Macrophage Migration Inhibitory Factor Predicts Outcome in Complex Aortic Surgery
Abstract
:1. Introduction
2. Results
2.1. Study Population
2.2. Complications, Re-Interventions, and Mortality
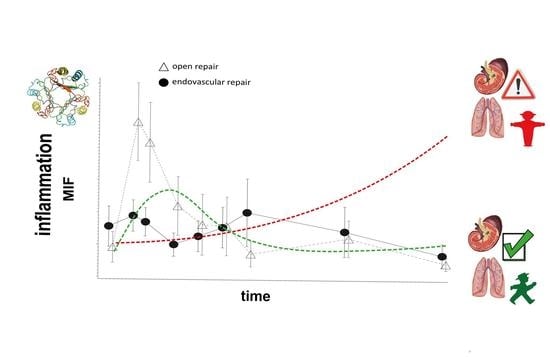
2.3. Higher MIF Release after OR vs. ER
2.4. Survival Rate and Discharge Modality
2.5. Acute Kidney Injury
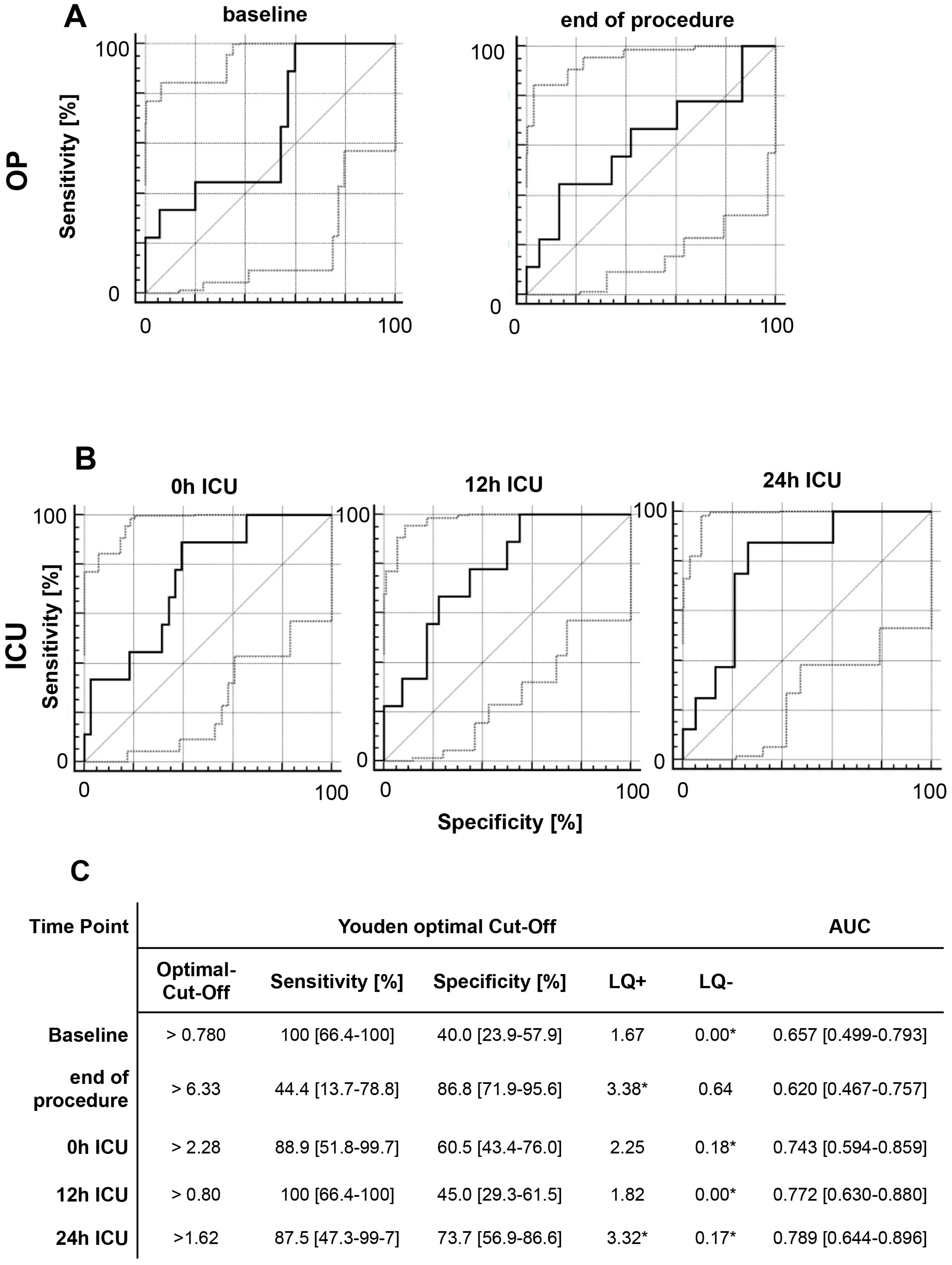
2.6. Multivariate Analysis of MIF Release Following OR and ER
3. Discussion
4. Material and Methods
4.1. Data Collection
4.2. Statistical Analyses
5. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
Abbreviations
| AG | Study concept, data collection, informed consent, statistical analysis, interpretation, writing, revision |
| CS | Statistical analysis, writing, revision |
| AF | Statistical analysis, writing, revision |
| TS | Interpretation, revision |
| LM | Data collection, revision |
| JK | Revision |
| GS | Data collection, revision |
| GM | Study concept, informed consent |
| MJ | Study concept, informed consent |
| JG | Statistical analysis, writing, revision |
References
- Schober, A.; Bernhagen, J.; Weber, C. Chemokine-like functions of MIF in atherosclerosis. J. Mol. Med. (Berl.) 2008, 86, 761–770. [Google Scholar] [CrossRef] [PubMed]
- Asare, Y.; Schmitt, M.; Bernhagen, J. The vascular biology of macrophage migration inhibitory factor (MIF). Expression and effects in inflammation, atherogenesis and angiogenesis. Thromb. Haemost. 2013, 109, 391–398. [Google Scholar] [CrossRef] [PubMed]
- Payen, D.; Lukaszewicz, A.C.; Legrand, M.; Gayat, E.; Faivre, V.; Megarbane, B.; Azoulay, E.; Fieux, F.; Charron, D.; Loiseau, P.; et al. A multicentre study of acute kidney injury in severe sepsis and septic shock: Association with inflammatory phenotype and HLA genotype. PLoS ONE 2012, 7, e35838. [Google Scholar] [CrossRef] [PubMed]
- Stefaniak, J.; Schiefer, J.; Miller, E.J.; Krenn, C.G.; Baron, D.M.; Faybik, P. Macrophage migration inhibitory factor as a potential predictor for requirement of renal replacement therapy after orthotopic liver transplantation. Liver Transpl. 2015, 21, 662–669. [Google Scholar] [CrossRef] [PubMed]
- Al-Abed, Y.; Dabideen, D.; Aljabari, B.; Valster, A.; Messmer, D.; Ochani, M.; Tanovic, M.; Ochani, K.; Bacher, M.; Nicoletti, F.; et al. ISO-1 binding to the tautomerase active site of MIF inhibits its pro-inflammatory activity and increases survival in severe sepsis. J. Biol. Chem. 2005, 280, 36541–36544. [Google Scholar] [CrossRef] [PubMed]
