Diurnal Hypothalamic-Pituitary-Adrenal Axis Measures and Inflammatory Marker Correlates in Major Depressive Disorder
Abstract
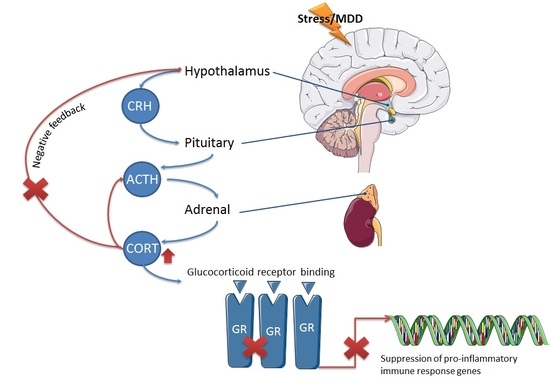
1. Introduction
2. Results
2.1. Demographics and Psychiatric Measurements
2.2. Salivary Cortisol Concentrations iIn Depressed Patients and Healthy Controls
2.3. Cortisol Awakening Response in Depressed Patients and Healthy Controls
2.4. Salivary Cortisone Concentrations in Depressed Patients and Healthy Controls
2.5. Relative Quantification of Whole Blood HSD11β-1 mRNA Expression in Depressed Patients and Healthy Controls
2.6. Whole Blood mRNA Expression of Inflammatory Cytokines
3. Discussion
3.1. Altered Cortisol Awakening Responses in MDD
3.2. Cortisone and HSD11β-1
3.3. Inflammatory Markers and Their Relation to HPA Axis Activity
3.4. Limitations
4. Materials and Methods
4.1. Recruitment
4.2. Psychiatric Rating Scales
4.3. Saliva Sample Collection
4.4. Cortisol and Cortisone Measurement by Liquid Chromatography-Mass Spectrometry (LC-MS)
4.5. Cortisol Awakening Response (CAR) Calculations
4.6. Measurement of Whole Blood mRNA Expression of HSD11β-1 and Inflammatory Cytokines by qPCR
4.7. Statistical Analysis
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
Abbreviations
| MDD | Major Depressive Disorder |
| CAR | cortisol awakening response |
| HPA | hypothalamic-pituitary-adrenal |
| HAM-D | Hamilton Depression rating scale |
| CES-D | Center for Epidemiology Scale–Depression |
| PSQI | Pittsburgh Sleep Quality Index |
| CTQ | Childhood Trauma Questionnaire |
| IL | interleukin |
| TNF | tumor necrosis factor |
| IFN | interferon |
| RQ | relative quantification |
| LC-MS | liquid chromatography-mass spectrometry |
| PSQI | qualitative polymerase chain reaction |
| AUC | area under curve |
| SSRI | selective serotonin reuptake inhibitor |
| mRNA | messenger ribonucleic acid |
| SD | standard deviation |
| SEM | standard error of the mean |
References
- Arsenault-Lapierre, G.; Kim, C.; Turecki, G. Psychiatric diagnoses in 3275 suicides: A meta-analysis. BMC Psychiatry 2004, 4, 37. [Google Scholar] [CrossRef] [PubMed]
- Kessler, R.C.; Berglund, P.; Demler, O.; Jin, R.; Merikangas, K.R.; Walters, E.E. Lifetime prevalence and age-of-onset distributions of DSM-IV disorders in the National Comorbidity Survey Replication. Arch. Gen. Psychiatry 2005, 62, 593–602. [Google Scholar] [CrossRef] [PubMed]
- Kessler, R.C.; Zhao, S.; Blazer, D.G.; Swartz, M. Prevalence, correlates, and course of minor depression and major depression in the National Comorbidity Survey. J. Affect. Disord. 1997, 45, 19–30. [Google Scholar] [CrossRef]
- Pariante, C.M.; Lightman, S.L. The HPA axis in major depression: Classical theories and new developments. Trends Neurosci. 2008, 31, 464–468. [Google Scholar] [CrossRef] [PubMed]
- O'Keane, V.; Frodl, T.; Dinan, T.G. A review of atypical depression in relation to the course of depression and changes in HPA axis organization. Psychoneuroendocrinology 2012, 37, 1589–1599. [Google Scholar] [CrossRef] [PubMed]
- Farrell, C.; O’Keane, V. Epigenetics and the glucocorticoid receptor: A review of the implications in depression. Psychiatry Res. 2016, 242, 349–356. [Google Scholar] [CrossRef] [PubMed]
