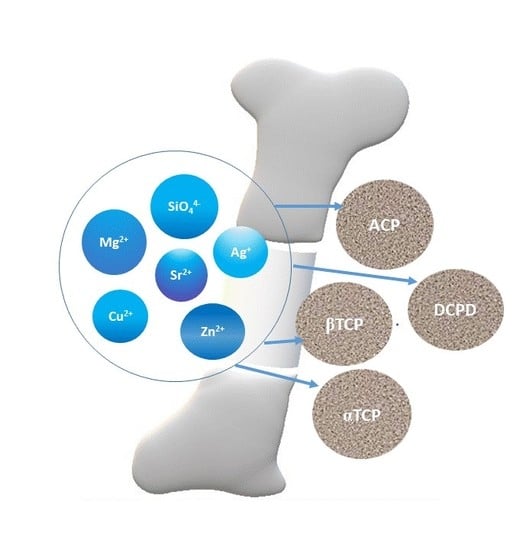
Ionic Substitutions in Non-Apatitic Calcium Phosphates
Abstract
:1. Introduction
2. The Most Relevant Ionic Substitution within the CaPs Crystal Structure
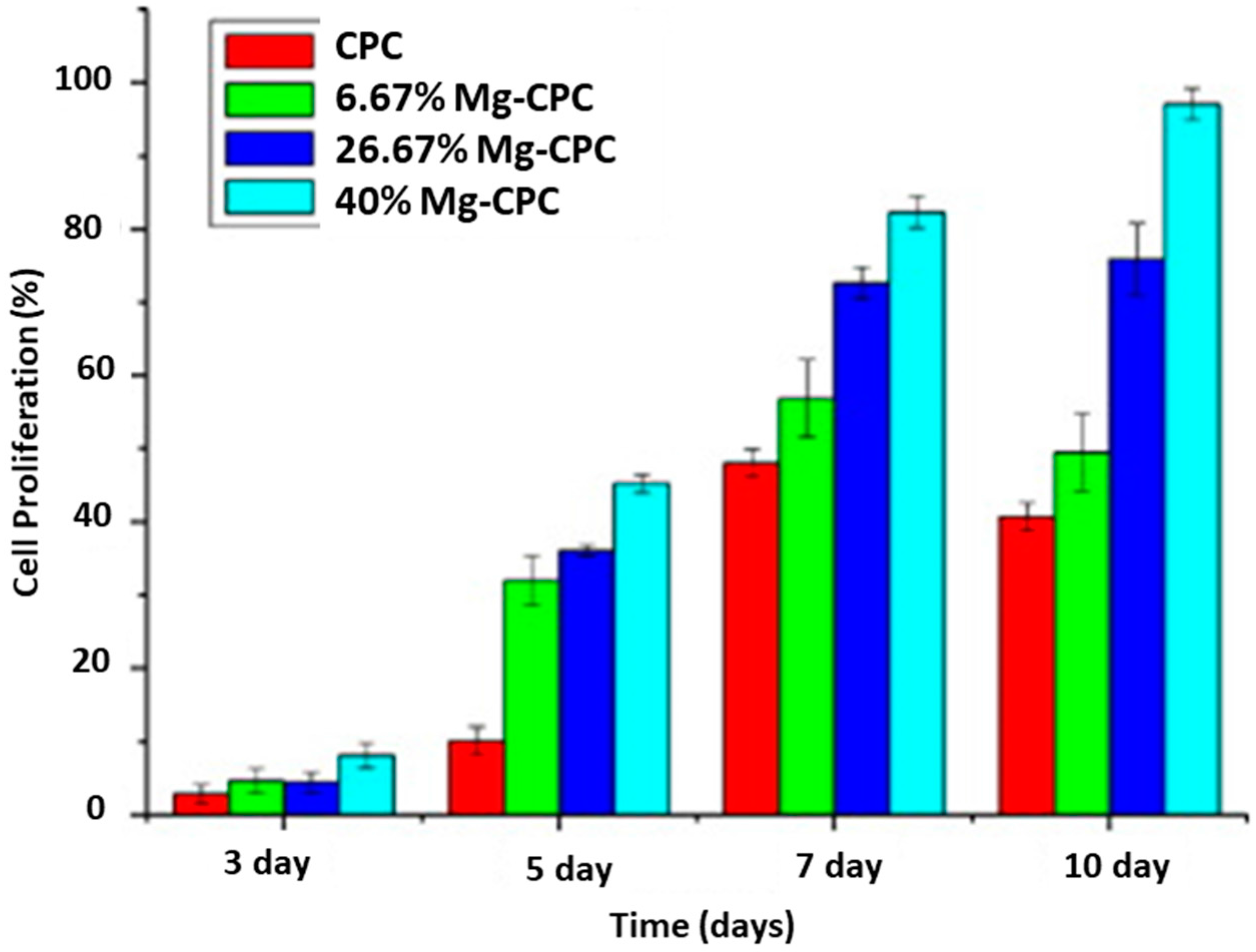
2.1. Magnesium (Mg2+)
2.2. Zinc (Zn2+)
2.3. Strontium (Sr2+)
2.4. Silicon (SiO32−, SiO42−)
3. Non-Apatitic Calcium Phosphates
3.1. Amorphous Calcium Phosphate (ACP)
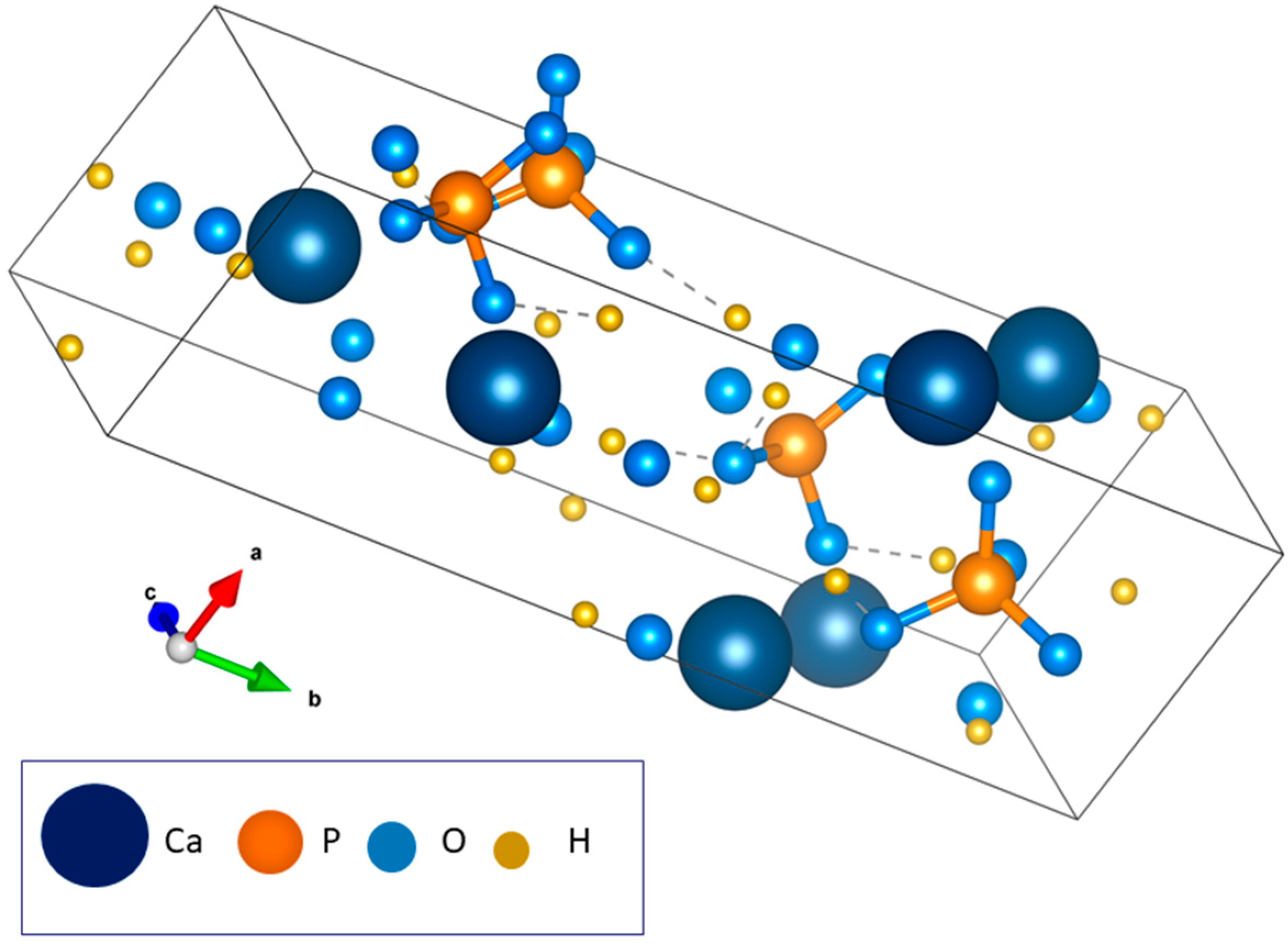
3.2. Dicalcium Phosphate Dihydrate (DCPD)
3.3. Tricalcium Phosphate (TCP)
- Thermal transformation of the precursor;
- Solid-state synthesis;
- Wet chemical precipitation;
- Sol-gel technique;
- Self-combustion method.
4. Ionic Substitutions in Non-Apatitic Calcium Phosphates
4.1. Substituted Amorphous Calcium Phosphate (ACP)
4.2. Substituted Dicalcium Phosphate Dihydrate (DCPD)
- Preparation of βTCP and βMgTCP powder via solid-state synthesis;
- Addition of MCPM to previously crushed and mixed powders;
- Addition of water to the composed powder;
- Mixing in mortar.
4.3. TCP Substituted with Foreign Ions
4.3.1. αTCP Substituted with Different Ions
4.3.2. βTCP Substituted with Foreign Ions
5. Conclusions
Acknowledgments
Conflicts of Interest
References
- Dorozhkin, S.V. Nanosized and nanocrystalline calcium orthophosphates. Acta Biomater. 2010, 6, 715–734. [Google Scholar] [CrossRef] [PubMed]
- Boanini, E.; Gazzano, M.; Bigi, A. Ionic substitutions in calcium phosphates synthesized at low temperature. Acta Biomater. 2010, 6, 1882–1894. [Google Scholar] [CrossRef] [PubMed]
- Dubnika, A.; Loca, D.; Rudovica, V.; Parekh, M.B.; Berzina-Cimdina, L. Functionalized silver doped hydroxyapatite scaffolds for controlled simultaneous silver ion and drug delivery. Ceram. Int. 2017, 43, 3698–3705. [Google Scholar] [CrossRef]
- Jafari, S.; Maleki-Dizaji, N.; Barar, J.; Barzegar-Jalali, M.; Rameshrad, M.; Adibkia, K. Methylprednisolone acetate-loaded hydroxyapatite nanoparticles as a potential drug delivery system for treatment of rheumatoid arthritis: In vitro and in vivo evaluations. Eur. J. Pharm. Sci. 2016, 91, 225–235. [Google Scholar] [CrossRef] [PubMed]
- Yu, W.-L.; Sun, T.-W.; Qi, C.; Zhao, H.-K.; Ding, Z.-Y.; Zhang, Z.-W.; Sun, B.-B.; Shen, J.; Chen, F.; Zhu, Y.-J.; et al. Enhanced osteogenesis and angiogenesis by mesoporous hydroxyapatite microspheres derived simvastatin sustained release system for superior bone regeneration. Sci. Rep. 2017, 7. [Google Scholar] [CrossRef] [PubMed]
- Kolanthai, E.; Ganesan, K.; Epple, M.; Kalkura, S.N. Synthesis of nanosized hydroxyapatite/agarose powders for bonefiller and drug delivery application. Mater. Today Commun. 2016, 8, 31–40. [Google Scholar] [CrossRef]
- Terukina, T.; Saito, H.; Tomita, Y.; Hattori, Y.; Otsuka, M. Development and effect of a sustainable and controllable simvastatin releasing device based on PLGA microspheres/carbonate apatite cement composite: In vitro evaluation for use as a drug delivery system from bone-like biomaterial. J. Drug Deliv. Sci. Technol. 2017, 37, 74–80. [Google Scholar] [CrossRef]
- Lei, Y.; Xu, Z.; Ke, Q.; Yin, W.; Chen, Y.; Zhang, C.; Guo, Y. Strontium hydroxyapatite/chitosan nanohybrid scaffolds with enhanced osteoinductivity for bone tissue engineering. Mater. Sci. Eng. C 2017, 72, 134–142. [Google Scholar] [CrossRef] [PubMed]
- Supova, M. Substituted hydroxyapatites for biomedical applications: A review. Ceram. Int. 2015, 41, 9203–9231. [Google Scholar] [CrossRef]
- Shepherd, J.H.; Shepherd, D.V.; Best, S.M. Substituted hydroxyapatites for bone repair. J. Mater. Sci. Mater. Med. 2012, 23, 2335–2347. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Widodo, J.; Lim, S.; Ooi, C.P. Synthesis and cytocompatibility of manganese (II) and iron (III) substituted hydroxyapatite nanoparticles. J. Mater. Sci. 2012, 47, 754–763. [Google Scholar] [CrossRef]
- Wang, Y.; Hao, H.; Li, Y.; Zhang, S. Selenium-substituted hydroxyapatite nanoparticles and their in vivo antitumor effect on hepatocellular carcinoma. Colloids Surf. B Biointerfaces 2016, 140, 297–306. [Google Scholar] [CrossRef]
- Mourino, V.; Cattalini, J.P.; Boccaccini, A.R. Metallic ions as therapeutic agents in tissue engineering scaffolds: An overview of their biological applications and strategies for new developments. J. R. Soc. Interface 2012, 9, 401–419. [Google Scholar] [CrossRef] [PubMed]
- Kolmas, J.; Groszyk, E.; Kwiatkowska-Różycka, D. Substituted hydroxyapatites with antibacterial properties. BioMed Res. Int. 2014, 2014. [Google Scholar] [CrossRef] [PubMed]
