Analgesic Mechanisms of Antidepressants for Neuropathic Pain
Abstract
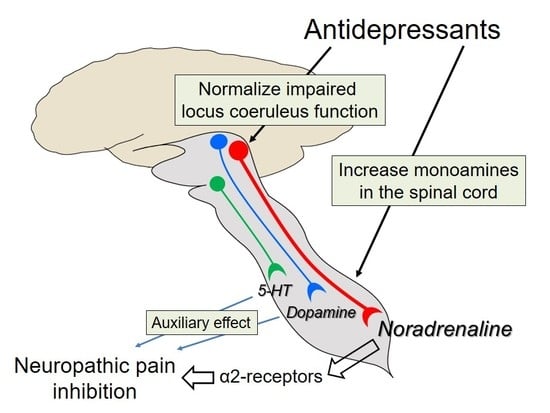
:1. Introduction
2. Effects of Antidepressants on Neuropathic Pain Differ from Their Effects on Depression
3. Noradrenaline Is Extremely Important for Inhibiting Neuropathic Pain
4. Noradrenaline Inhibits Neuropathic Pain in the Spinal Cord
5. Actions of Antidepressants on the Locus Coeruleus
6. The Role of 5-HT
7. The Role of Dopamine
8. Other Actions
9. Conclusions
Conflicts of Interest
References
- Finnerup, N.B.; Attal, N.; Haroutounian, S.; McNicol, E.; Baron, R.; Dworkin, R.H.; Gilron, I.; Haanpää, M.; Hansson, P.; Jensen, T.S.; et al. Pharmacotherapy for neuropathic pain in adults: A systematic review and meta-analysis. Lancet Neurol. 2015, 14, 162–173. [Google Scholar] [CrossRef]
- Finnerup, N.B.; Sindrup, S.H.; Jensen, T.S. The evidence for pharmacological treatment of neuropathic pain. Pain 2010, 150, 573–581. [Google Scholar] [CrossRef] [PubMed]
- Attal, N.; Cruccu, G.; Baron, R.; Haanpää, M.; Hansson, P.; Jensen, T.S.; Nurmikko, T. EFNS guidelines on the pharmacological treatment of neuropathic pain: 2010 revision. Eur. J. Neurol. 2010, 17, 1113–1123. [Google Scholar] [CrossRef] [PubMed]
- Dworkin, R.H.; O’Connor, B.; Backonja, M.; Farrar, J.T.; Finnerup, N.B.; Jensen, T.S.; Kalso, E.A.; Loeser, J.D.; Miaskowski, C.; Nurmikko, T.J.; et al. Pharmacologic management of neuropathic pain: Evidence-based recommendations. Pain 2007, 132, 237–251. [Google Scholar] [CrossRef] [PubMed]
- Calandre, E.P.; Rico-Villademoros, F.; Slim, M. An update on pharmacotherapy for the treatment of fibromyalgia. Expert Opin. Pharmacother. 2015, 16, 1347–1368. [Google Scholar] [CrossRef] [PubMed]
- Fishbain, D.A.; Cutler, R.B.; Rosomoff, H.L.; Rosomoff, R.S. Do antidepressants have an analgesic effect in psychogenic pain and somatoform pain disorder? A meta-analysis. Psychosom. Med. 1998, 60, 503–509. [Google Scholar] [CrossRef] [PubMed]
- Onghena, P.; van houdenhove, B. Antidepressant-induced analgesia in chronic non-malignant pain: A meta-analysis of 39 placebo-controlled studies. Pain 1992, 49, 205–219. [Google Scholar] [CrossRef]
- Micó, J.A.; Ardid, D.; Berrocoso, E.; Eschalier, A. Antidepressants and pain. Trends Pharmacol. Sci. 2006, 27, 348–354. [Google Scholar] [CrossRef] [PubMed]
- Dharmshaktu, P.; Tayal, V.; Kalra, B.S. Efficacy of antidepressants as analgesics: A review. J. Clin. Pharmacol. 2012, 52, 6–17. [Google Scholar] [CrossRef] [PubMed]
- Berton, O.; Nestler, E.J. New approaches to antidepressant drug discovery: Beyond monoamines. Nat. Rev. Neurosci. 2006, 7, 137–151. [Google Scholar] [CrossRef] [PubMed]
- Sindrup, S.H.; Otto, M.; Finnerup, N.B.; Jensen, T.S. Antidepressants in the treatment of neuropathic pain. Basic Clin. Pharmacol. Toxicol. 2005, 96, 399–409. [Google Scholar] [CrossRef] [PubMed]
