–280. [Google Scholar] [CrossRef] [PubMed]
- Pascapurnama, D.N.; Labayo, H.K.; Dapat, I.; Nagarajegowda, D.D.; Zhao, J.; Zhang, J.; Yamada, O.; Kikuchi, H.; Egawa, S.; Oshima, Y.; et al. Induction of Osteopontin by Dengue Virus-3 Infection in THP-1 Cells: Inhibition of the Synthesis by Brefelamide and Its Derivative. Front. Microbiol. 2017, 8, 521. [Google Scholar] [CrossRef] [PubMed]
- Schäfer, G.; Blumenthal, M.J.; Katz, A.A. Interaction of human tumor viruses with host cell surface receptors and cell entry. Viruses 2015, 7, 2592–2617. [Google Scholar] [CrossRef] [PubMed]
- Hao, C.; Cui, Y.; Owen, S.; Li, W.; Cheng, S.; Jiang, W.G. Human osteopontin: Potential clinical applications in cancer (Review). Int. J. Mol. Med. 2017, 39, 1327–1337. [Google Scholar] [CrossRef] [PubMed]
- Senger, D.; Wirth, D.F.; Hynes, R.O. Transformed mammalian cells secrete specific proteins and phosphoproteins. Cell 1979, 16, 885–893. [Google Scholar] [CrossRef]
- Briones-Orta, M.A.; Avendaño-Vázquez, S.E.; Aparicio-Bautista, D.I.; Coombes, J.D.; Weber, G.F.; Syn, W.K. Osteopontin splice variants and polymorphisms in cancer progression and prognosis. Biochim. Biophys. Acta 2017, 1868, 93–108. [Google Scholar] [CrossRef] [PubMed]
- Raab-Westphal, S.; Marshall, J.F.; Goodman, S.L. Integrins as Therapeutic Tartes: Success and Cancers. Cancers 2017, 9, 110. [Google Scholar] [CrossRef] [PubMed]
- Senbanjo, L.T.; Chellaiah, M.A. CD44: A Multifunctional Cell Surface Adhesion Receptor Is a Regulator of Progression and Metastasis of Cancer Cells. Front. Cell Dev. Biol. 2017, 5, 18. [Google Scholar] [CrossRef] [PubMed]
- Wei, R.; Wong, J.P.C.; Kwok, H.F. Osteopontin—A promising biomarker for cancer therapy. J. Cancer 2017, 8, 2173–2183. [Google Scholar] [CrossRef] [PubMed]
- Cappellano, G.; Orilieri, E.; Woldetsadik, A.D.; Boggio, E.; Soluri, M.F.; Comi, C.; Sblattero, D.; Chiocchetti, A.; Dianzani, U. Anti-cytokine autoantibodies in autoimmune diseases. Am. J. Clin. Exp. Immunol. 2012, 1, 136–146. [Google Scholar] [PubMed]
- Ying, X.; Zhao, Y.; Wang, J.L.; Zhou, X.; Zhao, J.; He, C.C.; Guo, X.J.; Jin, G.H.; Wang, L.J.; Zhu, Q.; et al. Serum anti-osteopontin autoantibody as a novel diagnostic and prognostic biomarker in patients with hepatocellular carcinoma. Oncol. Rep. 2014, 32, 1550–1556. [Google Scholar] [CrossRef] [PubMed]
- Dai, J.; Li, B.; Shi, J.; Peng, L.; Zhang, D.; Qian, W.; Hou, S.; Zhao, L.; Gao, J.; Cao, Z.; et al. A humanized anti-osteopontin antibody inhibits breast cancer growth and metastasis in vivo. Cancer Immunol. Immunother. 2010, 59, 355–366. [Google Scholar] [CrossRef] [PubMed]
- Shojaei, F.; Scott, N.; Kang, X.; Lappin, P.B.; Fitzgerald, A.A.; Karlicek, S.; Simmons, B.H.; Wu, A.; Lee, J.H.; Bergqvist, S.; et al. Osteopontin induces growth of metastatic tumors in a preclinical model of non-small lung cancer. J. Exp. Clin. Cancer Res. 2012, 31, 26. [Google Scholar] [CrossRef] [PubMed]
- Zhang, B.; Dai, J.; Wang, H.; Wei, H.; Zhao, J.; Guo, Y.; Fan, K. Anti-osteopontin monoclonal antibody prevents ovariectomy-induced osteoporosis in mice by promotion of osteoclast apoptosis. Biochem. Biophys. Res. Commun. 2014, 452, 795–800. [Google Scholar] [CrossRef] [PubMed]
- Cen, C.; Aziz, M.; Yang, W.L.; Nicastro, J.M.; Coppa, G.F.; Wang, P. Osteopontin Blockade Attenuates Renal Injury after Ischemia Reperfusion by Inhibiting NK Cell Infiltration. Shock 2017, 47, 52–60. [Google Scholar] [CrossRef] [PubMed]
- Chiovaro, F.; Chiquet-Ehrismann, R.; Chiquet, M. Transcriptional regulation of tenascin genes. Cell Adh. Migr. 2015, 9, 34–47. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Midwood, K.S.; Chiquet, M.; Tucker, R.P.; Orend, G. Tenascin-C at a glance. J. Cell Sci. 2016, 129, 4321–4327. [Google Scholar] [CrossRef] [PubMed]
- Tucker, R.P.; Chiquet-Ehrismann, R. Tenascin-C: Its functions as an integrin ligand. Int. J. Biochem. Cell Biol. 2015, 65, 165–168. [Google Scholar] [CrossRef] [PubMed]