- Kleemann, R.; Hausser, A.; Geiger, G.; Mischke, R.; Burger-Kentischer, A.; Flieger, O.; Johannes, F.J.; Roger, T.; Calandra, T.; Kapurniotu, A.; et al. Intracellular action of the cytokine MIF to modulate AP-1 activity and the cell cycle through Jab1. Nature 2000, 408, 211–216. [Google Scholar] [PubMed]
- Stoppe, C.; Grieb, G.; Rossaint, R.; Simons, D.; Coburn, M.; Gotzenich, A.; Strussmann, T.; Pallua, N.; Bernhagen, J.; Rex, S. High postoperative blood levels of macrophage migration inhibitory factor are associated with less organ dysfunction in patients after cardiac surgery. Mol. Med. 2012, 18, 843–850. [Google Scholar] [CrossRef] [PubMed]
- Roger, T.; Delaloye, J.; Chanson, A.L.; Giddey, M.; Le Roy, D.; Calandra, T. Macrophage migration inhibitory factor deficiency is associated with impaired killing of gram-negative bacteria by macrophages and increased susceptibility to Klebsiella pneumoniae sepsis. J. Infect. Dis. 2013, 207, 331–339. [Google Scholar] [CrossRef] [PubMed]
- Stoppe, C.; Rex, S.; Goetzenich, A.; Kraemer, S.; Emontzpohl, C.; Soppert, J.; Averdunk, L.; Sun, Y.; Rossaint, R.; Lue, H.; et al. Interaction of MIF Family Proteins in Myocardial Ischemia/Reperfusion Damage and Their Influence on Clinical Outcome of Cardiac Surgery Patients. Antioxid. Redox Signal. 2015, 23, 865–879. [Google Scholar] [CrossRef] [PubMed]
- Jacobs, M.J.; Mommertz, G.; Koeppel, T.A.; Langer, S.; Nijenhuis, R.J.; Mess, W.H.; Schurink, G.W. Surgical repair of thoracoabdominal aortic aneurysms. J. Cardiovasc. Surg. (Torino) 2007, 48, 49–58. [Google Scholar] [PubMed]
- Svensson, L.G.; Crawford, E.S.; Hess, K.R.; Coselli, J.S.; Safi, H.J. Experience with 1509 patients undergoing thoracoabdominal aortic operations. J. Vasc. Surg. 1993, 17, 357–368. [Google Scholar] [CrossRef]
- Coselli, J.S.; LeMaire, S.A.; Preventza, O.; de la Cruz, K.I.; Cooley, D.A.; Price, M.D.; Stolz, A.P.; Green, S.Y.; Arredondo, C.N.; Rosengart, T.K. Outcomes of 3309 thoracoabdominal aortic aneurysm repairs. J. Thorac. Cardiovasc. Surg. 2016, 151, 1323–1338. [Google Scholar] [CrossRef] [PubMed]
- Jacobs, M.J.; Schurink, G.W. Commentary on “Short-term outcome of spinal cord ischemia after endovascular repair of thoracoabdominal aortic aneurysms”. Eur. J. Vasc. Endovasc. Surg. 2015, 49, 410–411. [Google Scholar] [CrossRef] [PubMed]
- Dias, N.V.; Sonesson, B.; Kristmundsson, T.; Holm, H.; Resch, T. Short-term outcome of spinal cord ischemia after endovascular repair of thoracoabdominal aortic aneurysms. Eur. J. Vasc. Endovasc. Surg. 2015, 49, 403–409. [Google Scholar] [CrossRef] [PubMed]
- Aftab, M.; Coselli, J.S. Renal and visceral protection in thoracoabdominal aortic surgery. J. Thorac. Cardiovasc. Surg. 2014, 148, 2963–2966. [Google Scholar] [CrossRef] [PubMed]