- Fries, E.; Dettenborn, L.; Kirschbaum, C. The cortisol awakening response (CAR): Facts and future directions. Int. J. Psychophysiol. 2009, 72, 67–73. [Google Scholar] [CrossRef] [PubMed]
- Wust, S.; Wolf, J.; Hellhammer, D.H.; Federenko, I.; Schommer, N.; Kirschbaum, C. The cortisol awakening response—Normal values and confounds. Noise Health 2000, 2, 79–88. [Google Scholar] [PubMed]
- Holsboer, F. The corticosteroid receptor hypothesis of depression. Neuropsychopharmacology 2000, 23, 477–501. [Google Scholar] [CrossRef]
- Huber, T.J.; Issa, K.; Schik, G.; Wolf, O.T. The cortisol awakening response is blunted in psychotherapy inpatients suffering from depression. Psychoneuroendocrinology 2006, 31, 900–904. [Google Scholar] [CrossRef] [PubMed]
- Vreeburg, S.A.; Hoogendijk, W.J.; van Pelt, J.; Derijk, R.H.; Verhagen, J.C.; van Dyck, R.; Smit, J.H.; Zitman, F.G.; Penninx, B.W. Major depressive disorder and hypothalamic-pituitary-adrenal axis activity: Results from a large cohort study. Arch. Gen. Psychiatry 2009, 66, 617–626. [Google Scholar] [CrossRef] [PubMed]
- Coutinho, A.E.; Chapman, K.E. The anti-inflammatory and immunosuppressive effects of glucocorticoids, recent developments and mechanistic insights. Mol. Cell. Endocrinol. 2011, 335, 2–13. [Google Scholar] [CrossRef] [PubMed]
- Brown, E.S.; Chandler, P.A. Mood and cognitive changes during systemic corticosteroid therapy. Prim. Care Companion J. Clin. Psychiatry 2001, 3, 17–21. [Google Scholar] [CrossRef] [PubMed]
- Dekker, M.J.; Tiemeier, H.; Luijendijk, H.J.; Kuningas, M.; Hofman, A.; de Jong, F.H.; Stewart, P.M.; Koper, J.W.; Lamberts, S.W. The effect of common genetic variation in 11β-hydroxysteroid dehydrogenase type 1 on hypothalamic-pituitary-adrenal axis activity and incident depression. J. Clin. Endocrinol. Metab. 2012, 97, E233–E237. [Google Scholar] [CrossRef] [PubMed]
- Slattery, D.A.; Uzunov, D.P.; Cryan, J.F. 11-β hydroxysteroid type 1 knockout mice display an antidepressant-like phenotype in the forced swim test. Acta Neuropsychiatry. 2016, 28, 55–60. [Google Scholar] [CrossRef] [PubMed]
- Elenkov, I.J.; Webster, E.L.; Torpy, D.J.; Chrousos, G.P. Stress, corticotropin-releasing hormone, glucocorticoids, and the immune/inflammatory response: Acute and chronic effects. Ann. N. Y. Acad. Sci. 1999, 876, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Miura, H.; Ozaki, N.; Sawada, M.; Isobe, K.; Ohta, T.; Nagatsu, T. A link between stress and depression: Shifts in the balance between the kynurenine and serotonin pathways of tryptophan metabolism and the etiology and pathophysiology of depression. Stress 2008, 11, 198–209. [Google Scholar] [CrossRef] [PubMed]
- Sorrells, S.F.; Caso, J.R.; Munhoz, C.D.; Sapolsky, R.M. The stressed CNS: When glucocorticoids aggravate inflammation. Neuron 2009, 64, 33–39. [Google Scholar] [CrossRef] [PubMed]
- Raison, C.L.; Capuron, L.; Miller, A.H. Cytokines sing the blues: Inflammation and the pathogenesis of depression. Trends Immunol. 2006, 27, 24–31. [Google Scholar] [CrossRef] [PubMed]
- Maes, M. Major depression and activation of the inflammatory response system. Adv. Exp. Med. Boil. 1999, 461, 25–46. [Google Scholar]