- Chiquet-Ehrismann, R.; Tucker, R.P. Tenascins and the importance of adhesion modulation. Cold Spring Harb. Perspect. Biol. 2011, 3, a004960. [Google Scholar] [CrossRef] [PubMed]
- Imanaka-Yoshida, K.; Aoki, H. Tenascin-C and mechanotrasnduction in the development and diseases of cardiovascular system. Front. Physiol. 2014, 5, 283. [Google Scholar] [CrossRef] [PubMed]
- Yoshida, T.; Akatsuka, T.; Imanaka-Yoshida, K. Tenascin-C and integrins in cancer. Cell Adh. Migr. 2015, 9, 96–104. [Google Scholar] [CrossRef] [PubMed]
- Schumann, C.; Lepper, P.M.; Frank, H.; Schneiderbauer, R.; Wibmer, T.; Kropf, C.; Stoiber, K.M.; Rüdiger, S.; Kruska, L.; Krahn, T.; et al. Circulating biomarkers of tissue remodelling in pulmonary hypertension. Biomarkers 2010, 15, 523–532. [Google Scholar] [CrossRef] [PubMed]
- Page, T.H.; Charles, P.J.; Piccinini, A.M.; Nicolaidou, V.; Taylor, P.C.; Midwood, K.S. Raised circulating tenascin-C in rheumatoid arthritis. Arthritis Res. Ther. 2012, 14, R260. [Google Scholar] [CrossRef] [PubMed]
- Balasenthil, S.; Huang, Y.; Liu, S.; Marsh, T.; Chen, J.; Stass, S.A.; KuKuruga, D.; Brand, R.; Chen, N.; Frazier, M.L.; et al. A Plasma Biomarker Panel to Identify Surgically Resectable Early-Stage Pancreatic Cancer. J. Natl. Cancer Inst. 2017, 109. [Google Scholar] [CrossRef] [PubMed]
- Mock, A.; Warta, R.; Geisenberger, C.; Bischoff, R.; Schulte, A.; Lamszus, K.; Stadler, V.; Felgenhauer, T.; Schichor, C.; Schwartz, C.; et al. Printed peptide arrays identify prognostic TNC serumantibodies in glioblastoma patients. Oncotarget 2015, 6, 13579–13590. [Google Scholar] [CrossRef] [PubMed]
- Odaka, K.; Uehara, T.; Arano, Y.; Adachi, S.; Tadokoro, H.; Yoshida, K.; Hasegawa, H.; Imanaka-Yoshida, K.; Yoshida, T.; Hiroe, M.; et al. Noninvasive detection of cardiac repair after acute myocardial infarction in rats by 111In Fab fragment of monoclonal antibody specific for tenascin-C. Int. Heart J. 2008, 49, 481–492. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, N.; Odaka, K.; Uehara, T.; Imanaka-Yoshida, K.; Kato, Y.; Oyama, H.; Tadokoro, H.; Akizawa, H.; Tanada, S.; Hiroe, M.; et al. Toward in vivo imaging of heart disease using a radiolabeled single-chain Fv fragment targeting tenascin-C. Anal. Chem. 2011, 83, 9123–9130. [Google Scholar] [CrossRef] [PubMed]
- Kirk, J.A.; Cingolani, O.H. Thrombospondins in the transition from myocardial infarction to heart failure. J. Mol. Cell. Cardiol. 2016, 90, 102–110. [Google Scholar] [CrossRef] [PubMed]
- Lawler, J.W.; Slayter, H.S.; Coligan, J.E. Isolation and characterization of a high molecular weight glycoprotein from human blood platelets. J. Biol. Chem. 1978, 253, 8609–8616. [Google Scholar] [PubMed]
- Chen, H.; Herndon, M.E.; Lawler, J. The cell biology of thrombospondin-1. Matrix Biol. 2000, 19, 597–614. [Google Scholar] [CrossRef]
- Lawler, P.R.; Lawler, J. Molecular basis for the regulation of angiogenesis by thrombospondin-1 and -2. Cold Spring Harb. Perspect. Med. 2012, 2, a006627. [Google Scholar] [CrossRef] [PubMed]
- Dudek, A.Z.; Mahaseth, H. Circulating angiogenic cytokines in patients with advanced non-small cell lung cancer: Correlation with treatment response and survival. Cancer Investig. 2005, 23, 193–200. [Google Scholar] [CrossRef]
- Cymbaluk-Płoska, A.; Chudecka-Głaz, A.; Pius-Sadowska, E.; Machaliński, B.; Menkiszak, J. Thrombospondin-I concentrations behavior in plasma of patients with ovarian cancer. Cancer Biomark. 2017, 20, 31–39. [Google Scholar] [CrossRef] [PubMed]
- Kashihara, H.; Shimada, M.; Yoshikawa, K.; Higashijima, J.; Tokunaga, T.; Nishi, M.; Takasu, C.; Ishikawa, D. Correlation Between Thrombospondin-1 Expression in Non-cancer Tissue and Gastric Carcinogenesis. Anticancer Res. 2017, 37, 3547–3552. [Google Scholar] [PubMed]
- Jeanne, A.; Schneider, C.; Martiny, L.; Dedieu, S. Original insights on thrombospondin-1-related antireceptor strategies in cancer. Front. Pharmacol. 2015, 6, 252. [Google Scholar] [CrossRef] [PubMed]