- Hanssen, S.J.; Derikx, J.P.; Vermeulen Windsant, I.C.; Heijmans, J.H.; Koeppel, T.A.; Schurink, G.W.; Buurman, W.A.; Jacobs, M.J. Visceral injury and systemic inflammation in patients undergoing extracorporeal circulation during aortic surgery. Ann. Surg. 2008, 248, 117–125. [Google Scholar] [CrossRef] [PubMed]
- Mommertz, G.; Langer, S.; Koeppel, T.A.; Schurink, G.W.; Mess, W.H.; Jacobs, M.J. Brain and spinal cord protection during simultaneous aortic arch and thoracoabdominal aneurysm repair. J. Vasc. Surg. 2009, 49, 886–892. [Google Scholar] [CrossRef] [PubMed]
- Verzini, F.; Loschi, D.; De Rango, P.; Ferrer, C.; Simonte, G.; Coscarella, C.; Pogany, G.; Cao, P. Current results of total endovascular repair of thoracoabdominal aortic aneurysms. J. Cardiovasc. Surg. (Torino) 2014, 55, 9–19. [Google Scholar] [PubMed]
- Bisdas, T.; Panuccio, G.; Sugimoto, M.; Torsello, G.; Austermann, M. Risk factors for spinal cord ischemia after endovascular repair of thoracoabdominal aortic aneurysms. J. Vasc. Surg. 2015, 61, 1408–1416. [Google Scholar] [CrossRef] [PubMed]
- Herder, C.; Illig, T.; Baumert, J.; Muller, M.; Klopp, N.; Khuseyinova, N.; Meisinger, C.; Martin, S.; Thorand, B.; Koenig, W. Macrophage migration inhibitory factor (MIF) and risk for coronary heart disease: Results from the MONICA/KORA Augsburg case-cohort study, 1984–2002. Atherosclerosis 2008, 200, 380–388. [Google Scholar] [CrossRef] [PubMed]
- Gong, Z.; Xing, S.; Zheng, F.; Xing, Q. Increased expression of macrophage migration inhibitory factor in aorta of patients with coronary atherosclerosis. J. Cardiovasc. Surg. 2015, 56, 631–637. [Google Scholar]
- Stoppe, C.; Werker, T.; Rossaint, R.; Dollo, F.; Lue, H.; Wonisch, W.; Menon, A.; Goetzenich, A.; Bruells, C.S.; Coburn, M.; et al. What is the significance of perioperative release of macrophage migration inhibitory factor in cardiac surgery? Antioxid. Redox Signal. 2013, 19, 231–239. [Google Scholar] [CrossRef] [PubMed]
- Koga, K.; Kenessey, A.; Powell, S.R.; Sison, C.P.; Miller, E.J.; Ojamaa, K. Macrophage migration inhibitory factor provides cardioprotection during ischemia/reperfusion by reducing oxidative stress. Antioxid. Redox Signal. 2011, 14, 1191–1202. [Google Scholar] [CrossRef] [PubMed]
- Miller, E.J.; Li, J.; Leng, L.; McDonald, C.; Atsumi, T.; Bucala, R.; Young, L.H. Macrophage migration inhibitory factor stimulates AMP-activated protein kinase in the ischaemic heart. Nature 2008, 451, 578–582. [Google Scholar] [CrossRef] [PubMed]