- McQuay, H.J.; Tramèr, M.; Nye, B.A.; Carroll, D.; Wiffen, P.J.; Moore, R.A. A systematic review of antidepressants in neuropathic pain. Pain 1996, 68, 217–227. [Google Scholar] [CrossRef]
- Cook, R.J.; Sackett, D.L. The number needed to treat: A clinically useful measure of treatment effect. BMJ 1996, 310, 452–454. [Google Scholar] [CrossRef]
- Finnerup, N.B.; Otto, M.; McQuay, H.J.; Jensen, T.S.; Sindrup, S.H. Algorithm for neuropathic pain treatment: An evidence based proposal. Pain 2005, 118, 289–305. [Google Scholar] [CrossRef] [PubMed]
- Pan, H.L.; Wu, Z.Z.; Zhou, H.Y.; Chen, S.R.; Zhang, H.M.; Li, D.P. Modulation of pain transmission by, G-protein-coupled receptors. Pharmacol. Ther. 2008, 117, 141–161. [Google Scholar] [CrossRef] [PubMed]
- Kimura, M.; Saito, S.; Obata, H. Dexmedetomidine decreases hyperalgesia in neuropathic pain by increasing acetylcholine in the spinal cord. Neurosci. Lett. 2012, 529, 70–74. [Google Scholar] [CrossRef] [PubMed]
- Paqueron, X.; Conklin, D.; Eisenach, J.C. Plasticity in action of intrathecal clonidine to mechanical but not thermal nociception after peripheral nerve injury. Anesthesiology 2003, 99, 199–204. [Google Scholar] [CrossRef] [PubMed]
- Bantel, C.; Eisenach, J.C.; Duflo, F.; Tobin, J.R.; Childers, S.R. Spinal nerve ligation increases alpha2-adrenergic receptor, G-protein coupling in the spinal cord. Brain Res. 2005, 1038, 76–82. [Google Scholar] [CrossRef] [PubMed]
- Eisenach, J.C.; Zhang, Y.; Duflo, F. Alpha2-adrenoceptors inhibit the intracellular, Ca2+ response to electrical stimulation in normal and injured sensory neurons, with increased inhibition of calcitonin gene-related peptide expressing neurons after injury. Neuroscience 2005, 131, 189–197. [Google Scholar] [CrossRef] [PubMed]
- Pan, H.L.; Chen, S.R.; Eisenach, J.C. Intrathecal clonidine alleviates allodynia in neuropathic rats: Interaction with spinal muscarinic and nicotinic receptors. Anesthesiology 1999, 90, 509–514. [Google Scholar] [CrossRef] [PubMed]
- Paqueron, X.; Li, X.; Bantel, C.; Tobin, J.R.; Voytko, M.L.; Eisenach, J.C. An obligatory role for spinal cholinergic neurons in the antiallodynic effects of clonidine after peripheral nerve injury. Anesthesiology 2001, 94, 1074–1081. [Google Scholar] [CrossRef] [PubMed]
- Obata, H.; Li, X.; Eisenach, J.C. Alpha2-Adrenoceptor activation by clonidine enhances stimulation-evoked acetylcholine release from spinal cord tissue after nerve ligation in rats. Anesthesiology 2005, 102, 657–662. [Google Scholar] [CrossRef] [PubMed]
- Hayashida, K.; Eisenach, J.C. Spinal alpha2-adrenoceptor-mediated analgesia in neuropathic pain reflects brain-derived nerve growth factor and changes in spinal cholinergic neuronal function. Anesthesiology 2010, 113, 406–412. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.H.; Chung, J.M. An experimental model for peripheral neuropathy produced by segmental spinal nerve ligation in the rat. Pain 1992, 50, 355–563. [Google Scholar] [CrossRef]