- Ratnayake, J.T.B.; Mucalo, M.; Dias, G.J. Substituted hydroxyapatites for bone regeneration: A review of current trends. J. Biomed. Mater. Res. B 2017, 105, 1285–1299. [Google Scholar] [CrossRef] [PubMed]
- Graziani, G.; Bianchi, M.; Sassoni, E.; Russo, A.; Marcacci, M. Ion-substituted calcium phosphate coatings deposited by plasma-assisted techniques: A review. Mater. Sci. Eng. C 2017, 74, 219–229. [Google Scholar] [CrossRef] [PubMed]
- Combes, C.; Cazalbou, S.; Rey, C. Apatite biominerals. Minerals 2016, 6, 34. [Google Scholar] [CrossRef]
- Alshemary, Z.; Akrama, M.; Goh, Y.-F.; Tariq, U.; Butt, F.K.; Abdolahi, A.; Hussain, R. Synthesis, characterization, in vitro bioactivity and antimicrobial activity of magnesium and nickel doped silicate hydroxyapatite. Ceram. Int. 2015, 41, 11886–11898. [Google Scholar] [CrossRef]
- Castiglioni, S.; Cazzaniga, A.; Albisetti, W.; Maier, J.A.M. Magnesium and Osteoporosis: Current State of Knowledge and Future Research Directions. Nutrients 2013, 5, 3022–3033. [Google Scholar] [CrossRef] [PubMed]
- Rude, R.K.; Singer, F.R.; Gruber, H.E. Skeletal and Hormonal Effects of Magnesium Deficiency. J. Am. Coll. Nutr. 2009, 28, 131–141. [Google Scholar] [CrossRef] [PubMed]
- He, L.Y.; Zhang, X.M.; Liu, B.; Tian, Y.; Ma, W.H. Effect of magnesium ion on human osteoblast activity. Braz. J. Med. Biol. Res. 2016, 49, 1414–1431. [Google Scholar] [CrossRef] [PubMed]
- Smith, B.J.; Hermann, J. Aging, zinc, and bone health. In Bioactive Food as Dietary Interventions for the Aging Population; Watson, R.R., Preedy, V.R., Eds.; Academic Press: San Diego, CA, USA, 2013; pp. 433–443. [Google Scholar]
- Shepherd, D. Zinc-substituted hydroxyapatite for the inhibition of osteoporosis. In Hydroxyapatite (HAp) in Biomedical Applications; Mucalo, M., Ed.; Woodhead Publishing: Cambridge, UK, 2015; pp. 107–126. [Google Scholar]
- Kwiecień, M.; Winiarska-Mieczan, A.; Milczarek, A.; Tomaszewska, E.; Matras, J. Effects of zinc glycine chelate on growth performance, carcass characteristics, bone quality, and mineral content in bone of broiler chicken. Livest. Sci. 2016, 191, 43–50. [Google Scholar] [CrossRef]
- Hadley, K.B.; Newman, S.M.; Hunt, J.R. Dietary zinc reduces osteoclast resorption activities and increases markers of osteoblast differentiation, matrix maturation, and mineralization in the long bones of growing rats. J. Nutr. Biochem. 2010, 21, 297–303. [Google Scholar] [CrossRef] [PubMed]
- Querido, W.; Rossi, A.L.; Farina, M. The effects of strontium on bone mineral: A review on current knowledge and microanalytical approaches. Micron 2016, 80, 122–134. [Google Scholar] [CrossRef] [PubMed]
- Kaygili, O.; Keser, S.; Kom, M.; Eroksuz, Y.; Dorozhkin, S.V.; Ates, T.; Ozercan, I.H.; Tatar, C.; Yakuphanoglu, F. Strontium substituted hydroxyapatites: Synthesis and determination of their structural properties, in vitro and in vivo performance. Mater. Sci. Eng. C 2015, 55, 538–546. [Google Scholar] [CrossRef] [PubMed]
- Bonnelye, E.; Chabadel, A.; Saltel, F.; Jurdic, P. Dual effect of strontium ranelate: Stimulation of osteoblast differentiation and inhibition of osteoclast formation and resorption in vitro. Bone 2008, 42, 129–138. [Google Scholar] [CrossRef] [PubMed]
- Tat, K.S.; Pelletier, J.P.; Mineau, F.; Caron, J.; Martel-Pelletier, J. Strontium ranelate inhibits key factors affecting bone remodeling in human osteoarthritic subchondral bone osteoblasts. Bone 2011, 49, 559–567. [Google Scholar] [CrossRef] [PubMed]
- Pasqualetti, S.; Banfi, G.; Mariotti, M. The effects of strontium on skeletal development in zebrafish embryo. J. Trace Elem. Med. Biol. 2013, 27, 375–379. [Google Scholar] [CrossRef] [PubMed]
- Reginster, J.Y.; Neuprez, A.; Dardenne, N.; Beaudart, C.; Emonts, P.; Bruyere, O. Efficacy and safety of currently marketed antiosteoporosis medications. Best Pract. Res. Clin. Endocrinol. Metab. 2014, 28, 809–834. [Google Scholar] [CrossRef] [PubMed]
- Jurkić, M.L.; Cepanec, I.; Pavelić, S.K.; Pavelić, K. Biological and therapeutic effects of ortho-silicic acid and some ortho-silicic acid-releasing compounds: New perspectives for therapy. Nutr. Metab. 2013, 10, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Carlisle, E.M. Silicon: A Possible Factor in Bone Calcification. Science 1970, 167, 279–280. [Google Scholar] [CrossRef] [PubMed]
- Carlisle, E.M. Silicon: An essential element for the chick. Science 1972, 178, 619–621. [Google Scholar] [CrossRef] [PubMed]
