Real-Time Observation of the Interaction between Thioflavin T and an Amyloid Protein by Using High-Sensitivity Rheo-NMR
Abstract
:1. Introduction
2. Results
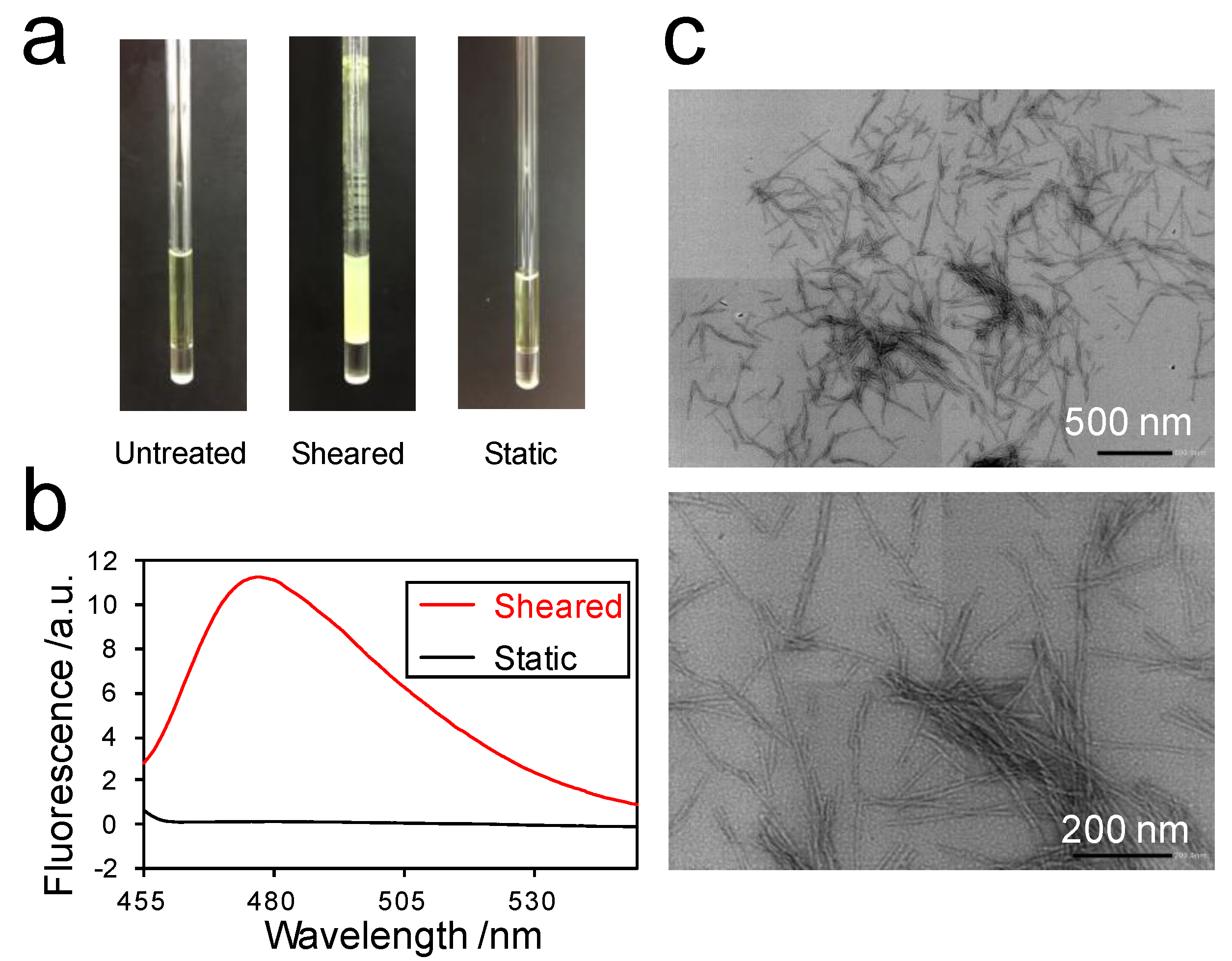
2.1. Amyloid Fibril Formation of Superoxide Dismutase 1 (SOD1) in the Rheo-NMR Instrument
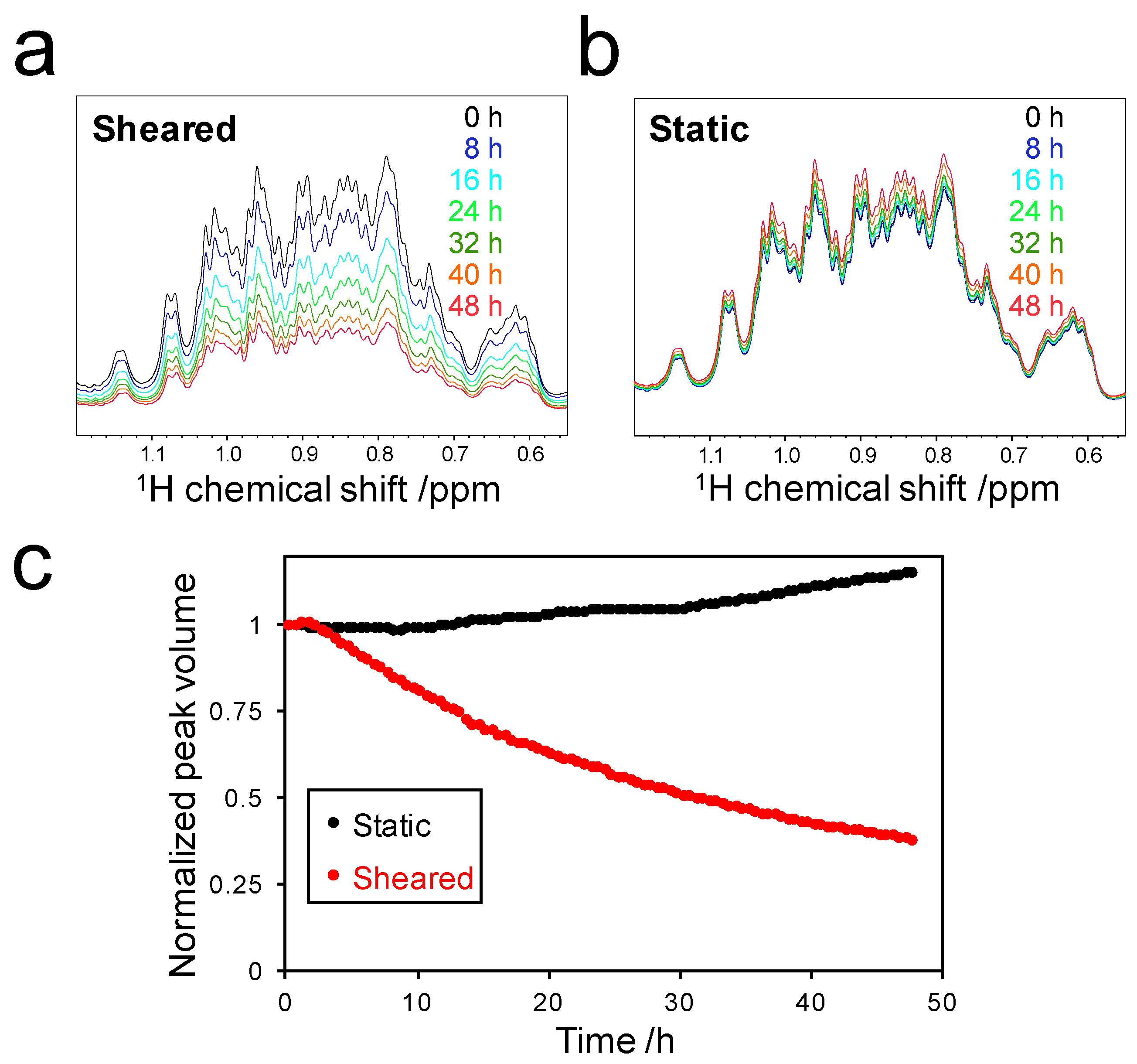
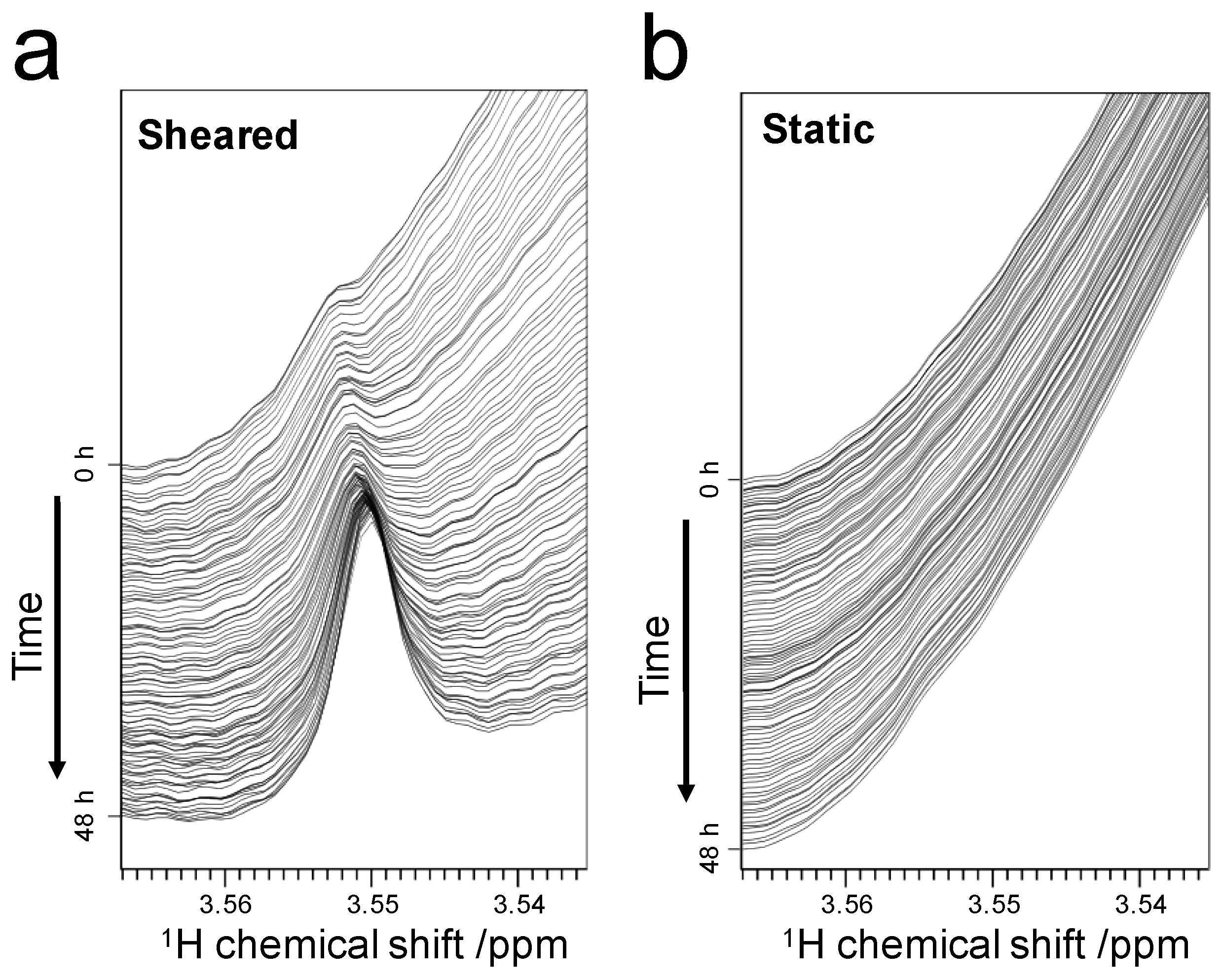
2.2. Decrease in Peak Volume of Soluble SOD1
2.3. Detection of the Interaction between Thioflavin T (ThT) and SOD1 Fibrils
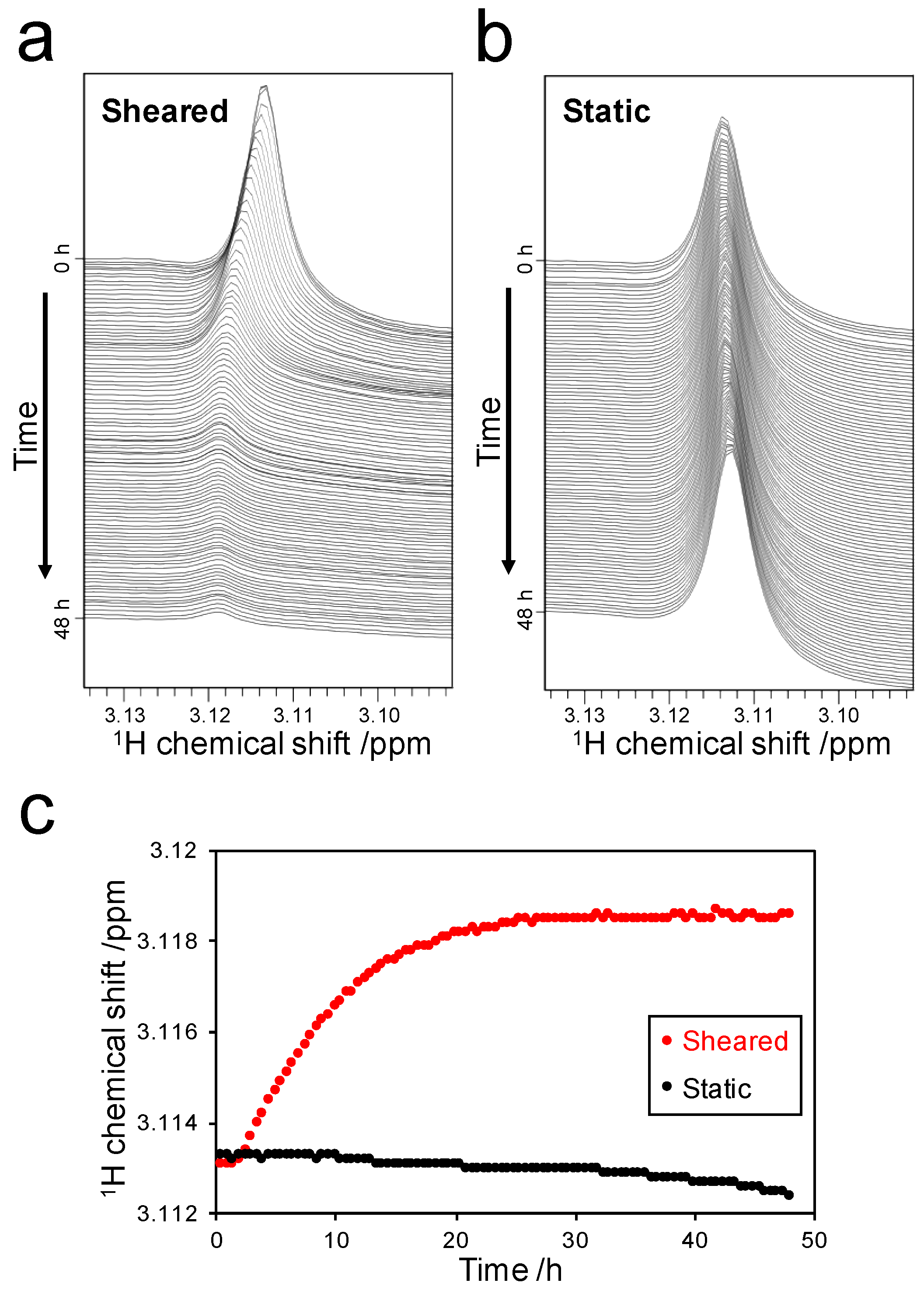
2.3.1. Weak-Interaction-Induced Chemical Shift Changes of the Dimethylamino (DMA) Signal
2.3.2. Detection of Strong Interactions between ThT and Fibrils
3. Discussion
3.1. Stoichiometry of ThT-Fibril Binding
3.2. Fluorescent-Dye-Based New Applications of Rheo-NMR
4. Materials and Methods
4.1. Protein Expression and Purification
4.2. Rheo-NMR Measurements
4.3. Thioflavin T Fluorescence Measurements
4.4. Transmission Electron Microscopy (TEM)
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
Abbreviations
| AD | Alzheimer’s disease |
| ALS | Amyotrophic lateral sclerosis |
| ANS | 1-Anilinonaphthalene-8-sulfonic acid |
| DMA | Dimethylamino |
| DSS | Dimethyl-2-silapentane-5-sulfonate |