- Lanquillon, S.; Krieg, J.C.; Bening-Abu-Shach, U.; Vedder, H. Cytokine production and treatment response in major depressive disorder. Neuropsychopharmacology 2000, 22, 370–379. [Google Scholar] [CrossRef]
- Alesci, S.; Martinez, P.E.; Kelkar, S.; Ilias, I.; Ronsaville, D.S.; Listwak, S.J.; Ayala, A.R.; Licinio, J.; Gold, H.K.; Kling, M.A.; et al. Major depression is associated with significant diurnal elevations in plasma interleukin-6 levels, a shift of its circadian rhythm, and loss of physiological complexity in its secretion: Clinical implications. J. Clin. Endocrinol. Metab. 2005, 90, 2522–2530. [Google Scholar] [CrossRef] [PubMed]
- Tuglu, C.; Kara, S.H.; Caliyurt, O.; Vardar, E.; Abay, E. Increased serum tumor necrosis factor-α levels and treatment response in major depressive disorder. Psychopharmacology 2003, 170, 429–433. [Google Scholar] [CrossRef] [PubMed]
- Thomas, A.J.; Davis, S.; Morris, C.; Jackson, E.; Harrison, R.; O'Brien, J.T. Increase in interleukin-1β in late-life depression. Am. J. Psychiatry 2005, 162, 175–177. [Google Scholar] [CrossRef] [PubMed]
- Taylor, A.E.; Keevil, B.; Huhtaniemi, I.T. Mass spectrometry and immunoassay: How to measure steroid hormones today and tomorrow. Eur. J. Endocrinol. 2015, 173, D1–D12. [Google Scholar] [CrossRef] [PubMed]
- Kushnir, M.M.; Rockwood, A.L.; Bergquist, J. Liquid chromatography–tandem mass spectrometry applications in endocrinology. Mass Spectrum. Rev. 2010, 29, 480–502. [Google Scholar] [CrossRef] [PubMed]
- Handelsman, D.J.; Wartofsky, L. Requirement for mass spectrometry sex steroid assays in the journal of clinical endocrinology and metabolism. J. Clin. Endocrinol. Metab. 2013, 98, 3971–3973. [Google Scholar] [CrossRef] [PubMed]
- Závada, M.; Šafarčik, K.; Topolčan, O. Some problems of radioimmunoassay control. J. Radioanal. Nucl. Chem. 1978, 46, 57–66. [Google Scholar] [CrossRef]
- Lopez-Duran, N.L.; Kovacs, M.; George, C.J. Hypothalamic-pituitary-adrenal axis dysregulation in depressed children and adolescents: A meta-analysis. Psychoneuroendocrinology 2009, 34, 1272–1283. [Google Scholar] [CrossRef] [PubMed]
- Kirschbaum, C.; Hellhammer, D.H. Salivary cortisol in psychobiological research: An overview. Neuropsychobiology 1989, 22, 150–169. [Google Scholar] [CrossRef] [PubMed]
- Edwards, S.; Clow, A.; Evans, P.; Hucklebridge, F. Exploration of the awakening cortisol response in relation to diurnal cortisol secretory activity. Life Sci. 2001, 68, 2093–2103. [Google Scholar] [CrossRef]
- Hsiao, F.-H.; Yang, T.-T.; Ho, R.T.H.; Jow, G.-M.; Ng, S.-M.; Chan, C.L.W.; Lai, Y.-M.; Chen, Y.-T.; Wang, K.-C. The self-perceived symptom distress and health-related conditions associated with morning to evening diurnal cortisol patterns in outpatients with major depressive disorder. Psychoneuroendocrinology 2010, 35, 503–515. [Google Scholar] [CrossRef] [PubMed]
- Unschuld, P.G.; Ising, M.; Roeske, D.; Erhardt, A.; Specht, M.; Kloiber, S.; Uhr, M.; Muller-Myhsok, B.; Holsboer, F.; Binder, E.B. Gender-specific association of galanin polymorphisms with HPA-axis dysregulation, symptom severity, and antidepressant treatment response. Neuropsychopharmacology 2010, 35, 1583–1592. [Google Scholar] [CrossRef] [PubMed]