- Baba, H.; Kohno, T.; Okamoto, M; Goldstein, P.A.; Shimoji, K.; Yoshimura, M. Muscarinic facilitation of, GABA release in substantia gelatinosa of the rat spinal dorsal horn. J. Physiol. 1998, 508, 83–93. [Google Scholar] [CrossRef] [PubMed]
- Kimura, M.; Hayashida, K.; Eisenach, J.C.; Saito, S.; Obata, H. Relief of hypersensitivity after nerve injury from systemic donepezil involves spinal cholinergic and γ-aminobutyric acid mechanisms. Anesthesiology 2013, 118, 173–180. [Google Scholar] [CrossRef] [PubMed]
- Ito, S.; Suto, T.; Saito, S.; Obata, H. Repeated administration of duloxetine suppresses neuropathic pain by accumulating effects of noradrenaline in the spinal cord. Anesth. Analg. 2017. [Google Scholar] [CrossRef] [PubMed]
- Hiroki, T.; Suto, T.; Saito, S.; Obata, H. Repeated administration of amitriptyline in neuropathic pain: Modulation of the noradrenergic descending inhibitory system. Anesth. Analg. 2017, 125, 1281–1288. [Google Scholar] [CrossRef] [PubMed]
- Nakajima, K.; Obata, H.; Iriuchijima, N.; Saito, S. An increase in spinal cord noradrenaline is a major contributor to the antihyperalgesic effect of antidepressants after peripheral nerve injury in the rat. Pain 2012, 153, 990–997. [Google Scholar] [CrossRef] [PubMed]
- Andersen, J.; Stuhr-Hansen, N.; Zachariassen, L.G.; Koldsø, H.; Schiøtt, B.; Strømgaard, K.; Kristensen, A.S. Molecular basis for selective serotonin reuptake inhibition by the antidepressant agent fluoxetine (Prozac). Mol. Pharmacol. 2014, 85, 703–714. [Google Scholar] [CrossRef] [PubMed]
- Owens, M.J.; Knight, D.L.; Nemeroff, C.B. Paroxetine binding to the rat norepinephrine transporter in vivo. Biol. Psychiatry 2000, 47, 842–845. [Google Scholar] [CrossRef]
- Chen, M.; Hoshino, H.; Saito, S.; Yang, Y.; Obata, H. Spinal dopaminergic involvement in the antihyperalgesic effect of antidepressants in a rat model of neuropathic pain. Neurosci. Lett. 2017, 649, 116–123. [Google Scholar] [CrossRef] [PubMed]
- Baker, K.G.; Tork, I.; Hornung, J.P.; Halasz, P. The human locus coeruleus complex: An immunohistochemical and three dimensional reconstruction study. Exp. Brain Res. 1989, 77, 257–270. [Google Scholar] [CrossRef] [PubMed]
- Goldman, G.; Coleman, P.D. Neuron numbers in locus coeruleus do not change with age in, Fisher 344 rat. Neurobiol. Aging 1981, 2, 33–36. [Google Scholar]
- Aston-Jones, G.; Bloom, F.E. Activity of norepinephrine-containing locus coeruleus neurons in behaving rats anticipates fluctuations in the sleep-waking cycle. J. Neurosci. 1981, 1, 876–886. [Google Scholar] [PubMed]
- Berridge, C.W.; Waterhouse, B.D. The locus coeruleus-noradrenergic system: Modulation of behavioral state and state-dependent cognitive processes. Brain Res. Rev. 2003, 42, 33–84. [Google Scholar] [CrossRef]
- Borodovitsyna, O.; Flamini, M.; Chandler, D. Noradrenergic modulation of cognition in health and disease. Neural. Plast. 2017, 2017. [Google Scholar] [CrossRef] [PubMed]