- Fiane, A.E.; Videm, V.; Lingaas, P.S.; Heggelund, L.; Nielsen, E.W.; Geiran, O.R.; Fung, M.; Mollnes, T.E. Mechanism of complement activation and its role in the inflammatory response after thoracoabdominal aortic aneurysm repair. Circulation 2003, 108, 849–856. [Google Scholar] [CrossRef] [PubMed]
- Welborn, M.B.; Oldenburg, H.S.; Hess, P.J.; Huber, T.S.; Martin, T.D.; Rauwerda, J.A.; Wesdorp, R.I.; Espat, N.J.; Copeland, E.M., 3rd; Moldawer, L.L.; et al. The relationship between visceral ischemia, proinflammatory cytokines, and organ injury in patients undergoing thoracoabdominal aortic aneurysm repair. Crit. Care Med. 2000, 28, 3191–3197. [Google Scholar] [CrossRef] [PubMed]
- Moris, D.N.; Kontos, M.I.; Mantonakis, E.I.; Athanasiou, A.K.; Spartalis, E.D.; Bakoyiannis, C.N.; Chrousos, G.P.; Georgopoulos, S.E. Concept of the aortic aneurysm repair-related surgical stress: A review of the literature. Int. J. Clin. Exp. Med. 2014, 7, 2402–2412. [Google Scholar] [PubMed]
- Swartbol, P.; Truedsson, L.; Norgren, L. The inflammatory response and its consequence for the clinical outcome following aortic aneurysm repair. Eur. J. Vasc. Endovasc. Surg. 2001, 21, 393–400. [Google Scholar] [CrossRef] [PubMed]
- White, D.A.; Su, Y.; Kanellakis, P.; Kiriazis, H.; Morand, E.F.; Bucala, R.; Dart, A.M.; Gao, X.M.; Du, X.J. Differential roles of cardiac and leukocyte derived macrophage migration inhibitory factor in inflammatory responses and cardiac remodelling post myocardial infarction. J. Mol. Cell. Cardiol. 2014, 69, 32–42. [Google Scholar] [CrossRef] [PubMed]
- Simons, D.; Grieb, G.; Hristov, M.; Pallua, N.; Weber, C.; Bernhagen, J.; Steffens, G. Hypoxia-induced endothelial secretion of macrophage migration inhibitory factor and role in endothelial progenitor cell recruitment. J. Cell. Mol. Med. 2011, 15, 668–678. [Google Scholar] [CrossRef] [PubMed]
- Nano, G.; Occhiuto, M.T.; Stegher, S.; Malacrida, G.; Cova, M.; Righini, P.; Tealdi, D.G.; Mazzaccaro, D. Postimplantation syndrome after endovascular aortic repair using the Anaconda endograft. Ann. Vasc. Surg. 2014, 28, 1409–1415. [Google Scholar] [CrossRef] [PubMed]
- Arnaoutoglou, E.; Kouvelos, G.; Papa, N.; Kallinteri, A.; Milionis, H.; Koulouras, V.; Matsagkas, M. Prospective evaluation of post-implantation inflammatory response after EVAR for AAA: Influence on patients’ 30 day outcome. Eur. J. Vasc. Endovasc. Surg. 2015, 49, 175–183. [Google Scholar] [CrossRef] [PubMed]
- Pohl, J.; Hendgen-Cotta, U.B.; Stock, P.; Luedike, P.; Rassaf, T. Elevated MIF-2 levels predict mortality in critically ill patients. J. Crit. Care 2017, 40, 52–57. [Google Scholar] [CrossRef] [PubMed]