- Knutsson, U.; Dahlgren, J.; Marcus, C.; Rosberg, S.; Bronnegard, M.; Stierna, P.; Albertsson-Wikland, K. Circadian cortisol rhythms in healthy boys and girls: Relationship with age, growth, body composition, and pubertal development. J. Clin. Endocrinol. Metab. 1997, 82, 536–540. [Google Scholar] [CrossRef] [PubMed]
- Hori, H.; Teraishi, T.; Sasayama, D.; Ozeki, Y.; Matsuo, J.; Kawamoto, Y.; Kinoshita, Y.; Hattori, K.; Higuchi, T.; Kunugi, H. Poor sleep is associated with exaggerated cortisol response to the combined dexamethasone/crh test in a non-clinical population. J. Psychiatr. Res. 2011, 45, 1257–1263. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.Y.; Hu, Z.Z.; Huang, Z.L.; Yang, S.R. [Role of stress in depression insomnia and sleep characteristics of commonly used animal stress models]. Yao Xue Xue Bao 2012, 47, 1–6. [Google Scholar] [PubMed]
- Glozier, N.; Martiniuk, A.; Patton, G.; Ivers, R.; Li, Q.A.; Hickie, I.; Senserrick, T.; Woodward, M.; Norton, R.; Stevenson, M. Short sleep duration in prevalent and persistent psychological distress in young adults: The drive study. Sleep 2010, 33, 1139–1145. [Google Scholar] [CrossRef] [PubMed]
- Fitzgerald, C.T.; Messias, E.; Buysse, D.J. Teen sleep and suicidality: Results from the youth risk behavior surveys of 2007 and 2009. J. Clin. Sleep Med. 2011, 7, 351–356. [Google Scholar] [PubMed]
- Copinschi, G.; Akseki, E.; Moreno-Reyes, R.; Leproult, R.; L’Hermite-Baleriaux, M.; Caufriez, A.; Vertongen, F.; Van Cauter, E. Effects of bedtime administration of zolpidem on circadian and sleep-related hormonal profiles in normal women. Sleep 1995, 18, 417–424. [Google Scholar] [CrossRef] [PubMed]
- Besnier, E.; Clavier, T.; Castel, H.; Gandolfo, P.; Morin, F.; Tonon, M.C.; Marguerite, C.; Veber, B.; Dureuil, B.; Compère, V. Modulation de l’axe hypothalamo-hypophyso-surrénalien par l’utilisation des agents hypnotiques dans le contexte chirurgical. Ann. Fr. Anesth. Réanim. 2014, 33, 256–265. [Google Scholar] [CrossRef] [PubMed]
- Stimson, R.H.; Andersson, J.; Andrew, R.; Redhead, D.N.; Karpe, F.; Hayes, P.C.; Olsson, T.; Walker, B.R. Cortisol release from adipose tissue by 11β-hydroxysteroid dehydrogenase type 1 in humans. Diabetes 2009, 58, 46–53. [Google Scholar] [CrossRef] [PubMed]
- Mihailova, S.; Ivanova-Genova, E.; Lukanov, T.; Stoyanova, V.; Milanova, V.; Naumova, E. A study of TNF-α, TGF-β, IL-10, IL-6, and IFN-γ gene polymorphisms in patients with depression. J. Neuroimmunol. 2016, 293, 123–128. [Google Scholar] [CrossRef] [PubMed]
- Fabbri, C.; Hosak, L.; Mossner, R.; Giegling, I.; Mandelli, L.; Bellivier, F.; Claes, S.; Collier, D.A.; Corrales, A.; Delisi, L.E.; et al. Consensus paper of the wfsbp task force on genetics: Genetics, epigenetics and gene expression markers of major depressive disorder and antidepressant response. World J. Boil. Psychiatry 2017, 18, 5–18. [Google Scholar] [CrossRef] [PubMed]
- Dinarello, C.A. Biology of interleukin 1. FASEB J. 1988, 2, 108–115. [Google Scholar] [PubMed]
- Van de Veerdonk, F.L.; Netea, M.G. New insights in the immunobiology of IL-1 family members. Front. Immunol. 2013, 4, 167. [Google Scholar] [CrossRef] [PubMed]
- Dantzer, R.; O'Connor, J.C.; Freund, G.G.; Johnson, R.W.; Kelley, K.W. From inflammation to sickness and depression: When the immune system subjugates the brain. Nat. Rev. Neurosci. 2008, 9, 46–56. [Google Scholar] [CrossRef] [PubMed]