| IAPP | Islet amyloid polypeptide |
| IPTG | Isopropyl 1-thio-β-d-galactopyranoside |
| LB | Luria-Bertani |
| NMR | Nuclear magnetic resonance |
| PD | Parkinson’s disease |
| SDS-PAGE | Sodium dodecyl sulfate polyacrylamide gel electrophoresis |
| SOD1 | Superoxide dismutase 1 |
| TEM | Transmission electron microscopy |
| ThT | Thioflavin T |
References
- Ross, C.A.; Poirier, M.A. Protein aggregation and neurodegenerative disease. Nat. Med. 2004, 10, S10–S17. [Google Scholar] [CrossRef] [PubMed]
- Conway, K.A.; Lee, S.-J.; Rochet, J.-C.; Ding, T.T.; Williamson, R.E.; Lansbury, P.T. Acceleration of oligomerization, not fibrillization, is a shared property of both α-synuclein mutations linked to early-onset Parkinson’s disease: Implications for pathogenesis and therapy. Proc. Natl. Acad. Sci. USA 2000, 97, 571–576. [Google Scholar] [CrossRef] [PubMed]
- Cohen, S.I.; Linse, S.; Luheshi, L.M.; Hellstrand, E.; White, D.A.; Rajah, L.; Otzen, D.E.; Vendruscolo, M.; Dobson, C.M.; Knowles, T.P. Proliferation of amyloid-β42 aggregates occurs through a secondary nucleation mechanism. Proc. Natl. Acad. Sci. USA 2013, 110, 9758–9763. [Google Scholar] [CrossRef] [PubMed]