- Lue, H.; Kleemann, R.; Calandra, T.; Roger, T.; Bernhagen, J. Macrophage migration inhibitory factor (MIF): Mechanisms of action and role in disease. Microbes Infect. 2002, 4, 449–460. [Google Scholar] [CrossRef]
- Pohl, J.; Papathanasiou, M.; Heisler, M.; Stock, P.; Kelm, M.; Hendgen-Cotta, U.B.; Rassaf, T.; Luedike, P. Renal replacement therapy neutralizes elevated MIF levels in septic shock. J. Intensive Care 2016, 4, 39. [Google Scholar] [CrossRef] [PubMed]
- Van Biesen, W.; Vanholder, R.; Lameire, N. Defining acute renal failure: RIFLE and beyond. Clin. J. Am. Soc. Nephrol. 2006, 1, 1314–1319. [Google Scholar] [CrossRef] [PubMed]
- Mehta, R.L.; Kellum, J.A.; Shah, S.V.; Molitoris, B.A.; Ronco, C.; Warnock, D.G.; Levin, A. Acute Kidney Injury Network: Report of an initiative to improve outcomes in acute kidney injury. Crit. Care 2007, 11, R31. [Google Scholar] [CrossRef] [PubMed]
- Sackett, D.L. Clinical epidemiology. what, who, and whither. J. Clin. Epidemiol. 2002, 55, 1161–1166. [Google Scholar] [CrossRef]



| All Patients | Open Surgery | Endovascular Surgery | P Value # | |
|---|---|---|---|---|
| (n = 52) | (44.2%; n = 29) | (55.8%; n = 23) | ||
| Patients characteristics and treatment | ||||
| Age, years | 64.5 ± 10.4 | 59.8 ± 10.7 | 70.48 ± 6.17 | 0.0001 * |
| Male gender | 39 (75.0%) | 22 (75.9%) | 17 (73.9%) | 0.8719 |
| BMI | 27.1 ± 3.9 | 26.4 ± 4.0 | 28.0 ± 3.8 | 0.1530 |
| Smoker | 22 (42.3%) | 10 (34.5%) | 12 (52.2%) | 0.1997 |
| Coronary artery disease | 21 (40.4%) | 13 (44.8%) | 8 (34.8%) | 0.5729 |
| Diabetes | 6 (11.54%) | 2 (6.9%) | 4 (17.4%) | 0.3870 |
| Hypertension | 47 (90.4%) | 27 (93.1%) | 20 (87.0%) | 0.6443 |
| Chronic kidney disease | 7 (13.5%) | 2 (6.9%) | 5 (21.7%) | 0.2192 |
| Procedure characteristics | ||||
| Operation time, min | 401.3 ± 99.0 | 403.4 ± 96.4 | 398.7 ± 103.9 | 0.8680 |
| Total ventilation time, min | 980 (IQA 570–1980) | 1212 (IQA 630–2372) | 885 (IQA 485–1590) | 0.2351 |
| In-hospital stay, days | 21 (IQA 11–32) | 26 (IQA 18–37) | 13.5 (IQA 9–23) | 0.2518 |
| ICU stay, days | 3 (IQA 1–7) | 5 IQA 1.5–7) | 2 (IQA 1–5) | 0.1060 |
| Baseline MIF, ng/mL | 1.40 (IQA 0.63–2.63) (Min: 0.09, Max: 12.75) | 1.09 (IQA 0.51–2.12) (Min: 0.09; Max: 10.45) | 1.93 (IQA 1.0–2.65) (Min: 0.24; Max: 12.75) | 0.1439 |
| Baseline serum creatinine, mg/dL | 0.99 (IQA: 0.86–1.20) | 0.92 (IQA: 0.85–1.05) | 1.14 (IQA: 0.91–1.28) | 0.0389 * |
| Morbidity and mortality | ||||
| AKI | 14 (26.9%) | 8 (27.6%) | 6 (26.1%) | 0.2449 |
| Need for temporary dialysis | 11 (21.2%) | 5 (17.2%) | 6 (26.1%) | 0.5066 |
| Permanent need for dialysis | 3 (5.7%) | 2 (6.8%) | 1 (4.3%) | 1 |
| SCI | 3 (5.7%) | 2 (6.8%) | 1 (4.3%) | 1 |
| Myocardial infarction | 0 | 0 | 0 | - |