- Owen, L.J.; Haslam, S.; Adaway, J.E.; Wood, P.; Glenn, C.; Keevil, B.G. A simplified liquid chromatography tandem mass spectrometry assay, using on-line solid-phase extraction, for the quantitation of cortisol in saliva and comparison with a routine DELFIA method. Ann. Clin. Biochem. 2010, 47, 131–136. [Google Scholar] [CrossRef] [PubMed]
- Maes, M.; Rief, W. Diagnostic classifications in depression and somatization should include biomarkers, such as disorders in the tryptophan catabolite (trycat) pathway. Psychiatry Res. 2012, 196, 243–249. [Google Scholar] [CrossRef] [PubMed]
- Anisman, H. Cascading effects of stressors and inflammatory immune system activation: Implications for major depressive disorder. J. Psychiatry Neurosci. 2009, 34, 4–20. [Google Scholar] [PubMed]
- Pace, T.W.; Hu, F.; Miller, A.H. Cytokine-effects on glucocorticoid receptor function: Relevance to glucocorticoid resistance and the pathophysiology and treatment of major depression. Brain Behav. Immun. 2007, 21, 9–19. [Google Scholar] [CrossRef] [PubMed]
- Bellavance, M.-A.; Rivest, S. The HPA–immune axis and the immunomodulatory actions of glucocorticoids in the brain. Front. Immunol. 2014, 5, 136. [Google Scholar] [CrossRef] [PubMed]
- Wolkow, A.; Aisbett, B.; Reynolds, J.; Ferguson, S.A.; Main, L.C. Relationships between inflammatory cytokine and cortisol responses in firefighters exposed to simulated wildfire suppression work and sleep restriction. Physiol. Rep. 2015, 3, e12604. [Google Scholar] [CrossRef] [PubMed]
- Otte, C.; Gold, S.M.; Penninx, B.W.; Pariante, C.M.; Etkin, A.; Fava, M.; Mohr, D.C.; Schatzberg, A.F. Major depressive disorder. Nat. Rev. Dis. Prim. 2016, 2, 16065. [Google Scholar] [CrossRef] [PubMed]
- Ho, R.C.; Neo, L.F.; Chua, A.N.; Cheak, A.A.; Mak, A. Research on psychoneuroimmunology: Does stress influence immunity and cause coronary artery disease? Ann. Acad. Med. Singap. 2010, 31, 191–196. [Google Scholar]
- Stalder, T.; Kirschbaum, C.; Kudielka, B.M.; Adam, E.K.; Pruessner, J.C.; Wust, S.; Dockray, S.; Smyth, N.; Evans, P.; Hellhammer, D.H.; et al. Assessment of the cortisol awakening response: Expert consensus guidelines. Psychoneuroendocrinology 2016, 63, 414–432. [Google Scholar] [CrossRef] [PubMed]
- Kirschbaum, C.; Hellhammer, D.H. Salivary cortisol in psychoneuroendocrine research: Recent developments and applications. Psychoneuroendocrinology 1994, 19, 313–333. [Google Scholar] [CrossRef]
- Kirschbaum, C.; Kudielka, B.; Gaab, J.; Schommer, M.; Hellhammer, D. Impact of Gender, Menstrual Cycle Phase, and Oral Contraceptives on the Activity of the Hypothalamus-Pituitary-Adrenal Axis. Psychosom. Med. 1999, 61, 154–162. [Google Scholar] [CrossRef] [PubMed]
- O’Donnell, K.J.; Bugge Jensen, A.; Freeman, L.; Khalife, N.; O’Connor, T.G.; Glover, V. Maternal prenatal anxiety and downregulation of placental 11β-HSD2. Psychoneuroendocrinology 2012, 37, 818–826. [Google Scholar] [CrossRef] [PubMed]