- Carlisle, E.M. Silicon: A requirement in bone formation independent of vitamin D1. Calcif. Tissue Int. 1981, 33, 27–34. [Google Scholar] [CrossRef] [PubMed]
- Carlisle, E.M. Silicon as an essential trace element in animal nutrition. Ciba Found. Symp. 1986, 121, 123–139. [Google Scholar] [CrossRef] [PubMed]
- Reffitt, D.M.; Ogston, N.; Jugdaohsingh, R.; Cheung, H.F.J.; Evans, B.A.J.; Thompson, R.P.H.; Powell, J.J.; Hampson, G.N. Orthosilicic acid stimulates collagen type 1 synthesis and osteoblastic differentiation in human osteoblast-like cells in vitro. Bone 2003, 32, 127–135. [Google Scholar] [CrossRef]
- Spector, T.D.; Calomme, M.R.; Anderson, S.H.; Clement, G.; Bevan, L.; Demeester, N.; Swaminathan, R.; Jugdaohsingh, R.; Berghe, D.A.; Powell, J.J. Choline-stabilized orthosilicic acid supplementation as an adjunct to Calcium/Vitamin D3 stimulates markers of bone formation in osteopenic females: A randomized, placebo-controlled trial. BMC Muscoskelet. Dis. 2008, 9, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Camaioni, A.; Cacciotti, I.; Campagnolo, L.; Bianco, A. Silicon-substituted hydroxyapatite for biomedical applications. In Hydroxyapatite (HAp) in Biomedical Applications; Mucalo, M., Ed.; Woodhead Publishing: Cambridge, UK, 2015; pp. 333–373. [Google Scholar]
- Jugdaohsingh, R.; Tucker, K.L.; Qiao, N.; Cupples, L.A.; Kiel, D.P.; Powell, J.J. Dietary Silicon Intake is Positively Associated with Bone Mineral Density in Men and Premenopausal Women of the Framingham Offspring Cohort. J. Bone Miner. Res. 2004, 19, 297–307. [Google Scholar] [CrossRef] [PubMed]
- Judgaohsingh, R. Silicon and Bone Health. J. Nutr. Health Aging 2007, 11, 99–110. [Google Scholar]
- Henstock, J.R.; Canham, L.T.; Anderson, S.I. Silicon: The evolution of its use in biomaterials. Acta Biomater. 2015, 11, 17–26. [Google Scholar] [CrossRef] [PubMed]
- Dorozhkin, S.V.; Epple, M. Biological and Medical Significance of Calcium Phosphates. Angew. Chem. Int. Ed. Engl. 2002, 41, 3130–3146. [Google Scholar] [CrossRef]
- LeGeros, Z.R.; Ito, A.; Ishikawa, K.; Sakae, T.; LeGeros, J.P. Fundamentals of Hydroxyapatite and Related Calcium Phosphates. In Advanced Biomaterials; Basu, B., Katti, D.S., Kumar, A., Eds.; Wiley: Newark, NJ, USA, 2009; pp. 19–52. [Google Scholar]
- Chow, L.C. Solubility of Calcium Phosphates. In Octacalcium Phosphate; Chow, L.C., Eanes, E.D., Eds.; Karger: Basel, Switzerland, 2001; Volume 18, pp. 94–111. [Google Scholar]
- Du, L.-W.; Bian, S.; Gou, B.-D.; Jiang, Y.; Huang, J.; Gao, Y.-X.; Zhao, Y.-D.; Wen, W.; Zhang, T.-L.; Wang, K. Structure of Clusters and Formation of Amorphous Calcium Phosphate and Hydroxyapatite: From the Perspective of Coordination Chemistry. Cryst. Growth Des. 2013, 13, 3103–3109. [Google Scholar] [CrossRef]
- Elliott, J.C. Structure and Chemistry of the Apatites and Other Calcium Orthophosphates; Elsevier Science: Amsterdam, The Netherlands, 1994; pp. 53–61. [Google Scholar]
- Combes, C.; Rey, C. Amorphous calcium phosphates: Synthesis, properties and uses in biomaterials. Acta Biomater. 2010, 6, 3362–3378. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dorozhkin, S.V. Amorphous calcium (ortho) phosphates. Acta Biomater. 2016, 6, 4457–4475. [Google Scholar] [CrossRef] [PubMed]
- Habraken, W.; Habibovic, P.; Epple, M.; Bohner, M. Calcium phosphates in biomedical applications: Materials for the future? Mater. Today 2016, 19, 69–87. [Google Scholar] [CrossRef]
- Lee, D.; Kumta, P.N. Chemical synthesis and stabilization of magnesium substituted brushite. Mater. Sci. Eng. C 2010, 30, 934–943. [Google Scholar] [CrossRef]
- Ferreira, A.; Oliveira, C.; Rocha, F. The different phases in the precipitation of dicalcium phosphate dehydrate. J. Cryst. Growth 2003, 252, 599–611. [Google Scholar] [CrossRef]
- Oliveira, A.; Ferreira, A.; Rocha, F. Dicalcium Phosphate Dihydrate: Precipitation Characterization and Crystal Growth. Chem. Eng. Res. Des. 2007, 85, 1655–1661. [Google Scholar] [CrossRef]
- Chen, G.G.; Luo, G.S.; Yang, L.M.; Xu, J.H.; Sun, Y.; Wang, J.D. Synthesis and size control of CaHPO4 particles in a two-liquid phase micro-mixing process. J. Cryst. Growth 2005, 279, 501–507. [Google Scholar] [CrossRef]
- Kumta, P.N.; Sfeir, C.; Lee, D.-H.; Olton, D.; Choi, D. Nanostructured calcium phosphates for biomedical applications: Novel synthesis and characterization. Acta Biomater. 2005, 1, 65–83. [Google Scholar] [CrossRef] [PubMed]