- Tsuruoka, M.; Tamaki, J.; Maeda, M.; Hayashi, B.; Inoue, T. Biological implications of coeruleospinal inhibition of nociceptive processing in the spinal cord. Front. Integr. Neurosci. 2012, 6. [Google Scholar] [CrossRef] [PubMed]
- Pompeiano, O. Vasopressin in the locus coeruleus and dorsal pontine tegmentum affects posture and vestibulospinal reflexes. Prog. Brain Res. 1998, 119, 537–554. [Google Scholar] [PubMed]
- Szabadi, E. Functional neuroanatomy of the central noradrenergic system. J. Psychopharmacol. 2013, 27, 659–693. [Google Scholar] [CrossRef] [PubMed]
- Kimura, M.; Suto, T.; Morado-Urbina, C.E.; Peters, C.M.; Eisenach, J.C.; Hayashida, K. Impaired pain-evoked analgesia after nerve injury in rats reflects altered glutamate regulation in the locus coeruleus. Anesthesiology 2015, 123, 899–908. [Google Scholar] [CrossRef] [PubMed]
- Howorth, P.W.; Teschemacher, A.G.; Pickering, A.E. Retrograde adenoviral vector targeting of nociresponsive pontospinal noradrenergic neurons in the rat in vivo. J. Comp. Neurol. 2009, 512, 141–157. [Google Scholar] [CrossRef] [PubMed]
- Peters, C.M.; Hayashida, K.; Suto, T.; Houle, T.T.; Aschenbrenner, C.A.; Martin, T.J.; Eisenach, J.C. Individual differences in acute pain-induced endogenous analgesia predict time to resolution of postoperative pain in the rat. Anesthesiology 2015, 122, 895–907. [Google Scholar] [CrossRef] [PubMed]
- Matsuoka, H.; Suto, T.; Saito, S.; Obata, H. Amitriptyline, but not pregabalin, reverses the attenuation of noxious stimulus-induced analgesia after nerve injury in rats. Anesth. Analg. 2016, 123, 504–510. [Google Scholar] [CrossRef] [PubMed]
- Singewald, N.; Philippu, A. Release of neurotransmitters in the locus coeruleus. Prog. Neurobiol. 1998, 56, 237–267. [Google Scholar] [CrossRef]
- Mateo, Y.; Fernández-Pastor, B.; Meana, J.J. Acute and chronic effects of desipramine and clorgyline on alpha(2)-adrenoceptors regulating noradrenergic transmission in the rat brain: A dual-probe microdialysis study. Br. J. Pharmacol. 2001, 133, 1362–1370. [Google Scholar] [CrossRef] [PubMed]
- Grandoso, L.; Pineda, J.; Ugedo, L. Comparative study of the effects of desipramine and reboxetine on locus coeruleus neurons in rat brain slices. Neuropharmacology 2004, 46, 815–823. [Google Scholar] [CrossRef] [PubMed]
- Grant, M.M.; Weiss, J.M. Effects of chronic antidepressant drug administration and electroconvulsive shock on locus coeruleus electrophysiologic activity. Biol. Psychiatry 2001, 49, 117–129. [Google Scholar] [CrossRef]
- Alba-Delgado, C.; Mico, J.A.; Sánchez-Blázquez, P.; Berrocoso, E. Analgesic antidepressants promote the responsiveness of locus coeruleus neurons to noxious stimulation: Implications for neuropathic pain. Pain 2012, 153, 1438–1449. [Google Scholar] [CrossRef] [PubMed]
- Kajitani, N.; Hisaoka-Nakashima, K.; Morioka, N.; Okada-Tsuchioka, M.; Kaneko, M.; Kasai, M.; Shibasaki, C.; Nakata, Y.; Takebayashi, M. Antidepressant acts on astrocytes leading to an increase in the expression of neurotrophic/growth factors: Differential regulation of, FGF-2 by noradrenaline. PLoS ONE 2012, 7, e51197. [Google Scholar] [CrossRef] [PubMed]