- Wyrwoll, C.; Keith, M.; Noble, J.; Stevenson, P.L.; Bombail, V.; Crombie, S.; Evans, L.C.; Bailey, M.A.; Wood, E.; Seckl, J.R.; et al. Fetal brain 11β-hydroxysteroid dehydrogenase type 2 selectively determines programming of adult depressive-like behaviors and cognitive function, but not anxiety behaviors in male mice. Psychoneuroendocrinology 2015, 59, 59–70. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Ho, R.C.; Mak, A. Interleukin (IL)-6, tumour necrosis factor alpha (TNF-α) and soluble interleukin-2 receptors (sIL-2R) are elevated in patients with major depressive disorder: A meta-analysis and meta-regression. J. Affect. Disord. 2012, 139, 230–239. [Google Scholar] [CrossRef] [PubMed]
- American Psychiatric Association. The Diagnostic and Statistical Manual, 5th ed.; American Psychiatric Publishing: Arlington, VA, USA, 2013. [Google Scholar]
- Hamilton, M. A rating scale for depression. J. Nneurol. Neur. Psychiatry 1960, 23, 56–62. [Google Scholar] [CrossRef]
- Hamilton, M. Rating depressive patients. J. Clin. Psychiatry 1980, 41, 21–24. [Google Scholar] [PubMed]
- Radloff, L. The CES-D scale: A self report depression scale for research in the general population. Appl. Psychol. Meas. 1977, 1, 385–401. [Google Scholar] [CrossRef]
- Buysse, D.J.; Reynolds, C.F., 3rd; Monk, T.H.; Berman, S.R.; Kupfer, D.J. The pittsburgh sleep quality index: A new instrument for psychiatric practice and research. Psychiatry Res. 1989, 28, 193–213. [Google Scholar] [CrossRef]
- Bernstein, D.P.; Stein, J.A.; Newcomb, M.D.; Walker, E.; Pogge, D.; Ahluvalia, T.; Stokes, J.; Handelsman, L.; Medrano, M.; Desmond, D.; et al. Development and validation of a brief screening version of the childhood trauma questionnaire. Child Abuse Negl. 2003, 27, 169–190. [Google Scholar] [CrossRef]
- Fekedulegn, D.B.; Andrew, M.E.; Burchfiel, C.M.; Violanti, J.M.; Hartley, T.A.; Charles, L.E.; Miller, D.B. Area under the curve and other summary indicators of repeated waking cortisol measurements. Psychosom. Med. 2007, 69, 651–659. [Google Scholar] [CrossRef] [PubMed]









| Variable | Patients (n = 57) | Controls (n = 40) | Statistics (p-Value) |
|---|---|---|---|
| Age (years) | 28.26 (8.41) | 27.48 (5.61) | Z= −0.018, p = 0.985 |
| Gender (female/male) | 37/20 | 27/13 | χ2 = 0.070, p = 0.831 |
| BMI | 24.96 (6.17) | 22.81 (3.25) | Z = −1.459, p = 0.145 |
| Smoking (Yes/No) | 23/34 | 10/30 | χ2 = 2.468, p = 0.133 |
| ISCED Education | 3.54 (1.54) | 6.30 (1.45) | Z = −6.194, p < 0.001 *** |
| HAM-D | 22.81 (5.14) | 2.92 (2.80) | Z = −8.193, p < 0.001 *** |
| CES-D | 39.04 (10.09) | 6.85 (6.60) | Z = −8.123, p < 0.001 *** |
| PSQI | 13.58 (3.36) | 4.13 (2.70) | Z = −7.786, p < 0.001 *** |
| CTQ Global Score | 44.50 (17.20) | 30.43 (7.67) | Z = −3.999, p < 0.001 *** |
| Early Life Adversity (Yes/No) | 35/22 | 8/32 | χ2 = 19.136, p < 0.001 *** |
| Antidepressant use (Yes/No) | 32/25 | 0/40 | χ2 = 33.511, p < 0.001 *** |
| Cortisol Measurement | Patients (n = 57) | Controls (n = 40) | Statistics (p-Value) |
|---|---|---|---|
| Cortisol T0 | 10.92 (1.34) | 7.54 (0.64) | Z = −2.275, p = 0.023 * |
| Cortisol T30 | 12.93 (1.03) | 11.00 (0.91) | Z = −1.156, p = 0.247 |
| Cortisol T60 | 9.58 (1.13) | 9.15 (0.85) | Z = −0.187, p = 0.852 |
| Average AM Cortisol (nM) | 11.46 (1.01) | 9.09 (0.58) | Z = −1.300, p = 0.194 |
| Cortisol T720 | 1.97 (0.29) | 2.07 (0.65) | Z = −0.801, p = 0.423 |
| Cortisol T750 | 2.12 (0.57) | 1.96 (0.53) | Z = −0.132, p = 0.895 |
| Average PM Cortisol (nM) | 2.36 (0.55) | 2.45 (0.68) | Z = −0.658, p = 0.510 |