- Ginebra, M.-P.; Canal, C.; Espanol, M.; Pastorino, D.; Montufar, E.B. Calcium phosphate cements as drug delivery materials. Adv. Drug Deliv. Rev. 2012, 64, 1090–1110. [Google Scholar] [CrossRef] [PubMed]
- Vallet-Regí, M.; González-Calbet, J.M. Calcium phosphates as substitution of bone tissues. Prog. Solid State Chem. 2004, 32, 1–31. [Google Scholar] [CrossRef]
- Dorozhkin, S.V. Bioceramics of calcium orthophosphates. Biomaterials 2010, 31, 1465–1485. [Google Scholar] [CrossRef] [PubMed]
- Kwon, S.-H.; Jun, Y.-K.; Hong, S.-H.; Kim, H.-E. Synthesis and dissolution behavior of β-TCP and HA/b-TCP composite powders. J. Eur. Ceram. Soc. 2003, 23, 1039–1045. [Google Scholar] [CrossRef]
- Kolmas, J.; Kaflak, A.; Zima, A.; Slosarczyk, A. Alpha-tricalcium phosphate synthesized by two different routes: Structural and spectroscopic characterization. Ceram. Int. 2015, 41, 5727–5733. [Google Scholar] [CrossRef]
- Carrodeguas, R.G.; De Aza, S. α-Tricalcium phosphate: Synthesis, properties and biomedical applications. Acta Biomater. 2011, 7, 3536–3546. [Google Scholar] [CrossRef] [PubMed]
- Somrani, S.; Rey, C.; Jemal, M. Thermal evolution of amorphous tricalcium phosphate. J. Mater. Chem. 2003, 13, 888–892. [Google Scholar] [CrossRef]
- Camiré, C.L.; Jegou, S.J.S.; Hansen, S.; McCarthy, I.; Lidgren, L. Hydration characteristics of α-tricalcium phosphates: Comparison of preparation routes. J. Appl. Biomater. Biomech. 2005, 3, 106–111. [Google Scholar] [PubMed]
- Jokic, B.; Jankovic-Castvan, I.; Veljovic, D.; Bucevac, D.; Obradovic-Djuricic, K.; Petrovic, R. Synthesis and settings behavior of alpha-TCP from calcium deficient hyroxyapatite obtained by hydrothermal method. J. Optoelectron. Adv. Mater. 2007, 9, 1904–1910. [Google Scholar]
- Bohner, M.; Brunner, T.J.; Doebelin, N.; Tang, R.K.; Stark, W.J. Effect of thermal treatments on the reactivity of nanosized tricalcium phosphate powders. J. Mater. Chem. 2008, 18, 4460–4467. [Google Scholar] [CrossRef]
- Döbelin, N.; Brunner, T.J.; Stark, W.J.; Eggimann, M.; Fisch, M.; Bohne, M. Phase evolution of thermally treated amorphous tricalcium phosphate nanoparticles. Key Eng. Mater. 2009, 396–398, 595–598. [Google Scholar] [CrossRef]
- Famery, R.; Richard, N.; Boch, P. Preparation of α- and β-tricalcium phosphate ceramics, with and without magnesium addition. Ceram. Int. 1994, 20, 327–336. [Google Scholar] [CrossRef]
- Durucan, C.; Brown, P.W. Reactivity of α-tricalcium phosphate. J. Mater. Sci. 2002, 37, 963–969. [Google Scholar] [CrossRef]
- Cai, S.; Wang, Y.W.; Li, J.Y.; Guan, Y.H.; Yao, K.D. Effects of dispersed medium and mineralizer CaF2 on the synthesis of alpha-tricalcium phosphate. J. Inorg. Mater. 2004, 19, 852–858. [Google Scholar]
- Camiré, C.L.; Gbureck, U.; Hirsiger, W.; Bohner, M. Correlating crystallinity and reactivity in α-tricalcium phosphate. Biomaterials 2005, 26, 2787–2794. [Google Scholar] [CrossRef] [PubMed]
- Wei, X.; Ugurlu, O.; Akinc, A. Hydrolysis of α-tricalcium phosphate in simulated body fluid and dehydration behaviour during the drying process. J. Am. Ceram. Soc. 2007, 90, 2315–2321. [Google Scholar] [CrossRef]
- Burkes, D.E.; Moore, J.J.; Ayers, R.A. Method for Producing Calcium Phosphate Powders Using an Auto-Ignition Combustion Synthesis Reaction. U.S. Patent Application No. 11/848,520, 15 May 2008. [Google Scholar]
- Ayers, R.A.; Simske, S.J.; Moore, J.J.; Castillo, M.; Gottoli, G. Manufacture of Porous Net-Shaped Materials Comprising Alpha or Beta Tricalcium Phosphate or Mixtures Thereof. U.S. Patent Application No. 8,545,786, 1 October 2013. [Google Scholar]
- Ayers, R.; Nielsen-Preiss, S.; Ferguson, V.; Gotolli, G.; Moore, J.J.; Kleebe, H.-J. Osteoblast-like cell mineralization induced by multiphasic calcium phosphate ceramic. Mater. Sci. Eng. C 2006, 26, 1333–1337. [Google Scholar] [CrossRef]
- Volkmer, T.M.; Bastos, L.L.; Sousa, V.C.; Santos, L.A. Obtainment of alpha-tricalcium phosphate by solution combustion synthesis method using urea ascombustible. Key Eng. Mater. 2009, 396–398, 591–594. [Google Scholar] [CrossRef]
- Ghosh, R.; Sarkar, R. Synthesis and characterization of sintered beta-tricalcium phosphate: A comparative study on the effect of preparation route. Mater. Sci. Eng. C 2016, 67, 345–352. [Google Scholar] [CrossRef] [PubMed]
- Sanosh, K.P.; Chu, M.-C.; Balakrishnan, A.; Kim, T.N.; Cho, S.-J. Sol-gel synthesis of pure nanosized β-tricalcium phosphate crystalline powders. Curr. Appl. Phys. 2010, 10, 68–71. [Google Scholar] [CrossRef]