- Coronella, J.; Li, L.; Johnson, K.; Pirie-Shepherd, S.; Roxas, G.; Levin, N. Selective activity against proliferating tumor endothelial cells by CVX-22, a thrombospondin-1 mimetic CovX-Body. Anticancer Res. 2009, 29, 2243–2252. [Google Scholar] [PubMed]
- Li, L.; Leedom, T.A.; Do, J.; Huang, H.; Lai, J.; Johnson, K.; Osothprarop, T.F.; Rizzo, J.D.; Doppalapudi, V.R.; Bradshaw, C.W.; et al. Antitumor efficacy of a thrombospondin 1 mimetic CovX-body. Transl. Oncol. 2011, 4, 249–257. [Google Scholar] [CrossRef] [PubMed]
- Takeshita, S.; Kikuno, R.; Tezuka, K.; Amann, E. Osteoblast-specific factor 2: Cloning of a putative bone adhesion protein with homology with the insect protein fasciclin I. Biochem. J. 1993, 294, 271–278. [Google Scholar] [CrossRef] [PubMed]
- Idolazzi, L.; Ridolo, E.; Fassio, A.; Gatti, D.; Montagni, M.; Caminati, M.; Martignago, I.; Incorvaia, C.; Senna, G. Periostin: The bone and beyond. Eur. J. Intern. Med. 2017, 38, 12–16. [Google Scholar] [CrossRef] [PubMed]
- Nuzzo, P.V.; Buzzatti, G.; Ricci, F.; Rubagotti, A.; Argellati, F.; Zinoli, L.; Boccardo, F. Periostin: A novel prognostic and therapeutic target for genitourinary cancer? Clin. Genitourin. Cancer 2014, 12, 301–311. [Google Scholar] [CrossRef] [PubMed]
- Gillan, L.; Matei, D.; Fishman, D.A.; Gerbin, C.S.; Karlan, B.Y.; Chang, D.D. Periostin secreted by epithelial ovarian carcinoma is a ligand for αvβ3 and αvβ5 integrins and promotes cell motility. Cancer Res. 2002, 62, 5358–5364. [Google Scholar] [PubMed]
- Baril, P.; Gangeswaran, R.; Mahon, P.C.; Caulee, K.; Kocher, H.M.; Harada, T.; Zhu, M.; Kalthoff, H.; Crnogorac-Jurcevic, T.; Lemoine, N.R. Periostin promotes invasiveness and resistance of pancreatic cancer cells to hypoxia-induced cell death: Role of the β4 integrin and the PI3k pathway. Oncogene 2007, 26, 2082–2094. [Google Scholar] [CrossRef] [PubMed]
- Moniuszko, T.; Wincewicz, A.; Koda, M.; Domysławska, I.; Sulkowski, S. Role of periostin in esophageal, gastric and colon cancer. Oncol. Lett. 2016, 12, 783–787. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Liu, B.A. Enhanced proliferation, invasion, and epithelial-mesenchymal transition of nicotine-promoted gastric cancer by periostin. World J. Gastroenterol. 2011, 17, 2674–2680. [Google Scholar] [CrossRef] [PubMed]
- Nuzzo, P.V.; Rubagotti, A.; Argellati, F.; Di Meglio, A.; Zanardi, E.; Zinoli, L.; Comite, P.; Mussap, M.; Boccardo, F. Prognostic Value of Preoperative Serum Levels of Periostin (PN) in Early Breast Cancer (BCa). Int. J. Mol. Sci. 2015, 16, 17181–17192. [Google Scholar] [CrossRef] [PubMed]
- Thuwajit, C.; Thuwajit, P.; Jamjantra, P.; Pairojkul, C.; Wongkham, S.; Bhudhisawasdi, V.; Ono, J.; Ohta, S.; Fujimoto, K.; Izuhara, K. Clustering of patients with intrahepatic cholangiocarcinoma based on serum periostin may be predictive of prognosis. Oncol. Lett. 2017, 14, 623–634. [Google Scholar] [CrossRef] [PubMed]
- Xu, C.H.; Wang, W.; Lin, Y.; Qian, L.H.; Zhang, X.W.; Wang, Q.B.; Yu, L.K. Diagnostic and prognostic value of serum periostin in patients with non-small cell lung cancer. Oncotarget 2017, 8, 18746–18753. [Google Scholar] [CrossRef] [PubMed]
- Field, S.; Uyttenhove, C.; Stroobant, V.; Cheou, P.; Donckers, D.; Coutelier, J.P.; Simpson, P.T.; Cummings, M.C.; Saunus, J.M.; Reid, L.E.; et al. Novel highly specific anti-periostin antibodies uncover the functional importance of the fascilin 1-1 domain and highlight preferential expression of periostin in aggressive breast cancer. Int. J. Cancer 2016, 138, 1959–1970. [Google Scholar] [CrossRef] [PubMed]
- Kyutoku, M.; Taniyama, Y.; Katsuragi, N.; Shimizu, H.; Kunugiza, Y.; Iekushi, K.; Koibuchi, N.; Sanada, F.; Oshita, Y.; Morishita, R. Role of periostin in cancer progression and metastasis: Inhibition of breast cancer progression and metastasis by anti-periostin antibody in a murine model. Int. J. Mol. Med. 2011, 28, 181–186. [Google Scholar] [CrossRef] [PubMed]
- Termine, J.D.; Kleinman, H.K.; Whitson, S.W.; Conn, K.M.; McGarvey, M.L.; Martin, G.R. Osteonectin, a bone-specific protein linking mineral to collagen. Cell 1981, 26, 99–105. [Google Scholar] [CrossRef]