| CAR Parameter | Patients (n = 57) | Controls (n = 41) | Statistics (p-Value) |
|---|---|---|---|
| AUCg | 681.96 (58.88) | 569.29 (38.95) | Z = −0.367, p = 0.367 |
| AUCi | 28.38 (63.97) | 128.78 (41.76) | Z = −1.295, p = 0.195 |
| Peak | 15.41 (1.36) | 12.68 (0.91) | Z = −1.120, p = 0.263 |
| Reactivity | −1.71 (1.41) | 1.37 (1.00) | Z = −1.962, p = 0.050 * |
| Intercept | 12.46 (1.24) | 8.41 (0.61) | Z = −2.045, p = 0.041 * |
| Slope | −0.039 (0.02) | 0.023 (0.02) | Z = −1.977, p = 0.048 * |
| Cortisone Measurement | Patients (n = 57) | Controls (n = 41) | Statistics (p-Value) |
|---|---|---|---|
| Cortisone T0 | 31.07 (2.03) | 27.59 (1.76) | Z = −1.045, p = 0.296 |
| Cortisone T30 | 39.85 (2.28) | 33.99 (1.76) | Z = −1.436, p = 0.151 |
| Cortisone T60 | 32.19 (2.22) | 30.63 (1.54) | Z = −0.155, p = 0.877 |
| Cortisone T720 | 10.93 (1.28) | 9.72 (1.33) | Z = −0.459, p = 0.646 |
| Cortisone T750 | 8.60 (1.00) | 8.83 (1.14) | Z = −0.579, p = 0.562 |
| Parameter | Definition | Formula |
|---|---|---|
| Diurnal variation | Change in cortisol concentration between morning time points and evening time points | =PM average − AM average |
| AUCg | Area under the curve with respect to ground | ={[(T30 cortisol value + T0 cortisol value)/2] × time difference} + {[(T60 cortisol value + T30 cortisol value)/2] × time difference} |
| AUCi | Area under the curve with respect to increase | =AUC g − [T0 cortisol value × (total time between first and last measurement)] |
| Peak | Highest concentration of cortisol recorded | =Maximum cortisol value |
| Reactivity | Change in cortisol between first and last morning measurement | =T60 value − T0 value |
| Slope | Slope of the regression line fitted through raw morning cortisol data | =correlation coefficient r × [SD of AM cortisol values/SD of AM time values] |
| Intercept | Intercept of the regression line fitted through raw cortisol data | =the mean of cortisol values − (slope × mean of 3 AM cortisol values) |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Doolin, K.; Farrell, C.; Tozzi, L.; Harkin, A.; Frodl, T.; O’Keane, V. Diurnal Hypothalamic-Pituitary-Adrenal Axis Measures and Inflammatory Marker Correlates in Major Depressive Disorder. Int. J. Mol. Sci. 2017, 18, 2226. https://doi.org/10.3390/ijms18102226
Doolin K, Farrell C, Tozzi L, Harkin A, Frodl T, O’Keane V. Diurnal Hypothalamic-Pituitary-Adrenal Axis Measures and Inflammatory Marker Correlates in Major Depressive Disorder. International Journal of Molecular Sciences. 2017; 18(10):2226. https://doi.org/10.3390/ijms18102226
Chicago/Turabian StyleDoolin, Kelly, Chloe Farrell, Leonardo Tozzi, Andrew Harkin, Thomas Frodl, and Veronica O’Keane. 2017. "Diurnal Hypothalamic-Pituitary-Adrenal Axis Measures and Inflammatory Marker Correlates in Major Depressive Disorder" International Journal of Molecular Sciences 18, no. 10: 2226. https://doi.org/10.3390/ijms18102226
APA StyleDoolin, K., Farrell, C., Tozzi, L., Harkin, A., Frodl, T., & O’Keane, V. (2017). Diurnal Hypothalamic-Pituitary-Adrenal Axis Measures and Inflammatory Marker Correlates in Major Depressive Disorder. International Journal of Molecular Sciences, 18(10), 2226. https://doi.org/10.3390/ijms18102226