- Reimers, J.M.; Loweth, J.A.; Wolf, M.E. BDNF contributes to both rapid and homeostatic alterations in, AMPA receptor surface expression innucleus accumbens medium spiny neurons. Eur. J. Neurosci. 2014, 39, 1159–1169. [Google Scholar] [CrossRef] [PubMed]
- Leventhal, L.; Smith, V.; Hornby, G.; Andree, T.H.; Brandt, M.R.; Rogers, K.E. Differential and synergistic effects of selective norepinephrine and serotonin reuptake inhibitors in rodent models of pain. J. Pharmacol. Exp. Ther. 2007, 320, 1178–1185. [Google Scholar] [CrossRef] [PubMed]
- Bardin, L. The complex role of serotonin and 5-HT receptors in chronic pain. Behav. Pharmacol. 2011, 22, 390–404. [Google Scholar] [CrossRef] [PubMed]
- Viguier, F.; Michot, B.; Hamon, M.; Bourgoin, S. Multiple roles of serotonin in pain control mechanisms—Implications of 5-HT7 and other 5-HT receptor types. Eur. J. Pharmacol. 2013, 716, 8–16. [Google Scholar] [CrossRef] [PubMed]
- Yoshimura, M.; Furue, H. Mechanisms for the anti-nociceptive actions of the descending noradrenergic and serotonergic systems in the spinal cord. J. Pharmacol. Sci. 2006, 101, 107–117. [Google Scholar] [CrossRef] [PubMed]
- Millan, M.J. Descending control of pain. Prog. Neurobiol. 2002, 66, 355–474. [Google Scholar] [CrossRef]
- Bliss, T.V.; Collingridge, G.L.; Kaang, B.K.; Zhuo, M. Synaptic plasticity in the anterior cingulate cortex in acute and chronic pain. Nat. Rev. Neurosci. 2016, 17, 485–496. [Google Scholar] [CrossRef] [PubMed]
- Fields, H.L.; Heinricher, M.M.; Mason, P. Neurotransmitters in nociceptive modulatory circuits. Annu. Rev. Neurosci. 1991, 14, 219–245. [Google Scholar] [CrossRef] [PubMed]
- Vanegas, H.; Schaible, H.G. Descending control of persistent pain: Inhibitory or facilitatory? Brain Res. Brain Res. Rev. 2004, 46, 295–309. [Google Scholar] [CrossRef] [PubMed]
- Kuner, R. Central mechanisms of pathological pain. Nat. Med. 2010, 16, 1258–1266. [Google Scholar] [CrossRef] [PubMed]
- Porreca, F.; Ossipov, M.H.; Gebhart, G.F. Chronic pain and medullary descending facilitation. Trends Neurosci. 2002, 25, 319–325. [Google Scholar] [CrossRef]
- Wei, F.; Dubner, R.; Zou, S.; Ren, K.; Bai, G.; Wei, D.; Guo, W. Molecular depletion of descending serotonin unmasks its novel facilitatory role in the development of persistent pain. J. Neurosci. 2010, 30, 8624–8636. [Google Scholar] [CrossRef] [PubMed]
- Rahman, W.; Suzuki, R.; Webber, M.; Hunt, S.P.; Dickenson, A.H. Depletion of endogenous spinal 5-HT attenuates the behavioural hypersensitivity to mechanical and cooling stimuli induced by spinal nerve ligation. Pain 2006, 123, 264–274. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, R.; Rahman, W.; Hunt, S.P.; Dickenson, A.H. Descending facilitatory control of mechanically evoked responses is enhanced in deep dorsal horn neurones following peripheral nerve injury. Brain Res. 2004, 1019, 68–76. [Google Scholar] [CrossRef] [PubMed]