- Said, N. Roles of SPARC in urothelial carcinogenesis, progression and metastasis. Oncotarget 2016, 7, 67574–67585. [Google Scholar] [CrossRef] [PubMed]
- Trombetta-Esilva, J.; Bradshaw, A.D. The Function of SPARC as a Mediator of Fibrosis. Open Rheumatol. J. 2012, 6, 146–155. [Google Scholar] [CrossRef] [PubMed]
- Said, N.; Frierson, H.F.; Sanchez-Carbayo, M.; Brekken, R.A.; Theodorescu, D. Ross of SPARC in bladder cancer enhances carcinogenesis and progression. J. Clin. Investig. 2013, 123, 751–766. [Google Scholar] [CrossRef] [PubMed]
- Shi, D.; Jiang, K.; Fu, Y.; Fang, R.; Liu, X.I.; Chen, J. Overexpression of SPARC correlates with poor prognosis in patients with cervical carcinoma and regulates cancer cell epithelial-mesenchymal transition. Oncol. Lett. 2016, 11, 3251–3258. [Google Scholar] [CrossRef] [PubMed]
- Vaz, J.; Ansari, D.; Sasor, A.; Andersson, R. SPARC: A Potential Prognostic and Therapeutic Target in Pancreatic Cancer. Pancreas 2015, 44, 1024–1035. [Google Scholar] [CrossRef] [PubMed]
- Mateo, F.; Meca-Cortés, O.; Celià-Terrassa, T.; Fernández, Y.; Abasolo, I.; Sánchez-Cid, L.; Bermudo, R.; Sagasta, A.; Rodríguez-Carunchio, L.; Pons, M.; et al. SPARC mediates metastatic cooperation between CSC and non-CSC prostate cancer cell subpopulations. Mol. Cancer 2014, 13, 237. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Grant, J.L.; Fishbein, M.C.; Hong, L.S.; Krysan, K.; Minna, J.D.; Shay, J.W.; Walser, T.C.; Dubinett, S.M. A novel molecular pathway for Snail-dependent, SPARC-mediated invasion in non-small cell lung cancer pathogenesis. Cancer Prev. Res. 2014, 7, 150–160. [Google Scholar] [CrossRef] [PubMed]
- Chiodoni, C.; Sangaletti, S.; Colombo, M.P. Matricellular proteins tune myeloid-derived suppressor Cell recruitment and function in breast cancer. J. Leukoc. Biol. 2017, 102, 287–292. [Google Scholar] [CrossRef] [PubMed]
- Maeda, N.; Fan, H.; Yoshikai, Y. Oncogenesis by retroviruses: Old and new paradigms. Rev. Med. Virol. 2008, 18, 387–405. [Google Scholar] [CrossRef] [PubMed]
- Katsuya, H.; Ishitsuka, K. Treatment advances and prognosis for patients with adult T-cell leukemia-lymphoma. J. Clin. Exp. Hematop. 2017, 17008. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, T. Adult T-cell leukemia: Molecular basis for clonal expansion and transformation of HTLV-1-infected T cells. Blood 2017, 129, 1071–1081. [Google Scholar] [CrossRef] [PubMed]
- Kogure, Y.; Kataoka, K. Genetic alterlations in adult T-cell leukemia/lymphoma. Cancer Sci. 2017. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Yamada, O.; Matsushita, Y.; Chagan-Yasutan, H.; Hattori, T. Transactivation of human osteopontin promoter by human T-cell leukemia virus type 1-encoded Tax protein. Leuk. Res. 2010, 34, 763–768. [Google Scholar] [CrossRef] [PubMed]
- Maeda, N.; Ohashi, T.; Chagan-Yasutan, H.; Hattori, T.; Takahashi, Y.; Harigae, H.; Hasegawa, H.; Yamada, Y.; Fujii, M.; Maenaka, K.; et al. Osteopontin-integrin interaction as a novel molecular target for antibody-mediated immunotherapy in adult T-cell leukemia. Retrovirology 2015, 12, 99. [Google Scholar] [CrossRef] [PubMed]
- Chagan-Yasutan, H.; Tsukasaki, K.; Takahashi, Y.; Oguma, S.; Harigae, H.; Ishii, N.; Zhang, J.; Fukumoto, M.; Hattori, T. Involvement of osteopontin and its signaling molecule CD44 in clinicopathological features of adult T cell leukemia. Leuk. Res. 2011, 35, 1484–1490. [Google Scholar] [CrossRef] [PubMed]
- Dai, J.; Peng, L.; Fan, K.; Wang, H.; Wei, R.; Ji, G.; Cai, J.; Lu, B.; Li, B.; Zhang, D.; et al. Osteopontin induces angiogenesis through activation of PI3K/AKT and ERK1/2 in endothelial cells. Oncogene 2009, 28, 3412–3422. [Google Scholar] [CrossRef] [PubMed]
- Chakraborty, G.; Jain, S.; Kundu, G.C. Osteopontin promotes vascular endothelial growth factor-dependent breast tumor growth and angiogenesis via autocrine and paracrine mechanisms. Cancer Res. 2008, 68, 152–161. [Google Scholar] [CrossRef] [PubMed]
