miRNome Profiling in Bicuspid Aortic Valve-Associated Aortopathy by Next-Generation Sequencing
Abstract
:1. Introduction
2. Results
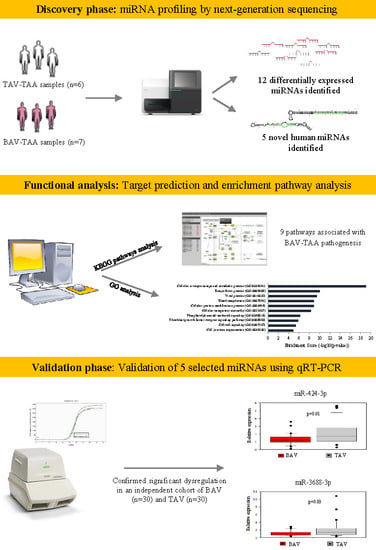
2.1. miRNA Profiling by Next-Generation Sequencing
2.2. Functional Analysis
2.3. Validation of Selected microRNAs Using qRT-PCR
3. Discussion
4. Materials and Methods
4.1. Study Population and Tissue Samples
4.2. RNA Extraction and Quantification
4.3. miRNA Sequencing and Data Analysis
4.4. Target Prediction and Enrichment Pathway Analysis
4.5. Quantification by qRT-PCR
4.6. Statistical Analysis
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Verma, S.; Siu, S.C. Aortic dilatation in patients with bicuspid aortic valve. N. Engl. J. Med. 2014, 370, 1920–1929. [Google Scholar] [CrossRef] [PubMed]
- Hiratzka, L.F.; Creager, M.A.; Isselbacher, E.M.; Svensson, L.G.; Nishimura, R.A.; Bonow, R.O.; Guyton, R.A.; Sundt, T.M., 3rd. Surgery for Aortic Dilatation in Patients with Bicuspid Aortic Valves: A Statement of Clarification from the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. J. Am. Coll. Cardiol. 2016, 67, 724–731. [Google Scholar] [CrossRef] [PubMed]
- Pape, L.A.; Tsai, T.T.; Isselbacher, E.M.; Oh, J.K.; O’gara, P.T.; Evangelista, A.; Fattori, G.; Meinhardt, S.; Trimarchi, E.; Bossone, T.; et al. Aortic diameter ≥ 5.5 cm is not a good predictor of type A aortic dissection: Observations from the International Registry of Acute Aortic Dissection (IRAD). Circulation 2007, 116, 1120–1127. [Google Scholar] [CrossRef] [PubMed]
- Andreassi, M.G.; Della Corte, A. Genetics of bicuspid aortic valve aortopathy. Curr. Opin. Cardiol. 2016, 31, 585–592. [Google Scholar] [CrossRef] [PubMed]
- Kjellqvist, S.; Maleki, S.; Olsson, T.; Chwastyniak, M.; Branca, R.M.; Lehtiö, J.; Pinet, F.; Franco-Cereceda, A.; Eriksson, P. A combined proteomic and transcriptomic approach shows diverging molecular mechanisms in thoracic aortic aneurysm development in patients with tricuspid- and bicuspid aortic valve. Mol. Cell. Proteom. 2013, 12, 407–425. [Google Scholar] [CrossRef] [PubMed]
- Rocchiccioli, S.; Cecchettini, A.; Panesi, P.; Farneti, P.A.; Mariani, M.; Ucciferri, N.; Citti, L.; Andreassi, M.G.; Foffa, I. Hypothesis-free secretome analysis of thoracic aortic aneurysm reinforces the central role of TGF-β cascade in patients with bicuspid aortic valve. J. Cardiol. 2017, 69, 570–576. [Google Scholar] [CrossRef] [PubMed]
- Schmiedel, J.M.; Klemm, S.L.; Zheng, Y.; Sahay, A.; Blüthgen, N.; Marks, D.S.; van Oudenaarden, A. Gene expression. MicroRNA control of protein expression noise. Science 2015, 348, 128–132. [Google Scholar] [CrossRef] [PubMed]
- Fu, X.M.; Zhou, Y.Z.; Cheng, Z.; Liao, X.B.; Zhou, X.M. MicroRNAs: Novel Players in Aortic Aneurysm. Biomed. Res. Int. 2015, 2015, 831641. [Google Scholar] [CrossRef] [PubMed]
- Jones, J.A.; Stroud, R.E.; O’Quinn, E.C.; Black, L.E.; Barth, J.L.; Elefteriades, J.A.; Bavaria, J.E.; Gorman, J.H.; Gorman, R.C.; Spinale, F.G.; et al. Selective microRNA suppression in human thoracic aneurysms: Relationship of miR-29a to aortic size and proteolytic induction. Circ. Cardiovasc. Genet. 2011, 4, 605–613. [Google Scholar] [CrossRef] [PubMed]