- Thomas, D.; Su, S.; Qiu, J.; Pantoya, M.L. Microwave synthesis of functionally graded tricalcium phosphate for osteoconduction. Mater. Today 2016, 9, 47–53. [Google Scholar] [CrossRef]
- Nabiyouni, M.; Ren, Y.; Bhaduri, S.B. Magnesium substitution in the structure of orthopaedic nanoparticles: A comparison between amorphous magnesium phosphates, calcium magnesium phosphates and hydroxyapatites. Mater. Sci. Eng. C 2015, 52, 11–17. [Google Scholar] [CrossRef] [PubMed]
- Dong, G.; Zheng, Y.; He, L.; Wu, G.; Deng, C. The effect of silicon doping on the transformation of amorphous calcium phosphate to silicon-substituted α-tricalcium phosphate by heat treatment. Ceram. Int. 2016, 42, 883–890. [Google Scholar] [CrossRef]
- Kato, N.; Hatoko, Y.; Yamamoto, E.; Furuzono, T.; Hontsu, S. Preparation and application of a potassium-substituted calcium phosphate sheet as a dental material for treating dentin hypersensitivity. Key Eng. Mater. 2017, 720, 102–107. [Google Scholar] [CrossRef]
- Yu, T.; Gao, C.; Ye, J.; Zhang, M. Synthesis and characterization of a novel silver-substituted calcium phosphate cement. J. Mater. Sci. Technol. 2014, 30, 686–691. [Google Scholar] [CrossRef]
- Alkhraisat, M.H.; Cabrejos-Azama, J.; Rodríguez, C.R.; Blanco Jerez, L.; López-Cabarcos, E. Magnesium substitution in brushite cements. Mater. Sci. Eng. C 2013, 33, 475–481. [Google Scholar] [CrossRef] [PubMed]
- Cabrejos-Azama, J.; Alkhraisat, M.H.; Rueda, C.; Torres, J.; Blanco, L.; López-Cabarcos, E. Magnesium substitution in brushite cements for enhanced bone tissue regeneration. Mater. Sci. Eng. C 2014, 43, 403–410. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cabrejos-Azama, J.; Alkhraisat, M.H.; Rueda, C.; Torres, J.; Pintado, C.; Blanco, L.; López-Cabarcos, E. Magnesium substitution in brushite cements: Efficacy of a new biomaterial loaded with vancomycin for the treatment of Staphylococcus aureus infections. Mater. Sci. Eng. C 2016, 61, 72–78. [Google Scholar] [CrossRef] [PubMed]
- Saleh, T.; Ling, L.S.; Hussain, R. Injectable magnesium-doped brushite cement for controlled drug release application. J. Mater. Sci. 2016, 51, 7427–7439. [Google Scholar] [CrossRef]
- Geffers, M.; Barralet, J.E.; Groll, J.; Gbureck, U. Dual-setting brushite—Silica gel cements. Acta Biomater. 2015, 11, 467–476. [Google Scholar] [CrossRef] [PubMed]
- Alkhraisat, M.H.; Mariño, F.T.; Rodríguez, C.R.; Jerez, L.B.; Cabarcos, E.L. Combined effect of strontium and pyrophosphate on the properties of brushite cements. Acta Biomater. 2008, 4, 664–670. [Google Scholar] [CrossRef] [PubMed]
- Alkhraisat, M.H.; Rueda, C.; Cabarcos, E.L. Strontium ion substitution in brushite crystals: The role of strontium chloride. J. Funct. Biomater. 2011, 2, 31–38. [Google Scholar] [CrossRef] [PubMed]
- Ewald, A.; Kappel, C.; Vorndran, E.; Moseke, C.; Gelinsky, M.; Gbureck, U. The effect of Cu(II)-loaded brushite scaffolds on growth and activity of osteoblastic cells. J. Biomed. Mater. Res. A 2012, 100, 2392–2400. [Google Scholar] [CrossRef] [PubMed]
- Nishikawa, H.; Honda, M.; Yokota, T.; Shimizu, Y.; Aizawa, M. Preparation of Spherical Zn-Substituted Tricalcium Phosphate Powder by Ultrasonic Spray-Pyrolysis Technique and Its Characterization. J. Nanomater. 2016, 2016, 1–8. [Google Scholar] [CrossRef]
- El-dek, S.I.; Mansour, S.F.; Ahmed, M.A.; Ahmed, M.K. Microstructural feature of flower like Fe brushite. Prog. Nat. Sci. 2017, 27, 520–526. [Google Scholar] [CrossRef]
- Guerra-López, J.R.; Güida, J.A.; Ramos, G.; Punte, M. The influence of nickel on brushite structure stabilization. J. Mol. Struct. 2017, 1137, 720–724. [Google Scholar] [CrossRef]
- Correia, M.B.; Junior, J.P.G.; Macedo, M.C.S.S.; Resende, C.X.; dos Santos, E.A. Effect of Mg2+ on acidic calcium phosphate phases grown by electrodeposition. J. Cryst. Growth 2017, 475, 328–333. [Google Scholar] [CrossRef]
- Torres, P.M.C.; Marote, A.; Cerqueira, A.R.; Calado, A.J.; Abrantes, J.C.C.; Olhero, S.; da Cruz e Silva, O.A.B.; Vieira, S.I.; Ferreira, J.M.F. Injectable MnSr-doped brushite bone cements with improved biological performance. J. Mater. Chem. B 2017, 5, 2775–2787. [Google Scholar] [CrossRef]
- Vahabzadeh, S.; Bandyopadhyay, A.; Bose, S.; Mandal, R.; Nandi, S.K. IGF-loaded silicon and zinc doped brushite cement: Physico-mechanical characterization and in vivo ostogenesis evaluation. Integr. Biol. 2015, 7, 1561–1573. [Google Scholar] [CrossRef] [PubMed]