- Bannister, K.; Patel, R.; Goncalves, L.; Townson, L.; Dickenson, A.H. Diffuse noxious inhibitory controls and nerve injury: Restoring an imbalance between descending monoamine inhibitions and facilitations. Pain 2015, 156, 1803–1811. [Google Scholar] [CrossRef] [PubMed]
- Avila-Rojas, S.H.; Velázquez-Lagunas, I.; Salinas-Abarca, A.B.; Barragán-Iglesias, P.; Pineda-Farias, J.B.; Granados-Soto, V. Role of spinal 5-HT5A, and 5-HT1A/1B/1D, receptors in neuropathic pain induced by spinal nerve ligation in rats. Brain Res. 2015, 1622, 377–385. [Google Scholar] [CrossRef] [PubMed]
- Okazaki, R.; Namba, H.; Yoshida, H.; Okai, H.; Miura, T.; Kawamura, M. The antiallodynic effect of, Neurotropin is mediated via activation of descending pain inhibitory systems in rats with spinal nerve ligation. Anesth. Analg. 2008, 107, 1064–1069. [Google Scholar] [CrossRef] [PubMed]
- Obata, H.; Saito, S.; Sasaki, M.; Ishizaki, K.; Goto, F. Antiallodynic effect of intrathecally administered 5-HT(2) agonists in rats with nerve ligation. Pain 2001, 90, 173–179. [Google Scholar] [CrossRef]
- Sasaki, M.; Obata, H.; Saito, S.; Goto, F. Antinociception with intrathecal alpha-methyl-5-hydroxytryptamine, a 5-hydroxytryptamine 2A/2C receptor agonist, in two rat models of sustained pain. Anesth. Analg. 2003, 96, 1072–1078. [Google Scholar] [CrossRef] [PubMed]
- Song, Z.; Meyerson, B.A.; Linderoth, B. Spinal 5-HT receptors that contribute to the pain-relieving effects of spinal cord stimulation in a rat model of neuropathy. Pain 2011, 152, 1666–1673. [Google Scholar] [CrossRef] [PubMed]
- Wattiez, A.S; Dupuis, A.; Privat, A.M.; Chalus, M.; Chapuy, E.; Aissouni, Y.; Eschalier, A.; Courteix, C. Disruption of 5-HT2A-PDZ protein interaction differently affects the analgesic efficacy of, SSRI, SNRI and, TCA in the treatment of traumatic neuropathic pain in rats. Neuropharmacology 2017, 125, 308–318. [Google Scholar] [CrossRef] [PubMed]
- Sałat, K.; Kołaczkowski, M.; Furgała, A.; Rojek, A.; Śniecikowska, J.; Varney, M.A.; Newman-Tancredi, A. Antinociceptive, antiallodynic and antihyperalgesic effects of the 5-HT1A receptor selective agonist, NLX-112 in mouse models of pain. Neuropharmacology 2017, 125, 181–188. [Google Scholar] [CrossRef]
- Fleetwood-walker, S.M.; Hope, P.J.; Mitchell, R. Antinociceptive actions of descending dopaminergic tracts on cat and rat dorsal horn somatosensory neurones. J. Physiol. 1988, 399, 335–348. [Google Scholar] [CrossRef] [PubMed]
- Taniguchi, W.; Nakatsuka, T.; Miyazaki, N.; Yamada, H.; Takeda, D.; Fujita, T.; Kumamoto, E.; Yoshida, M. In vivo patch-clamp analysis of dopaminergic antinociceptive actions on substantia gelatinosa neurons in the spinal cord. Pain 2011, 152, 95–105. [Google Scholar] [CrossRef] [PubMed]
