Essential Oils from Neotropical Piper Species and Their Biological Activities
Abstract
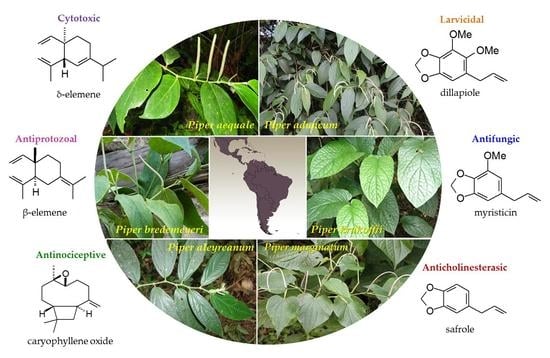
1. Introduction
2. Volatile Profiles
3. Biological Activities
3.1. Antibacterial and Antifungal Activity
3.2. Antiprotozoal Activity
3.3. Anticholinesterase Potential
3.4. Anti-Inflammatory and Antinociceptive Effects
3.5. Cytotoxic Activity
4. Composition-Bioactivity Correlation
5. Conclusions
Author Contributions
Conflicts of Interest
Abbreviations
| ACh | Acetylcholine |
| AChE | Acetylcholine esterase |
| DL | Detection limit |
| DPPH | 2,2-Diphenyl-1-picrylhydrazyl (radical) |
| EO | Essential oil |
| FIOCRUZ | Fundação Oswaldo Cruz (Oswaldo Cruz Foundation) |
| GC-MS | Gas chromatography-mass spectrometry |
| HD | Hydrodistillation |
| IC50 | Median inhibitory concentration |
| ID50 | Median inhibitory dose |
| LC50 | Median lethal concentration |
| MFC | Minimum fungicidal concentration |
| MH | Monoterpene hydrocarbons |
| MIC | Minimum inhibitory concentration |
| MWHD | Microwave-assisted hydrodistillation |
| OM | Oxygenated monoterpenoids |
| OS | Oxygenated sesquiterpenoids |
| PCA | Principal component analysis |
| PP | Phenylpropanoids |
| RI | Retention index |
| SD | Steam distillation |
| SH | Sesquiterpene hydrocarbons |
| spp. | Species (plural) |
| TLC | Thin-layer chromatography |
Appendix A
| Piper species | Collection Site | Essential Oil | Major Components (>5%) | Bioactivity of EO | Ref. |
|---|---|---|---|---|---|
| P. abutiloides Kunth | Cultivated (State University of Campinas, São Paulo, Brazil) | Leaf (HD) | --- | Antibacterial (Escherichia coli, MIC 700 μg/mL) | [52] |
| P. acutifolium Ruiz & Pav. | La Florida, Cajamarca, Peru | Leaf (HD) | (E)-β-Ocimene (8.1%), α-copaene (6.1%), β-caryophyllene (7.9%), allo-aromadendrene (6.0%), α-cadinene (6.7%), δ-cadinene (6.8%), dillapiole (5.9%) | --- | [99] |
| P. aduncum L. | Serra do Navio, Amapá state, Brazil | Aerial parts (HD) | Limonene (5.2%), γ-terpinene (7.1%), terpinen-4-ol (11.0%), piperitone (15.1%), dillapiole (31.5%) | --- | [15] |
| P. aduncum L. | Melgaço, Pará state, Brazil | Aerial parts (HD) | γ-Terpinene (6.5%), terpinen-4-ol (7.3%), piperitone (13.9%), dillapiole (50.8%) | --- | [15] |
| P. aduncum L. | Benfica, Pará state, Brazil | Aerial parts (HD) | Piperitone (7.0%), dillapiole (56.3%) | --- | [15] |
| P. aduncum L. | Belém, Pará state, Brazil | Aerial parts (HD) | Dillapiole (82.2%) | --- | [15] |
| P. aduncum L. | Belém, Pará state, Brazil | Aerial parts (HD) | Dillapiole (86.9%) | --- | [15] |
| P. aduncum L. | Manaus, Amazonas state, Brazil | Aerial parts (HD) | Dillapiole (91.1%) | --- | [15] |
| P. aduncum L. | Manaus-Caracaraí, Amazonas, Brazil | Aerial parts (HD) | Dillapiole (97.3%) | --- | [15] |
| P. aduncum L. | Cruzero do Sul, Acre state, Brazil | Aerial parts (HD) | Dillapiole (88.1%) | --- | [15] |
| P. aduncum L. | Pinar del Río, Cuba | Leaf (HD) | Dillapiole (82.2%) | --- | [27] |
| P. aduncum L. | Valle del Sajta, Cochabamba, Bolivia | Leaf (HD) | α-Pinene (9.0%), β-pinene (7.1%), limonene (5.0%), 1,8-cineole (40.5%), asaricin (12.9%) | --- | [100] |
| P. aduncum L. | Altos de Campana National Park, Panama | Leaf (HD) | α-Pinene (8.8%), linalool (8.6%), β-caryophyllene (17.4%), aromadendrene (13.4%) | --- | [100] |
| P. aduncum L. | Reserva da Ripasa, Ibaté, São Paulo state, Brazil | Leaf (HD) | (E)-β-Ocimene (5.0%), linalool (31.7%), β-caryophyllene (9.1%), α-humulene (5.5%), bicyclogermacrene (11.2%), (E)-nerolidol (10.4%) | Antifungal, TLC bioautography (Cladosporium sphareospermum) | [59] |