- Liao, M.; Zou, S.; Weng, J.; Hou, L.; Yang, L.; Zhao, Z.; Bao, J.; Jing, Z. A microRNA profile comparison between thoracic aortic dissection and normal thoracic aorta indicates the potential role of microRNAs in contributing to thoracic aortic dissection pathogenesis. J. Vasc. Surg. 2011, 53, 1341–1349. [Google Scholar] [CrossRef] [PubMed]
- Patuzzo, C.; Pasquali, A.; Malerba, G.; Trabetti, E.; Pignatti, P.; Tessari, M.; Faggian, A. A Preliminary microRNA Analysis of Non Syndromic Thoracic Aortic Aneurysms. Balkan J. Med. Genet. 2012, 15, 51–55. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.J.; Huang, B.; Yang, Y.M.; Zhang, L.; Su, W.J.; Tian, L.; Lu, T.Y.; Zhang, S.; Fan, X.H.; Hui, R.T. Differential expression of microRNAs in aortic tissue and plasma in patients with acute aortic dissection. J. Geriatr. Cardiol. 2015, 12, 655–661. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Song, H.F.; Li, S.H.; Guo, J.; Tsang, K.; Tumiati, L.; Butany, J.; Yau, T.M.; Ouzounian, M.; Fu, S.; et al. Progressive Aortic Dilation Is Regulated by miR-17-Associated miRNAs. J. Am. Coll. Cardiol. 2016, 67, 2965–2977. [Google Scholar] [CrossRef] [PubMed]
- Licholai, S.; Blaż, M.; Kapelak, B.; Sanak, M. Unbiased Profile of MicroRNA Expression in Ascending Aortic Aneurysm Tissue Appoints Molecular Pathways Contributing to the Pathology. Ann. Thorac. Surg. 2016, 102, 1245–1252. [Google Scholar] [CrossRef] [PubMed]
- Ikonomidis, J.S.; Ivey, C.R.; Wheeler, J.B.; Akerman, A.W.; Rice, A.; Patel, R.K.; Stroud, R.E.; Shah, A.A.; Hughes, C.G.; Ferrari, G.; et al. Plasma biomarkers for distinguishing etiologic subtypes of thoracic aortic aneurysm disease. J. Thorac. Cardiovasc. Surg. 2013, 145, 1326–1333. [Google Scholar] [CrossRef] [PubMed]
- Pei, H.; Tian, C.; Sun, X.; Qian, X.; Liu, P.; Liu, W.; Chang, Q. Overexpression of MicroRNA-145 Promotes Ascending Aortic Aneurysm Media Remodeling through TGF-β1. Eur. J. Vasc. Endovasc. Surg. 2015, 49, 52–59. [Google Scholar] [CrossRef] [PubMed]
- Albinsson, S.; Della Corte, A.; Alajbegovic, A.; Krawczyk, K.K.; Bancone, C.; Galderisi, U.; Cipollaro, M.; De Feo, M.; Forte, A. Patients with bicuspid and tricuspid aortic valve exhibit distinct regional microrna signatures in mildly dilated ascending aorta. Heart Vessels 2017, 32, 750–767. [Google Scholar] [CrossRef] [PubMed]
- Tam, S.; de Borja, R.; Tsao, M.S.; McPherson, J.D. Robust global microRNA expression profiling using next-generation sequencing technologies. Lab. Investig. 2014, 94, 350–358. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Micaelo, N.; Beltrán-Debón, R.; Baiges, I.; Faiges, M.; Alegret, J.M. Specific circulating microRNA signature of bicuspid aortic valve disease. J. Transl. Med. 2017, 15, 76. [Google Scholar] [CrossRef] [PubMed]