- Naiki, H.; Higuchi, K.; Hosokawa, M.; Takeda, T. Fluorometric determination of amyloid fibrils in vitro using the fluorescent dye, thioflavine T. Anal. Biochem. 1989, 177, 244–249. [Google Scholar] [CrossRef]
- Robbins, K.J.; Liu, G.; Lin, G.; Lazo, N.D. Detection of strongly bound thioflavin T species in amyloid fibrils by ligand-detected 1H NMR. J. Phys. Chem. Lett. 2011, 2, 735–740. [Google Scholar] [CrossRef]
- Hill, E.K.; Krebs, B.; Goodall, D.G.; Howlett, G.J.; Dunstan, D.E. Shear flow induces amyloid fibril formation. Biomacromolecules 2006, 7, 10–13. [Google Scholar] [CrossRef] [PubMed]
- Morimoto, D.; Walinda, E.; Fukada, H.; Sou, Y.-S.; Kageyama, S.; Hoshino, M.; Fujii, T.; Tsuchiya, H.; Saeki, Y.; Arita, K.; et al. The unexpected role of polyubiquitin chains in the formation of fibrillar aggregates. Nat. Commun. 2015, 6, 6116. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stathopulos, P.B.; Scholz, G.A.; Hwang, Y.-M.; Rumfeldt, J.A.O.; Lepock, J.R.; Meiering, E.M. Sonication of proteins causes formation of aggregates that resemble amyloid. Protein Sci. 2004, 13, 3017–3027. [Google Scholar] [CrossRef] [PubMed]
- Sasahara, K.; Yagi, H.; Naiki, H.; Goto, Y. Heat-induced conversion of β2-microglobulin and hen egg-white lysozyme into amyloid fibrils. J. Mol. Biol. 2007, 372, 981–991. [Google Scholar] [CrossRef] [PubMed]