- El-Sabban, M.E.; Merhi, R.A.; Haidar, H.A.; Arnulf, B.; Khoury, H.; Basbous, J.; Nijmeh, J.; de Thé, H.; Hermine, O.; Bazarbachi, A. Human T-cell lymphotropic virus type 1-transformed cells induce angiogenesis and establish functional gap junctions with endothelial cells. Blood 2002, 99, 3383–3389. [Google Scholar] [CrossRef] [PubMed]
- Bazarbachi, A.; Abou Merhi, R.; Gessain, A.; Talhouk, R.; El-Khoury, H.; Nasr, R.; Gout, O.; Sulahian, R.; Homaidan, F.; de Thé, H.; et al. Human T-cell lymphotropic virus type I-infected cells extravasate through the endothelial barrier by a local angiogenesis-like mechanism. Cancer Res. 2004, 64, 2039–2046. [Google Scholar] [CrossRef] [PubMed]
- Watters, K.M.; Dean, J.; Gautier, V.; Hall, W.W.; Sheehy, N. Tax 1-independent induction of vascular endothelial growth factor in adult T-cell leukemia caused by human T-cell leukemia virus type 1. J. Virol. 2010, 84, 5222–5228. [Google Scholar] [CrossRef] [PubMed]
- Ohyashiki, J.H.; Hamamura, R.; Kobayashi, C.; Zhang, Y.; Ohyashiki, K. A network biology approach evaluating the anticancer effects of bortezomib identifies SPARC as a therapeutic target in adult T-cell leukemia cells. Adv. Appl. Bioinform. Chem. 2008, 1, 85–98. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Koh, S.S.; Lee, C.G. Hepatitis B Virus X Protein and Hepatocarcinogenesis. Int. J. Mol. Sci. 2016, 17, 940. [Google Scholar] [CrossRef] [PubMed]
- Xu, W.; Yu, J.; Wong, V.W. Mechanism and prediction of HCC development in HBV infection. Best Pract. Res. Clin. Gastroenterol. 2017, 31, 291–298. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Ye, L.H.; Zhang, X.D. A mutant of hepatitis B virus X protein (HBxΔ127) enhances hepatoma cell migration via osteopontin involving 5-lipoxygenase. Acta Pharmacol. Sin. 2010, 31, 593–600. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; You, X.; Wang, Q.; Zhang, T.; Du, Y.; Lv, N.; Zhang, Z.; Zhang, S.; Shan, C.; Ye, L.; et al. Hepatitis B virus X protein drives multiple cross-talk cascade loops involving NF-κB, 5-LOX, OPN and Capn4 to promote cell migration. PLoS ONE 2012, 7, e31458. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.; Li, T.; Wang, Y.; Pan, Y.; Ning, H.; Hui, X.; Xie, H.; Wang, J.; Han, Y.; Liu, Z.; et al. Elevated plasma osteopontin level is predictive of cirrhosis in patients with hepatitis B infection. Int. J. Clin. Pract. 2008, 62, 1056–1062. [Google Scholar] [CrossRef] [PubMed]
- Da Costa, A.N.; Plymoth, A.; Santos-Silva, D.; Ortiz-Cuaran, S.; Camey, S.; Guilloreau, P.; Sangrajrang, S.; Khuhaprema, T.; Mendy, M.; Lesi, O.A.; et al. Osteopontin and latent-TGF β binding-protein 2 as potential diagnostic markers for HBV-related hepatocellular carcinoma. Int. J. Cancer 2015, 136, 172–181. [Google Scholar] [CrossRef] [PubMed]
- Xie, H.; Song, J.; Du, R.; Liu, K.; Wang, J.; Tang, H.; Bai, F.; Liang, J.; Lin, T.; Liu, J.; et al. Prognostic significance of osteopontin in hepatitis B virus-related hepatocellular carcinoma. Dig. Liver Dis. 2007, 39, 167–172. [Google Scholar] [CrossRef] [PubMed]
- Cui, G.; Chen, J.; He, J.; Lu, C.; Wei, Y.; Wang, L.; Xu, X.; Li, L.; Uede, T.; Diao, H. Osteopontin promotes dendritic cell maturation and function in response to HBV antigens. Drug Des. Dev. Ther. 2015, 9, 3003–3016. [Google Scholar] [CrossRef]
- Zhang, B.; Wang, Z.; Deng, B.; Wu, X.; Liu, J.; Feng, X. Identification of Enolase 1 and Thrombospondin-1 as serum biomarkers in HBV hepatic fibrosis by proteomics. Proteome Sci. 2013, 11, 30. [Google Scholar] [CrossRef] [PubMed]
- Bastos, J.C.; Padilla, M.A.; Caserta, L.C.; Miotto, N.; Vigami, A.G.; Arns, C.W. Hepatitis C virus: Promising discoveries and new treatments. World J. Gastroenterol. 2016, 22, 6393–6401. [Google Scholar] [CrossRef] [PubMed]
- Vescovo, T.; Refolo, G.; Vitagliano, G.; Fimia, G.M.; Piacentini, M. Molecular mechanisms of hepatitis C virus-induced hepatocellular carcinoma. Clin. Microbiol. Infect. 2016, 22, 853–861. [Google Scholar] [CrossRef] [PubMed]