| Pneumonia | 10 (19.2%) | 7 (24.1%) | 3 (13.0%) | 0.48 |
| Tracheotomy | 10 (19.2%) | 7 (24.1%) | 3 (13.0%) | 0.4815 |
| Sepsis | 6 (11.5%) | 4 (13.7%) | 2 (8.6%) | 0.68 |
| Surgical revisions | 6 (11.5%) | 4 (13.7%) | 2 (8.6%) | 0.68 |
| 90-Day mortality | 5 (10.42%) | 4 (13.8%) | 1 (4.4%) | 0.3686 |
| Univariate Analysis of log(MIF) over Time | |||||
|---|---|---|---|---|---|
| Den DF | F Value | P-Value | Slope Estimator | SD (Estimator) | |
| Continuous variable | |||||
| Age | 47.7 | 0.03 | 0.8604 | −0.00239 | 0.01351 |
| BMI | 47.8 | 0.34 | 0.5605 | −0.02082 | 0.03551 |
| Baseline MIF, logarithmized | 42.2 | 11.44 | 0.0016 * | 0.4456 | 0.1317 |
| Categorical variable | |||||
| Gender (male) | 48 | 0.07 | 0.7942 | −0.0857 | 0.3267 |
| Endovascular repair | 48.2 | 0.01 | 0.9172 | −0.02937 | 0.2809 |
| Continuous variable (repeated measurements) | |||||
| Serum creatinine | 171 | 3.11 | 0.0796 | 0.2094 | 0.1187 |
| Apache II | 137 | 12.92 | 0.0005 * | 0.04273 | 0.01189 |
| Den DF | F-Value | P | |
|---|---|---|---|
| Operation method (endovascular) | 41.5 | 0.92 | 0.3421 |
| Baseline value (MIF), logarithmized | 41.3 | 12.5 | 0.001 * |
| Time point (Reference: time point 4) | 217 | 12.37 | <0.0001 * |
| Different time points during surgery | 217 | 2.65 | 0.0121 * |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gombert, A.; Stoppe, C.; Foldenauer, A.C.; Schuerholz, T.; Martin, L.; Kalder, J.; Schälte, G.; Marx, G.; Jacobs, M.; Grommes, J. Macrophage Migration Inhibitory Factor Predicts Outcome in Complex Aortic Surgery. Int. J. Mol. Sci. 2017, 18, 2374. https://doi.org/10.3390/ijms18112374
Gombert A, Stoppe C, Foldenauer AC, Schuerholz T, Martin L, Kalder J, Schälte G, Marx G, Jacobs M, Grommes J. Macrophage Migration Inhibitory Factor Predicts Outcome in Complex Aortic Surgery. International Journal of Molecular Sciences. 2017; 18(11):2374. https://doi.org/10.3390/ijms18112374
Chicago/Turabian StyleGombert, Alexander, Christian Stoppe, Ann Christina Foldenauer, Tobias Schuerholz, Lukas Martin, Johannes Kalder, Gereon Schälte, Gernot Marx, Michael Jacobs, and Jochen Grommes. 2017. "Macrophage Migration Inhibitory Factor Predicts Outcome in Complex Aortic Surgery" International Journal of Molecular Sciences 18, no. 11: 2374. https://doi.org/10.3390/ijms18112374
APA StyleGombert, A., Stoppe, C., Foldenauer, A. C., Schuerholz, T., Martin, L., Kalder, J., Schälte, G., Marx, G., Jacobs, M., & Grommes, J. (2017). Macrophage Migration Inhibitory Factor Predicts Outcome in Complex Aortic Surgery. International Journal of Molecular Sciences, 18(11), 2374. https://doi.org/10.3390/ijms18112374