- Morimoto, D.; Walinda, E.; Iwakawa, N.; Nishizawa, M.; Kawata, Y.; Yamamoto, A.; Shirakawa, M.; Scheler, U.; Sugase, K. High-sensitivity Rheo-NMR spectroscopy for protein studies. Anal. Chem. 2017, 89, 7286–7290. [Google Scholar] [CrossRef] [PubMed]
- Elam, J.S.; Taylor, A.B.; Strange, R.; Antonyuk, S.; Doucette, P.A.; Rodriguez, J.A.; Hasnain, S.S.; Hayward, L.J.; Valentine, J.S.; Yeates, T.O.; et al. Amyloid-like filaments and water-filled nanotubes formed by SOD1 mutant proteins linked to familial ALS. Nat. Struct. Mol. Biol. 2003, 10, 461–467. [Google Scholar] [CrossRef] [PubMed]
- Danielsson, J.; Kurnik, M.; Lang, L.; Oliveberg, M. Cutting off functional loops from homodimeric enzyme superoxide dismutase 1 (SOD1) leaves monomeric β-barrels. J. Biol. Chem. 2011, 286, 33070–33083. [Google Scholar] [CrossRef] [PubMed]
- Arosio, P.; Knowles, T.P.; Linse, S. On the lag phase in amyloid fibril formation. Phys. Chem. Chem. Phys. 2015, 17, 7606–7618. [Google Scholar] [CrossRef] [PubMed]
- Bolognesi, B.; Kumita, J.R.; Barros, T.P.; Esbjorner, E.K.; Luheshi, L.M.; Crowther, D.C.; Wilson, M.R.; Dobson, C.M.; Favrin, G.; Yerbury, J.J. ANS binding reveals common features of cytotoxic amyloid species. ACS Chem. Biol. 2010, 5, 735–740. [Google Scholar] [CrossRef] [PubMed]