- Presser, L.D.; Haskett, A.; Waris, G. Hepatitis C virus-induced furin and thrombospondin-1 activate TGF-β1: Role of TGF-β1 in HCV replication. Virology 2011, 412, 284–296. [Google Scholar] [CrossRef] [PubMed]
- Benzoubir, N.; Lejamtel, C.; Battaglia, S.; Testoni, B.; Benassi, B.; Gondeau, C.; Perrin-Cocon, L.; Desterke, C.; Thiers, V.; Samuel, D.; et al. HCV core-mediated activation of latent TGF-β via thrombospondin drives the crosstalk between hepatocytes and stromal environment. J. Hepatol. 2013, 59, 1160–1168. [Google Scholar] [CrossRef] [PubMed]
- Bose, S.K.; Meyer, K.; Di Bisceglie, A.M.; Ray, R.B.; Ray, R. Hepatitis C virus induces epithelial-mesenchymal transition in primary human hepatocytes. J. Virol. 2012, 86, 13621–13628. [Google Scholar] [CrossRef] [PubMed]
- Kwon, Y.C.; Bose, S.K.; Steele, R.; Meyer, K.; Di Bisceglie, A.M.; Ray, R.B.; Ray, R. Promotion of Cancer Stem-Like Cell Properties in Hepatitis C Virus-Infected Hepatocytes. J. Virol. 2015, 89, 11549–11556. [Google Scholar] [CrossRef] [PubMed]
- Sasaki, R.; Devhare, P.; Ray, R.B.; Ray, R. Hepatitis C virus induced tumor initiating cancer stem-like cells activate stromal fibroblasts in xenograft tumor model. Hepatology 2017. [Google Scholar] [CrossRef] [PubMed]
- Huang, W.; Zhu, G.; Huang, M.; Lou, G.; Liu, Y.; Wang, S. Plasma osteopontin concentration correlates with the severity of hepatic fibrosis and inflammation in HCV-infected subjects. Clin. Chim. Acta 2010, 411, 675–678. [Google Scholar] [CrossRef] [PubMed]
- Abu El Makarem, M.A.; Abdel-Aleem, A.; Ali, A.; Saber, R.; Shatat, M.; Rahem, D.A.; Sayed, D. Diagnostic significance of plasma osteopontin in hepatitis C virus-related hepatocellular carcinoma. Ann. Hepatol. 2011, 10, 296–305. [Google Scholar] [PubMed]
- Matsue, Y.; Tsutsumi, M.; Hayashi, N.; Saito, T.; Tsuchishima, M.; Toshikuni, N.; Arisawa, T.; George, J. Serum osteopontin predicts degree of hepatic fibrosis and serves as a biomarker in patients with hepatitis C virus infection. PLoS ONE 2015, 10, e0118744. [Google Scholar] [CrossRef] [PubMed]
- Cabiati, M.; Gaggini, M.; Cesare, M.M.; Caselli, C.; De Simone, P.; Filipponi, F.; Basta, G.; Gastaldelli, A.; Del Ry, S. Osteopontin in hepatocellular carcinoma: A possible biomarker for diagnosis and follow-up. Cytokine 2017, 99, 59–65. [Google Scholar] [CrossRef] [PubMed]
- Chen, R.X.; Xia, Y.H.; Xue, T.C.; Zhang, H.; Ye, S.L. Down-regulation of osteopontin inhibits metastasis of hepatocellular carcinoma cells via a mechanism involving MMP-2 and uPA. Oncol. Rep. 2011, 25, 803–808. [Google Scholar] [CrossRef] [PubMed]
- Iqbal, J.; McRae, S.; Banaudha, K.; Mai, T.; Waris, G. Mechanism of hepatitis C virus (HCV)-induced osteopontin and its role in epithelial to mesenchymal transition of hepatocytes. J. Biol. Chem. 2013, 288, 36994–37009. [Google Scholar] [CrossRef] [PubMed]
- Shanmugasundaram, S.; You, J. Targeting Persistent Human Papillomavirus Infection. Viruses 2017, 9, 229. [Google Scholar] [CrossRef]
- Senapati, R.; Senapati, N.N.; Dwibedi, B. Molecular mechanisms of HPV mediated neoplastic progression. Infect. Agent. Cancer 2016, 11, 59. [Google Scholar] [CrossRef] [PubMed]
- Bequet-Romero, M.; López-Ocejo, O. Angiogenesis modulators expression in culture cell lines positives for HPV-16 oncoproteins. Biochem. Biophys. Res. Commun. 2000, 277, 55–61. [Google Scholar] [CrossRef] [PubMed]
- Toussaint-Smith, E.; Donner, D.B.; Roman, A. Expression of human papillomavirus type 16 E6 and E7 oncoproteins in primary foreskin keratinocytes is sufficient to alter the expression of angiogenic factors. Oncogene 2004, 23, 2988–2995. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.; Li, F.; Mead, L.; White, H.; Walker, J.; Ingram, D.A.; Roman, A. Human papillomavirus causes an angiogenic switch in keratinocytes which is sufficient to alter endothelial cell behavior. Virology 2007, 367, 168–174. [Google Scholar] [CrossRef] [PubMed]