- Wei, X.; Akinc, M. Crystal structure analysis of Si- and Zn-codoped tricalcium phosphate by neutron powder diffraction. J. Am. Ceram. Soc. 2007, 90, 2709–2715. [Google Scholar] [CrossRef]
- Reid, J.W.; Tuck, L.; Sayer, M.; Fargo, K.; Hendry, J.A. Synthesis and characterization of single-phase silicon-substituted α-tricalcium phosphate. Biomaterials 2006, 27, 2916–2925. [Google Scholar] [CrossRef] [PubMed]
- Duncan, J.; Hayakawa, S.; Osaka, A.; MacDonald, J.F.; Hanna, J.V.; Skakle, J.M.S.; Gibson, I.R. Furthering the understanding of silicate-substitution in α-tricalcium phosphate: An X-ray diffraction, X-ray fluorescence and solid-state nuclear magnetic resonance study. Acta Biomater. 2014, 10, 1443–1450. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Motisuke, M.; Mestres, G.; Reno, C.O.; Carrodeguas, R.G.; Zavaglia, C.A.C.; Ginebra, M.P. Influence of Si substitution on the reactivity of α-tricalcium phosphate. Mater. Sci. Eng. C 2017, 75, 816–821. [Google Scholar] [CrossRef] [PubMed]
- Kamitakahara, M.; Tatsukawa, E.; Shibata, Y.; Umemoto, S.; Yokoi, T.; Ioku, K.; Ikeda, T. Effect of silicate incorporation on in vivo responses of α-tricalcium phosphate ceramics. J. Mater. Sci. Mater. Med. 2016, 27, 97. [Google Scholar] [CrossRef] [PubMed]
- Mate-Sanchez de Val, J.E.; Calvo-Guirado, J.L.; Delgado-Ruiz, R.A.; Ramirez-Fernandez, M.P.; Negri, B.; Abboud, M.; Martinez, I.M.; de Aza, P.N. Physical properties, mechanical behavior, and electron microscopy study of a new α-TCP block graft with silicon in an animal model. J. Biomed. Mater. Res. A 2012, 100, 3446–3454. [Google Scholar] [CrossRef] [PubMed]
- Mate-Sanchez de Val, J.E.; Calvo-Guirado, J.L.; Delgado-Ruiz, R.A.; Ramirez-Fernandez, M.P.; Martinez, I.M.; Granero-Marin, J.M.; Negri, B.; Chiva-Garcia, F.; Martinez-Gonzalez, J.M.; de Aza, P.N. New block graft of α-TCP with silicon in critical size defects in rabbits: Chemical characterization, histological, histomorphometric and micro-CT study. Ceram. Int. 2012, 38, 1563–1570. [Google Scholar] [CrossRef]
- Radovanovíc, P.Z.; Jokíc, B.; Veljovíc, D.; Dimitrijevíc, S.; Kojic, V.; Petrovíc, R.; Janáckovíc, A. Antimicrobial activity and biocompatibility of Ag+- and Cu2+-doped biphasic hydroxyapatite/α-tricalcium phosphate obtained from hydrothermally synthesized Ag+- and Cu2+-doped hydroxyapatite. Appl. Surf. Sci. 2014, 307, 513–519. [Google Scholar] [CrossRef]
- Reid, J.W.; Fargo, K.; Hendry, J.A.; Sayer, M. The influence of trace magnesium content on the phase composition of silicon-stabilized calcium phosphate powders. Mater. Lett. 2007, 61, 3851–3854. [Google Scholar] [CrossRef]
- Tong, C.; Zhu, Y.; Xu, C.; Li, Y. Preliminary observation of self-reduction of Eu ions in α-Ca3(PO4)2 phosphors prepared in air condition. Phys. B Condens. Matter 2016, 500, 20–23. [Google Scholar] [CrossRef]
- Pina, S.; Torres, P.M.; Goetz-Neunhoeffer, F.; Neubauer, J.; Ferreira, J.M.F. Newly developed Sr-substituted α-TCP bone cements. Acta Biomater. 2010, 6, 928–935. [Google Scholar] [CrossRef] [PubMed]
- Bose, S.; Tarafder, S.; Banerjee, S.S.; Davies, N.M.; Bandyopadhyay, A. Understanding in vivo response and mechanical property variation in MgO, SrO and SiO2 doped β-TCP. Bone 2011, 48, 1282–1290. [Google Scholar] [CrossRef] [PubMed]
- Singh, R.K.; Kannan, S. Synthesis, Structural analysis, Mechanical, antibacterial and Hemolytic activity of Mg2+ and Cu2+ co-substitutions in β-Ca3(PO4)2. Mater. Sci. Eng. C 2014, 45, 530–538. [Google Scholar] [CrossRef] [PubMed]
- Matsumoto, N.; Sato, K.; Yoshida, K.; Hashimoto, K.; Toda, Y. Preparation and characterization of α-tricalcium phosphate co-doped with monovalent and divalent antibacterial metal ions. Acta Biomater. 2009, 5, 3157–3164. [Google Scholar] [CrossRef] [PubMed]
- García-Páez, H.; García Carrodeguas, R.; De Aza, A.H.; Baudín, C.; Pena, P. Effect of Mg and Si co-substitution on microstructure and strength of tricalcium phosphate ceramics. J. Mech. Behav. Biomed. Mater. 2014, 30, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Banerjee, S.S.; Tarafder, S.; Davies, N.M.; Bandyopadhyay, A.; Bose, S. Understanding the influence of MgO and SrO binary doping on the mechanical and biological properties of β-TCP ceramics. Acta Biomater. 2010, 6, 4167–4174. [Google Scholar] [CrossRef] [PubMed]
- Bigi, A.; Foresti, E.; Gandolfi, M.; Gazzano, M.; Roveri, N. Isomorphous Substitutions in β-Tricalcium Phosphate: The Different Effects of Zinc and Strontium. J. Inorg. Biochem. 1997, 66, 259–265. [Google Scholar] [CrossRef]