- Baskakov, I.V.; Legname, G.; Baldwin, M.A.; Prusiner, S.B.; Cohen, F.E. Pathway complexity of prion protein assembly into amyloid. J. Biol. Chem. 2002, 277, 21140–21148. [Google Scholar] [CrossRef] [PubMed]
- Mena, R.; Edwards, P.; Pérez-Olvera, O.; Wischik, C.M. Monitoring pathological assembly of tau and β-amyloid proteins in Alzheimer’s disease. Acta Neuropathol. 1995, 89, 50–56. [Google Scholar] [CrossRef] [PubMed]
- McParland, V.J.; Kad, N.M.; Kalverda, A.P.; Brown, A.; Kirwin-Jones, P.; Hunter, M.G.; Sunde, M.; Radford, S.E. Partially unfolded states of β2-microglobulin and amyloid formation in vitro. Biochemistry 2000, 39, 8735–8746. [Google Scholar] [CrossRef] [PubMed]
- Iwakawa, N.; Morimoto, D.; Walinda, E.; Sugase, K.; Shirakawa, M. Backbone resonance assignments of monomeric SOD1 in dilute and crowded environments. Biomol. NMR Assign. 2017, 11, 81–84. [Google Scholar] [CrossRef] [PubMed]
- Hwang, T.-L.; Shaka, A.J. Water suppression that works. Excitation sculpting using arbitrary wave-forms and pulsed-field gradients. J. Magn. Reson. A 1995, 112, 275–279. [Google Scholar] [CrossRef]