- Bao, L.; Si, Q.; Jia, L.; Ren, X.; Ma, R.; Wang, Y. Detection of human papillomavirus and expression of osteopontin in cervical cancer specimens. Mol. Med. Rep. 2015, 11, 447–453. [Google Scholar] [CrossRef] [PubMed]
- Richards, R.M.; Lowy, D.R.; Schiller, J.T.; Day, P.M. Cleavage of the papillomavirus minor capsid protein, L2, at a furin consensus site is necessary for infection. Proc. Natl. Acad. Sci. USA 2006, 103, 1522–1527. [Google Scholar] [CrossRef] [PubMed]
- Kumar, V.; Behera, R.; Lohite, K.; Karnik, S.; Kundu, G.C. p38 kinase is crucial for osteopontin-induced furin expression that supports cervical cancer progression. Cancer Res. 2010, 70, 10381–10391. [Google Scholar] [CrossRef] [PubMed]
- Tiitta, O.; Wahlström, T.; Paavonen, J.; Linnala, A.; Sharma, S.; Gould, V.E.; Virtanen, I. Enhanced tenascin expression in cervical and vulvar koilocytotic lesions. Am. J. Pathol. 1992, 141, 907–913. [Google Scholar] [PubMed]
- Pöllänen, R.; Soini, Y.; Vuopala, S.; Läärä, E.; Lehto, V.P. Tenascin in human papillomavirus associated lesions of the uterine cervix. J. Clin. Pathol. 1996, 49, 521–523. [Google Scholar] [CrossRef] [PubMed]
- Spurgeon, M.E.; Lambert, P.F. Human Papillomavirus and the Stroma: Bidirectional Crosstalk during the Virus Life Cycle and Carcinogenesis. Viruses 2017, 9, 219. [Google Scholar] [CrossRef] [PubMed]
- Ali, A.S.; Al-Shraim, M.; Al-Hakami, A.M.; Jones, I.M. Epstein-Barr Virus: Clinical and Epidemiological Revisits and Genetic Basis of Oncogenesis. Open Virol. J. 2015, 9, 7–28. [Google Scholar] [CrossRef] [PubMed]
- Jha, H.C.; Pei, Y.; Robertson, E.S. Epstein-Barr Virus: Diseases Linked to Infection and Transformation. Front. Microbiol. 2016, 7, 1602. [Google Scholar] [CrossRef] [PubMed]
- Tang, W.; Morgan, D.R.; Meyers, M.O.; Dominguez, R.L.; Martinez, E.; Kakudo, K.; Kuan, P.F.; Banet, N.; Muallem, H.; Woodward, K.; et al. Epstein-Barr virus infected gastric adenocarcinoma expresses latent and lytic viral transcripts and has a distinct human gene expression profile. Infect. Agent. Cancer 2012, 7, 21. [Google Scholar] [CrossRef] [PubMed]
- Luo, W.; Yao, K. Molecular characterization and clinical implications of spindle cells in nasopharyngeal carcinoma: A novel molecule-morphology model of tumor progression proposed. PLoS ONE 2013, 8, e83135. [Google Scholar] [CrossRef] [PubMed]
- Aneja, K.K.; Yuan, Y. Reactivation and Lytic Replication of Kaposi’s Sarcoma-Associated Herpesvirus: An Update. Front. Microbiol. 2017, 8, 613. [Google Scholar] [CrossRef] [PubMed]
- Dittmer, D.P.; Damania, B. Kaposi’s sarcoma-associated herpesvirus: Immunobiology, oncogenesis, and therapy. J. Clin. Investig. 2016, 126, 3165–3175. [Google Scholar] [CrossRef] [PubMed]
- Kang, S.; Myoung, J. Primary lymphocyte infection models for KSHV and its putative tumorigenesis mechanisms in B cell lymphomas. J. Microbiol. 2017, 55, 319–329. [Google Scholar] [CrossRef] [PubMed]
- Kaaya, E.E.; Castaños-Velez, E.; Amir, H.; Lema, L.; Luande, J.; Kitinya, J.; Patarroyo, M.; Biberfeld, P. Expression of adhesion molecules in endemic and epidemic Kaposi’s sarcoma. Histopathology 1996, 29, 337–346. [Google Scholar] [CrossRef] [PubMed]
- Taraboletti, G.; Benelli, R.; Borsotti, P.; Rusnati, M.; Presta, M.; Giavazzi, R.; Ruco, L.; Albini, A. Thrombospondin-1 inhibits Kaposi’s sarcoma (KS) cell and HIV-1 Tat-induced angiogenesis and is poorly expressed in KS lesions. J. Pathol. 1999, 188, 76–81. [Google Scholar] [CrossRef]
- Samols, M.A.; Skalsky, R.L.; Maldonado, A.M.; Riva, A.; Lopez, M.C.; Baker, H.V.; Renee, R. Identification of cellular genes targeted by KSHV-encoded microRNAs. PLoS Pathog. 2007, 3, e65. [Google Scholar] [CrossRef] [PubMed]
- Cao, Y.; Qiao, J.; Lin, Z.; Zabaleta, J.; Dai, L.; Qin, Z. Up-regulation of tumor suppressor genes by exogenous dhC16-Cer contributes to its anti-cancer activity in primary effusion lymphoma. Oncotarget 2017, 8, 15220–15229. [Google Scholar] [CrossRef] [PubMed]