- Hoshino, H.; Obata, H.; Nakajima, K.; Mieda, R.; Saito, S. The antihyperalgesic effects of intrathecal bupropion, a dopamine and noradrenaline reuptake inhibitor, in a rat model of neuropathic pain. Anesth. Analg. 2015, 120, 460–466. [Google Scholar] [CrossRef] [PubMed]
- Morón, J.A.; Brockington, A.; Wise, R.A.; Rocha, B.A.; Hope, B.T. Dopamine uptake through the norepinephrine transporter in brain regions with low levels of the dopamine transporter: Evidence from knock-out mouse lines. J. Neurosci. 2002, 22, 389–395. [Google Scholar] [PubMed]
- Taylor, B.K.; Joshi, C.; Uppal, H. Stimulation of dopamine, D2 receptors in the nucleus accumbens inhibits inflammatory pain. Brain. Res. 2003, 987, 135–143. [Google Scholar] [CrossRef]
- Moradi, M.; Yazdnian, M.; Haghparast, A. Role of dopamine, D2-like receptors within the ventral tegmental area and nucleus accumbens in antinociception induced by lateral hypothalamus stimulation. Behav. Brain Res. 2015, 292, 508–514. [Google Scholar] [CrossRef] [PubMed]
- Wakaizumi, K.; Kondo, T.; Hamada, Y.; Narita, M.; Kawabe, R.; Narita, H.; Watanabe, M.; Kato, S.; Senba, E.; Kobayashi, K.; et al. Involvement of mesolimbic dopaminergic network in neuropathic pain relief by treadmill exercise: A study for specific neural control with, Gi-DREADD in mice. Mol. Pain 2016, 12. [Google Scholar] [CrossRef] [PubMed]
- Kihara, T.; Ikeda, M. Effects of duloxetine, a new serotonin and norepinephrine uptake inhibitor, on extracellular monoamine levels in rat frontal cortex. J. Pharmacol. Exp. Ther. 1995, 272, 177–183. [Google Scholar] [PubMed]
- Magnusson, J.E.; Fisher, K. The involvement of dopamine in nociception: The role of, D(1) and, D(2) receptors in the dorsolateral striatum. Brain Res. 2000, 855, 260–266. [Google Scholar] [CrossRef]
- Ansah, O.B.; Leite-Almeida, H.; Wei, H.; Pertovaara, A. Striatal dopamine, D2 receptors attenuate neuropathic hypersensitivity in the rat. Exp. Neurol. 2007, 205, 536–546. [Google Scholar] [CrossRef] [PubMed]
- Dick, I.E.; Brochu, R.M.; Purohit, Y.; Kaczorowski, G.J.; Martin, W.J.; Priest, B.T. Sodium channel blockade may contribute to the analgesic efficacy of antidepressants. J. Pain 2007, 8, 315–324. [Google Scholar] [CrossRef] [PubMed]
- Sudoh, Y.; Cahoon, E.E.; Gerner, P.; Wang, G.K. Tricyclic antidepressants as long-acting local anesthetics. Pain 2003, 103, 49–55. [Google Scholar] [CrossRef]
- Kalso, E. Sodium channel blockers in neuropathic pain. Curr. Pharm. Des. 2005, 11, 3005–3011. [Google Scholar] [CrossRef] [PubMed]
- Devor, M. Sodium channels and mechanisms of neuropathic pain. J. Pain 2006, 7 (Suppl. S1), S3–S12. [Google Scholar] [CrossRef] [PubMed]
- Barygin, O.I.; Nagaeva, E.I.; Tikhonov, D.B.; Belinskaya, D.A.; Vanchakova, N.P.; Shestakova, N.N. Inhibition of the, NMDA and, AMPA receptor channels by antidepressants and antipsychotics. Brain Res. 2017, 1660, 58–66. [Google Scholar] [CrossRef] [PubMed]
- Kohno, T.; Kimura, M.; Sasaki, M.; Obata, H.; Amaya, F.; Saito, S. Milnacipran inhibits glutamatergic, N-methyl-d-aspartate receptor activity in spinal dorsal horn neurons. Mol. Pain 2012, 8. [Google Scholar] [CrossRef] [PubMed]