- Mitra, R.; Lin, C.C.; Eischen, C.M.; Bandyopadhyay, S.; Zhao, Z. Concordant dysregulation of miR-5p and miR-3p arms of the same precursor microRNA may be a mechanism in inducing cell proliferation and tumorigenesis: A lung cancer study. RNA 2015, 21, 1055–1065. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, G.; Subramanian, I.V.; Adhikari, N.; Zhang, X.; Joshi, H.P.; Basi, D.; Chandrashekhar, Y.S.; Hall, J.L.; Roy, S.; Zeng, Y.; et al. Hypoxia-induced microRNA-424 expression in human endothelial cells regulates HIF-α isoforms and promotes angiogenesis. J. Clin. Investig. 2010, 120, 4141–4154. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, K.; Satoh, M.; Takahashi, Y.; Osaki, T.; Nasu, T.; Tamada, M.; Okabayashi, H.; Nakamura, M.; Morino, Y. Dysregulation of ossification-related miRNAs in circulating osteogenic progenitor cells obtained from patients with aortic stenosis. Clin. Sci. 2016, 130, 1115–1124. [Google Scholar] [CrossRef] [PubMed]
- Xiao, X.; Huang, C.; Zhao, C.; Gou, X.; Senavirathna, L.K.; Hinsdale, M.; Lloyd, P.; Liu, L. Regulation of myofibroblast differentiation by miR-424 during epithelial-to-mesenchymal transition. Arch. Biochem. Biophys. 2015, 566, 49–57. [Google Scholar] [CrossRef] [PubMed]
- Wei, S.; Li, Q.; Li, Z.; Wang, L.; Zhang, L.; Xu, Z. miR-424-5p promotes proliferation of gastric cancer by targeting Smad3 through TGF-β signaling pathway. Oncotarget 2016, 7, 75185–75196. [Google Scholar] [CrossRef] [PubMed]
- Koshiol, J.; Wang, E.; Zhao, Y.; Marincola, F.; Landi, M.T. Strengths and limitations of laboratory procedures for microRNA detection. Cancer Epidemiol. Biomark. Prev. 2010, 19, 907–911. [Google Scholar] [CrossRef] [PubMed]
- Nelson, P.T.; Wang, W.X.; Wilfred, B.R.; Tang, G. Technical variables in high-throughput miRNA expression profiling: Much work remains to be done. Biochim. Biophys. Acta 2008, 779, 758–765. [Google Scholar] [CrossRef] [PubMed]
- Git, A.; Dvinge, H.; Salmon-Divon, M.; Osborne, M.; Kutter, C.; Hadfield, J.; Bertone, P.; Caldas, C. Systematic comparison of microarray profiling, real-time PCR, and next-generation sequencing technologies for measuring differential microRNA expression. RNA 2010, 16, 991–1006. [Google Scholar] [CrossRef] [PubMed]
- Baumgartner, H.; Hung, J.; Bermejo, J.; Chambers, J.B.; Evangelista, A.; Griffin, B.P.; Iung, B.; Otto, C.M.; Pellikka, P.A.; Quiñones, M. American Society of Echocardiography, European Association of Echocardiography. Echocardiographic assessment of valve stenosis: EAE/ASE recommendations for clinical practice. J. Am. Soc. Echocardiogr. 2009, 22, 1–23. [Google Scholar] [CrossRef] [PubMed]
- Giurato, G.; De Filippo, M.R.; Rinaldi, A.; Hashim, A.; Nassa, G.; Ravo, M.; Rizzo, F.; Tarallo, R.; Weisz, A. iMir: An integrated pipeline for high-throughput analysis of small non-coding RNA data obtained by smallRNA-Seq. BMC Bioinform. 2013, 14, 362. [Google Scholar] [CrossRef] [PubMed]
- Vlachos, I.S.; Zagganas, K.; Paraskevopoulou, M.D.; Georgakilas, G.; Karagkouni, D.; Vergoulis, T.; Dalamagas, T.; Hatzigeorgiou, A.G. DIANA-miRPath v3.0: Deciphering microRNA function with experimental support. Nucleic Acids Res. 2015, 43, W460–W466. [Google Scholar] [CrossRef] [PubMed]



| miRNAs | FDR-Adjusted p-Value | Fold Change |
|---|---|---|
| miR-203a-3p | 0.031 | 1.83 |