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Iwakawa, N.; Morimoto, D.; Walinda, E.; Kawata, Y.; Shirakawa, M.; Sugase, K. Real-Time Observation of the Interaction between Thioflavin T and an Amyloid Protein by Using High-Sensitivity Rheo-NMR. Int. J. Mol. Sci. 2017, 18, 2271. https://doi.org/10.3390/ijms18112271
Iwakawa N, Morimoto D, Walinda E, Kawata Y, Shirakawa M, Sugase K. Real-Time Observation of the Interaction between Thioflavin T and an Amyloid Protein by Using High-Sensitivity Rheo-NMR. International Journal of Molecular Sciences. 2017; 18(11):2271. https://doi.org/10.3390/ijms18112271
Chicago/Turabian StyleIwakawa, Naoto, Daichi Morimoto, Erik Walinda, Yasushi Kawata, Masahiro Shirakawa, and Kenji Sugase. 2017. "Real-Time Observation of the Interaction between Thioflavin T and an Amyloid Protein by Using High-Sensitivity Rheo-NMR" International Journal of Molecular Sciences 18, no. 11: 2271. https://doi.org/10.3390/ijms18112271
APA StyleIwakawa, N., Morimoto, D., Walinda, E., Kawata, Y., Shirakawa, M., & Sugase, K. (2017). Real-Time Observation of the Interaction between Thioflavin T and an Amyloid Protein by Using High-Sensitivity Rheo-NMR. International Journal of Molecular Sciences, 18(11), 2271. https://doi.org/10.3390/ijms18112271