- Alkhraisat, M.H.; Rueda, C.; Cabrejos-Azama, J.; Lucas-Aparicio, J.; Marińo, F.T.; Torres García-Denche, J.; Jerez, L.B.; Gbureck, U.; Cabarcos, E.L. Loading and release of doxycycline hyclate from strontium-substituted calcium phosphate cement. Acta Biomater. 2010, 6, 1522–1528. [Google Scholar] [CrossRef] [PubMed]
- Araújo, C.; Sader, M.S.; Moreira, E.L.; Moraes, V.C.A.; LeGeros, R.Z.; Soares, G.A. Maximum substitution of magnesium for calcium sites in Mg-β-TCP structure determined by X-ray powder diffraction with the Rietveld refinement. Mater. Chem. Phys. 2009, 118, 337–340. [Google Scholar] [CrossRef]
- Enderle, R.; Götz-Neunhoeffer, F.; Göbbels, M.; Müller, F.A.; Greil, P. Influence of magnesium doping on the phase transformation temperature of β-TCP ceramics examined by Rietveld refinement. Biomaterials 2005, 26, 3379–3384. [Google Scholar] [CrossRef] [PubMed]
- Kannan, S.; Ventura, J.M.; Ferreira, J.M.F. Aqueous precipitation method for the formation of Mg-stabilized β-tricalcium phosphate: An X-ray diffraction study. Ceram. Int. 2007, 33, 637–641. [Google Scholar] [CrossRef]
- Kannan, S.; Lemos, I.A.F.; Rocha, J.H.G.; Ferreira, J.M.F. Synthesis and characterization of magnesium substituted biphasic mixtures of controlled hydroxyapatite/β-tricalcium phosphate ratios. J. Solid State Chem. 2005, 178, 3190–3196. [Google Scholar] [CrossRef]
- Marchi, A.; Dantas, A.C.S.; Greil, P.; Bressiani, J.C.; Bressiani, A.H.A.; Müller, F.A. Influence of Mg-substitution on the physicochemical properties of calcium phosphate powders. Mater. Res. Bull. 2007, 42, 1040–1050. [Google Scholar] [CrossRef]
- Zhou, H.; Hou, S.; Zhang, M.; Chai, H.; Liu, Y.; Bhaduri, S.; Yang, L.; Deng, L. Synthesis of β-TCP and CPP containing biphasic calcium phosphates by a robust technique. Ceram. Int. 2016, 42, 11032–11038. [Google Scholar] [CrossRef]
- Ryu, H.-S.; Hong, K.S.; Lee, J.-K.; Kim, D.J.; Lee, J.H.; Chang, B.-S.; Lee, D.-H.; Lee, C.-K.; Chung, S.-S. Magnesia-doped HA/β-TCP ceramics and evaluation of their biocompatibility. Biomaterials 2004, 25, 393–401. [Google Scholar] [CrossRef]
- Nikaido, T.; Tsuru, K.; Munar, M.; Maruta, M.; Matsuya, S.; Nakamura, S.; Ishikawa, K. Fabrication of β-TCP foam: Effects of magnesium oxide as phase stabilizer on its properties. Ceram. Int. 2015, 41, 14245–14250. [Google Scholar] [CrossRef]
- Salma-Ancane, A.; Stipniece, L.; Putnins, A.; Berzina-Cimdina, L. Development of Mg-containing porous β-tricalcium phosphate scaffolds for bone repair. Ceram. Int. 2015, 41, 4996–5004. [Google Scholar] [CrossRef]
- Wang, S.; Liu, R.; Yao, J.; Wang, Y.; Li, H.; Dao, R.; Guan, J.; Tang, G. Fabrication of mesoporous magnesium substituted β-tricalcium phosphate nanospheres by self-transformation and assembly involving EDTA ions. Microporous Microporous Mater. 2013, 179, 172–181. [Google Scholar] [CrossRef]
- Tavares, D.; Castro, L.O.; Soares, G.D.A.; Alves, G.G.; Granjeiro, J.M. Synthesis and cytotoxicity evaluation of granular magnesium substituted β-tricalcium phosphate. J. Appl. Oral Sci. 2013, 21, 37–42. [Google Scholar] [CrossRef]
- Grigg, T.; Mee, M.; Mallinson, P.M.; Fong, S.K.; Gan, Z.; Dupree, R.; Holland, D. Cation substitution in β-tricalcium phosphate investigated using multi-nuclear, solid-state NMR. J. Solid State Chem. 2014, 212, 227–236. [Google Scholar] [CrossRef]
- Kannan, S.; Ventura, J.M.G.; Lemos, A.F.; Barba, A.; Ferreira, J.M.F. Effect of sodium addition on the preparation of hydroxyapatites and biphasic ceramics. Ceram. Int. 2008, 34, 7–13. [Google Scholar] [CrossRef]
- Kannan, S.; Ventura, J.M.G.; Ferreira, J.M.F. Synthesis and thermal stability of potassium substituted hydroxyapatites and hydroxyapatite/β-tricalcium phosphate mixtures. Ceram. Int. 2007, 33, 1489–1494. [Google Scholar] [CrossRef]
- Kannan, S.; Rebelo, A.; Lemos, A.F.; Barba, A.; Ferreira, J.M.F. Synthesis and mechanical behaviour of chlorapatite and chlorapatite/β-TCP composites. J. Eur. Ceram. Soc. 2007, 27, 2287–2294. [Google Scholar] [CrossRef]
- Matsumoto, A.; Yoshida, K.; Hashimoto, K.; Toda, Y. Thermal stability of β-tricalcium phosphate doped with monovalent metal ions. Mater. Res. Bull. 2009, 44, 1889–1894. [Google Scholar] [CrossRef]
- Meenambal, R.; Singh, R.K.; Kumar, P.N.; Kannan, S. Synthesis, structure, thermal stability, mechanical and antibacterial behaviour of lanthanum (La3+) substitutions in β-tricalcium phosphate. Mater. Sci. Eng. C 2014, 43, 598–606. [Google Scholar] [CrossRef] [PubMed]