| miR-377-5p | 0.030 | 2.15 |
| miR-5684 | 0.036 | −4.64 |
| miR-664b-3p | 0.021 | −2.35 |
| miR-424-3p | 0.006 | −2.55 |
| miR-550a-3p | 0.011 | −3.24 |
| miR-3158-3p | 0.009 | −4.35 |
| miR-3688-3p | 0.017 | −3.83 |
| miR-511-3p | 0.046 | −2.36 |
| miR-340-3p | 0.033 | −2.11 |
| miR-210-5p | 0.018 | −2.98 |
| miR-486-3p | 0.016 | −5.04 |
| miRNA Candidate | Sequence (5′-3′) | Chromosome Position | ΔG (kcal/mol) | Orthologous miRNA |
|---|---|---|---|---|
| 1 | TAAGGGCTGGGTCGGTTGGGCTGGG | 2:133038057-133038161 | −40.30 | mmu-miR-6937-5p, bta-miR-2487 |
| 2 | GGAGCCTCGGTTGGCCTCGGATAGC | 22:22210613-22210687 | −22.50 | bta-miR-2904 |
| 3 | GTGGAGGACTGAGAAGGTGAGGC | 5:76376302-76376432 | −43.00 | mmu-miR-1940 |
| 4 | AAAGGTAGATAGAACAGGTCTTGTT | 15:83424755-83424829 | −21.60 | bta-miR-1839, cgr-miR-1839-5p, cfa-miR-1839, mmu-miR-1839-5p, eca-miR-1839, ssc-miR-1839-5p, rno-miR-1839-5p, chi-miR-1839 |
| 5 | GCCGGCGGGAGCCCCGGGGAGAGT | 5:71146764-71146846 | −33.90 | mmu-miR-2137 |
| Variables | Discovery Cohort | Validation Cohort | ||||
|---|---|---|---|---|---|---|
| BAV (n = 7) | TAV (n = 6) | p Value | BAV (n = 30) | TAV (n = 30) | p Value | |
| Age (years ± SD) | 56.2 ± 17 | 61.6 ± 10 | 0.5 | 59.5 ± 12 | 68.5 ± 9.5 | 0.003 |
| Gender, male (n, %) | 7 (100%) | 4 (66%) | 0.09 | 26 (86%) | 16 (53%) | 0.003 |
| Ascending aortic diameter (mm) | 48.8 ± 3.2 | 48.5 ± 3.8 | 0.8 | 49.5 ± 5 | 53.7 ± 4.9 | 0.002 |
| Aortic Stenosis (n, %) | 6 (86%) | 1 (16%) | 0.01 | 13 (43%) | 0 (0%) | <0.0001 |
| Aortic valve insufficiency (n, %) | 1 (14%) | 5 (83%) | 0.01 | 10 (33%) | 25 (83%) | <0.0001 |
| Hypertension (n, %) | 4 (57%) | 3 (50%) | 0.8 | 18 (60%) | 21 (70%) | 0.3 |
| Smoking habit (n, %) | 4 (57%) | 1 (16%) | 0.1 | 7 (23%) | 3 (10%) | 0.2 |
| Diabetes (n, %) | 1 (14%) | 0 (0%) | 0.3 | 0 (0%) | 2 (6%) | 0.1 |
| Dyslipidemia (n, %) | 0 (0%) | 1 (16%) | 0.5 | 7 (23%) | 9 (30%) | 0.5 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Borghini, A.; Foffa, I.; Pulignani, S.; Vecoli, C.; Ait-Ali, L.; Andreassi, M.G. miRNome Profiling in Bicuspid Aortic Valve-Associated Aortopathy by Next-Generation Sequencing. Int. J. Mol. Sci. 2017, 18, 2498. https://doi.org/10.3390/ijms18112498
Borghini A, Foffa I, Pulignani S, Vecoli C, Ait-Ali L, Andreassi MG. miRNome Profiling in Bicuspid Aortic Valve-Associated Aortopathy by Next-Generation Sequencing. International Journal of Molecular Sciences. 2017; 18(11):2498. https://doi.org/10.3390/ijms18112498
Chicago/Turabian StyleBorghini, Andrea, Ilenia Foffa, Silvia Pulignani, Cecilia Vecoli, Lamia Ait-Ali, and Maria Grazia Andreassi. 2017. "miRNome Profiling in Bicuspid Aortic Valve-Associated Aortopathy by Next-Generation Sequencing" International Journal of Molecular Sciences 18, no. 11: 2498. https://doi.org/10.3390/ijms18112498
APA StyleBorghini, A., Foffa, I., Pulignani, S., Vecoli, C., Ait-Ali, L., & Andreassi, M. G. (2017). miRNome Profiling in Bicuspid Aortic Valve-Associated Aortopathy by Next-Generation Sequencing. International Journal of Molecular Sciences, 18(11), 2498. https://doi.org/10.3390/ijms18112498