- Feng, H.; Shuda, M.; Chang, Y.; Moore, P.S. Clonal integration of a polyomavirus in human Merkel cell carcinoma. Science 2008, 319, 1096–1100. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; MacDonald, M.; You, J. Merkel cell polyomavirus infection and Merkel cell carcinoma. Curr. Opin. Virol. 2016, 20, 20–27. [Google Scholar] [CrossRef] [PubMed]
- Koljonen, V.; Jahkola, T.; Tukiainen, E.; Granroth, G.; Haglund, C.; Böhling, T. Tenascin-C in primary Merkel cell carcinoma. J. Clin. Pathol. 2005, 58, 297–300. [Google Scholar] [CrossRef] [PubMed]
- Koljonen, V.; Böhling, T.; Tukiainen, E.; Haglund, C.; Jahkola, T. Tenascin-C expression in Merkel cell carcinoma lymph node metastasis. APMIS 2006, 114, 39–42. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Yang, R.; Payne, A.S.; Schowalter, R.M.; Spurgeon, M.E.; Lambert, P.F.; Xu, X.; Buck, C.B.; You, J. Identifying the Target Cells and Mechanisms of Merkel Cell Polyomavirus Infection. Cell Host Microbe 2016, 19, 775–787. [Google Scholar] [CrossRef] [PubMed]
- Shuda, M.; Kwun, H.J.; Feng, H.; Chang, Y.; Moore, P.S. Human Merkel cell polyomavirus small T antigen is an oncoprotein targeting the 4E-BP1 translation regulator. J. Clin. Investig. 2011, 121, 3623–3634. [Google Scholar] [CrossRef] [PubMed]
- Maeda, N.; Matsuda, A.; Maenaka, K. Antibody-mediated molecular-targeted therapy for adult T-cell leukemia: Recent progress and future challenges in the treatment of cancers. Cancer Cell Microenviron. 2016, 3, e1201. [Google Scholar] [CrossRef]


| Viruses | Viral Genes/Oncogenes | Matricellular Proteins | Phenomena/Mechanisms | References |
|---|---|---|---|---|
| HTLV-I | Tax | OPN | Transcriptional upregulation of OPN resulted in the activation of PI3K/Akt pathway | [63] |
| ? | OPN | Stromal cell-derived OPN involved in the tumorigenesis and metastasis | [64,65] | |
| ? | SPARC | SPARC inhibition resulted in caspase 3-dependent apoptosis by bortezomib | [71] | |
| HBV | HBx | OPN | 5-LOX-dependent upregulation of OPN promoted cell migration | [74,75] |
| ? | OPN | Elevated production in cirrhosis and HCC | [76,77,78] | |
| ? | TSP-1 | Elevated production in fibrosis | [80] | |
| HCV | NS3/4A, NS5A | TSP-1 | Proteolytic activation of TGF-β by intracellular TSP-1 | [83] |
| Core | TSP-1 | Increased secretion of TSP-1 activated TGF-β | [84] | |
| ? | OPN | Antisense ODNs suppressed lung metastasis via downregulating MMP-2 and uPA | [92] | |
| HPV | E6, E7 | TSP-1 | Decreased expression in keratinocytes | [96,97,98] |
| ? | OPN | Induction of furin via p38 and NF-kB resulted in cancer progression | [99] | |
| ? | Tenascin * | Expressed during the premalignant stage | [103] | |
| EBV | ? | OPN | Elevated expression | [107] |
| LMP1, EBER | SPARC POSTN | Elevated expression | [107,108] | |
| KSHV | ? | Tenascin * | Expressed in the vessel walls | [112] |
| ? | TSP-1 | Viral miRNA-dependent TSP-1 reduction decreased TGF-β activity | [113,114] | |
| ? | OPN | Viral miRNA-dependent reduction | [114] | |
| MCPyV | sT antigen? | TNC | Tumorigenesis and metastasis | [118,119] |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Maeda, N.; Maenaka, K. The Roles of Matricellular Proteins in Oncogenic Virus-Induced Cancers and Their Potential Utilities as Therapeutic Targets. Int. J. Mol. Sci. 2017, 18, 2198. https://doi.org/10.3390/ijms18102198
Maeda N, Maenaka K. The Roles of Matricellular Proteins in Oncogenic Virus-Induced Cancers and Their Potential Utilities as Therapeutic Targets. International Journal of Molecular Sciences. 2017; 18(10):2198. https://doi.org/10.3390/ijms18102198
Chicago/Turabian StyleMaeda, Naoyoshi, and Katsumi Maenaka. 2017. "The Roles of Matricellular Proteins in Oncogenic Virus-Induced Cancers and Their Potential Utilities as Therapeutic Targets" International Journal of Molecular Sciences 18, no. 10: 2198. https://doi.org/10.3390/ijms18102198
APA StyleMaeda, N., & Maenaka, K. (2017). The Roles of Matricellular Proteins in Oncogenic Virus-Induced Cancers and Their Potential Utilities as Therapeutic Targets. International Journal of Molecular Sciences, 18(10), 2198. https://doi.org/10.3390/ijms18102198