- Mayer, A.; Cuisinier, F.J.G.; Gdalya, S.; Popov, I. TEM study of the morphology of Mn2+-doped calcium hydroxyapatite and β-tricalcium phosphate. J. Inorg. Biochem. 2008, 102, 311–317. [Google Scholar] [CrossRef] [PubMed]
- Ananth, P.; Shanmugam, S.; Jose, S.P.; Nathanael, A.J.; Oh, T.H.; Mangalaraj, D.; Ballamurugan, A.M. Structural and chemical analysis of silica-doped β-TCP ceramic coatings on surgical grade 316L SS for possible biomedical application. J. Asian Ceram. Soc. 2015, 3, 317–324. [Google Scholar] [CrossRef]
- Kawabata, A.; Yamamoto, T.; Kitada, A. Substitution mechanism of Zn ions in β-tricalcium phosphate. Phys. B Condens. Matter 2011, 406, 890–894. [Google Scholar] [CrossRef]
- Gokcekaya, O.; Ueda, K.; Ogasawara, K.; Kanetaka, H.; Narushima, T. In vitro evaluation of Ag-incorporated β-tricalcium phosphate. Mater. Sci. Eng. C 2017, 75, 926–933. [Google Scholar] [CrossRef] [PubMed]
- Rau, J.V.; Wu, V.M.; Graziani, V.; Fadeeva, I.V.; Fomin, A.S.; Fosca, M.; Uskoković, V. The bone building blues: Self-hardening copper-doped calcium phosphate cement and its in vitro assessment against mammalian cells and bacteria. Mater. Sci. Eng. C 2017, 79, 270–279. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Wu, C.; Li, H.; Yuen, J.; Chang, J.; Xiao, Y. Preparation, characterization and in vitro angiogenic capacity of cobalt substituted β-tricalcium phosphate ceramics. J. Mater. Chem. 2012, 22, 21686–21694. [Google Scholar] [CrossRef]
- Zhu, H.; Guo, D.; Qi, W.; Xu, K. Development of Sr-incorporated biphasic calcium phosphate bone cement. Biomed. Mater. 2017, 12, 015016. [Google Scholar] [CrossRef] [PubMed]
- Singh, R.K.; Srivastava, M.; Prasad, N.K.; Kannan, S. Structural analysis and hyperthermia effect of Fe3+/Ni2+ co-substitutions in β-Ca3(PO4)2. J. Alloys Compd. 2017, 725, 393–402. [Google Scholar] [CrossRef]
- Singh, R.K.; Srivastava, M.; Prasad, N.K.; Awasthi, S.; Dhayalan, A.; Kannan, S. Iron doped β-tricalcium phosphate: Synthesis, characterization, hyperthermia effect, biocompatibility and mechanical evaluation. Mater. Sci. Eng. C 2017, 78, 715–726. [Google Scholar] [CrossRef] [PubMed]
- Gomes, S.; Kaur, A.; Greneche, J.-M.; Nedelec, J.-M. Atomic scale modeling of iron-doped biphasic calcium phosphate bioceramics. Acta Biomater. 2017, 50, 78–88. [Google Scholar] [CrossRef] [PubMed]
- Kannan, S.; Goetz-Neunhoeffer, F.; Neubauer, J.; Pina, S.; Torres, P.M.C.; Ferreira, J.M.F. Synthesis and structural characterization of strontium-and magnesium-co-substituted β-tricalcium phosphate. Acta Biomater. 2010, 6, 571–576. [Google Scholar] [CrossRef] [PubMed]
- Matsumoto, N.; Yoshida, K.; Hashimoto, K.; Toda, Y. Preparation of beta-tricalcium phosphate powder substituted with Na/Mg ions by polymerized complex method. J. Am. Ceram. Soc. 2010, 93, 3663–3670. [Google Scholar] [CrossRef]
- Meenambal, R.; Kumar, P.N.; Poojar, P.; Geethanath, S.; Kannan, S. Simultaneous substitutions of Gd3+ and Dy3+ in β-Ca3(PO4)2 as a potential multifunctional bio-probe. Mater. Des. 2017, 120, 336–344. [Google Scholar] [CrossRef]
- El Khouri, A.; Elaatmani, M.; Ventura, G.D.; Sodo, A.; Rizzi, R.; Rossi, M.; Capitelli, A. Synthesis, structure refinement and vibrational spectroscopy of new rare-earth tricalcium phosphates Ca9RE(PO4)7 (RE = La, Pr, Nd, Eu, Gd, Dy, Tm, Yb). Ceram. Int. 2017, 43, 15645–15653. [Google Scholar] [CrossRef]
- Goldberg, M.A.; Smirnov, V.V.; Protsenko, P.V.; Antonova, O.S.; Smirnov, S.V.; Fomina, A.A.; Konovalov, A.A.; Leonov, A.V.; Ashmarin, A.A.; Barinov, S.M. Influence of aluminium substitutions on phase composition and morphology of β-tricalcium phosphate nanopowders. Ceram. Int. 2017, 43, 13881–13884. [Google Scholar] [CrossRef]







© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Laskus, A.; Kolmas, J. Ionic Substitutions in Non-Apatitic Calcium Phosphates. Int. J. Mol. Sci. 2017, 18, 2542. https://doi.org/10.3390/ijms18122542
Laskus A, Kolmas J. Ionic Substitutions in Non-Apatitic Calcium Phosphates. International Journal of Molecular Sciences. 2017; 18(12):2542. https://doi.org/10.3390/ijms18122542
Chicago/Turabian StyleLaskus, Aleksandra, and Joanna Kolmas. 2017. "Ionic Substitutions in Non-Apatitic Calcium Phosphates" International Journal of Molecular Sciences 18, no. 12: 2542. https://doi.org/10.3390/ijms18122542
APA StyleLaskus, A., & Kolmas, J. (2017). Ionic Substitutions in Non-Apatitic Calcium Phosphates. International Journal of Molecular Sciences, 18(12), 2542. https://doi.org/10.3390/ijms18122542