- Dickenson, A.H; Chapman, V.; Green, G.M. The pharmacology of excitatory and inhibitory amino acid-mediated events in the transmission and modulation of pain in the spinal cord. Gen. Pharmacol. 1997, 28, 633–638. [Google Scholar] [CrossRef]
- Herrero, J.F.; Laird, J.M.; López-García, J.A. Wind-up of spinal cord neurones and pain sensation: Much ado about something? Prog. Neurobiol. 2000, 61, 169–203. [Google Scholar] [CrossRef]
- Yokogawa, F.; Kiuchi, Y.; Ishikawa, Y.; Otsuka, N.; Masud, Y, Oguchi; Hosoyamada, A. An investigation of monoamine receptors involved in antinociceptive effects of antidepressants. Anesth. Analg. 2002, 95, 163–168. [Google Scholar] [CrossRef] [PubMed]
- Antkiewicz-Michaluk, L.; Romańska, I.; Michaluk, J.; Vetulani, J. Role of calcium channels in effects of antidepressant drugs on responsiveness to pain. Psychopharmacology (Berl) 1991, 105, 269–274. [Google Scholar] [CrossRef] [PubMed]
- Galeotti, N.; Ghelardini, C.; Bartolini, A. Involvement of potassium channels in amitriptyline and clomipramine analgesia. Neuropharmacology 2001, 40, 75–84. [Google Scholar] [CrossRef]
- Phillis, J.W.; Wu, P.H. The effect of various centrally active drugs on adenosine uptake by the central nervous system. Comp. Biochem. Physiol. 1982, 72C, 179–187. [Google Scholar] [CrossRef]
- McCarson, K.E.; Duric, V.; Reisman, S.A.; Winter, M.; Enna, S.J. GABA(B) receptor function and subunit expression in the rat spinal cord as indicators of stress and the antinociceptive response to antidepressants. Brain Res. 2006, 1068, 109–117. [Google Scholar] [CrossRef] [PubMed]
- Isenberg, K.E.; Cicero, T.J. Possible involvement of opiate receptors in the pharmacological profiles of antidepressant compounds. Eur. J. Pharmacol. 1984, 103, 57–63. [Google Scholar] [CrossRef]
- Yaron, I.; Shirazi, I.; Judovich, R.; Levartovsky, D.; Caspi, D.; Yaron, M. Fluoxetine and amitriptyline inhibit nitric oxide, prostaglandin, E2, and hyaluronic acid production in human synovial cells and synovial tissue cultures. Arthritis Rheumatol. 1999, 42, 2561–2568. [Google Scholar] [CrossRef]



© 2017 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Obata, H. Analgesic Mechanisms of Antidepressants for Neuropathic Pain. Int. J. Mol. Sci. 2017, 18, 2483. https://doi.org/10.3390/ijms18112483
Obata H. Analgesic Mechanisms of Antidepressants for Neuropathic Pain. International Journal of Molecular Sciences. 2017; 18(11):2483. https://doi.org/10.3390/ijms18112483
Chicago/Turabian StyleObata, Hideaki. 2017. "Analgesic Mechanisms of Antidepressants for Neuropathic Pain" International Journal of Molecular Sciences 18, no. 11: 2483. https://doi.org/10.3390/ijms18112483
APA StyleObata, H. (2017). Analgesic Mechanisms of Antidepressants for Neuropathic Pain. International Journal of Molecular Sciences, 18(11), 2483. https://doi.org/10.3390/ijms18112483