| P. aduncum L. | Reserva da Ripasa, Ibaté, São Paulo state, Brazil | Floral (HD) a | α-Terpinene (6.8%), (Z)-β-ocimene (5.6%), (E)-β-ocimene (11.1%), γ-terpinene (12.0%), linalool (41.2%), (E)-nerolidol (6.1%) | --- | [59] |
| P. aduncum L. | Reserva da Ripasa, Ibaté, São Paulo state, Brazil | Stem (HD) | α-Pinene (7.2%), β-pinene (14.2%), limonene (8.7%), (Z)-β-ocimene (5.5%), (E)-β-Ocimene (13.3%), linalool (11.8%), β-caryophyllene (7.6%), α-humulene (6.3%), (E)-nerolidol (10.6%) | Antifungal, TLC bioautography (Cladosporium cladosporioides, Cladosporium sphareospermum) | [59] |
| P. aduncum L. | Brejo da Madre de Deus, Matas Serranas, Pernambuco state, Brazil | Leaf (HD) | (E)-Nerolidol (80.6–82.5%), longipinanol (2.4–5.6%) | --- | [26] |
| P. aduncum L. | Serra Negra, Matas Serranas, Pernambuco state, Brazil | Leaf (HD) | (E)-Nerolidol (79.2–81.2%), longipinanol (11.1–13.6%) | --- | [26] |
| P. aduncum L. | Cultivated (State University of Campinas, São Paulo, Brazil) | Leaf (HD) | --- | Antibacterial (Escherichia coli, MIC 500 μg/mL) | [52] |
| P. aduncum L. | Bulo Bulo, Bolivia | Leaf (SD) | α-Pinene (8.0–8.9%), β-pinene (6.6–7.0%), 1,8-cineole (42.0–42.5%), (E)-β-ocimene (6.4%), bicyclogermacrene (3.8–6.0%), asaricin (9.2–10.5%) | --- | [101] |
| P. aduncum L. | Wasak'entsa reserve, Ecuador | Aerial parts (HD) | (E)-β-Ocimene (10.4%), piperitone (8.5%), dillapiole (45.9%) | Antifungal activity against dermatophytes (Trichophyton mentagrophytes, MIC 500 μg/mL, IC50 92.7 μg/mL; Trichophyton tonsurans, MIC 500 μg/mL, IC50 108.7 μg/mL; Nantzzia cajetani, IC50 195 μg/mL) | [65] |
| P. aduncum L. | Ducke Reserve, Manaus, Amazonas state, Brazil | Leaf (HD) | Dillapiole (94.8%) | Acaricidal (Rhipicephalus (Boophilus) microplus, LC50 9.3 mg/mL) | [28] |
| P. aduncum L. | Santo Antonio do Tauá, Pará state, Brazil | Aerial parts (HD) | Dillapiole (86.9%) | Larvicidal and insecticidal activity against mosquitoes (Anopheles marajoara, LC50 50.9 μg/mL, 417 μg/mL, respectively; Aedes aegypti, LC50 54.5 μg/mL, 401 μg/mL, respectively) | [16] |
| P. aduncum L. | Araraquara, São Paulo state, Brazil | Leaf (HD) | (E)-β-Ocimene (5.0%), linalool (31.8%), β-caryophyllene (9.3%), α-humulene (5.5%), bicyclogermacrene (11.3%), (E)-nerolidol (10.3%) | Antifungal, broth dilution assay (Cryptococcus neoformans, MIC 62.5 μg/mL) | [35] |
| P. aduncum L. | Brazlândia, Distrito Federal, Brazil | Leaf (HD) | β-Phellandrene (6.8%), γ-terpinene (8.3%), terpinen-4-ol (15.0%), piperitone (22.7%), asaricin (5.6%) | --- | [16] |
| P. aduncum L. | Parque do Guará, Distrito Federal, Brazil | Leaf (HD) | β-Phellandrene (6.6%), γ-terpinene(8.2%), terpinen-4-ol (16.8%), piperitone (24.9%) | --- | [16] |
| P. aduncum L. | Córrego Bananal, Distrito Federal, Brazil | Leaf (HD) | (E)-β-Ocimene (11.6%), terpinen-4-ol (6.7%), piperitone (11.0%), asaricin (15.8%) | --- | [16] |
| P. aduncum L. | Fazenda Água Limpa, Distrito Federal, Brazil | Leaf (HD) | Piperitone (16.3%), dillapiole (49.5%) | --- | [16] |
| P. aduncum L. | Mata de Dois Irmãos, Recife, Pernambuco, Brazil | Leaf (HD) | Dillapiole (79.0%) | --- | [29] |
| P. aduncum L. | Belém, Pará state, Brazil | Aerial parts (HD) | Dillapiole (64.4%) | Insecticidal (Solenopsis saevissima, IC50 135 μg/mL) | [41] |
| P. aduncum L. | Bocaiuva, Minas Gerais state, Brazil | Leaf (HD) | α-Pinene (14.2%), β-pinene (9.0%), 1,8-cineole (57.2%) | --- | [25] |
| P. aduncum L. | Montes Claros, Minas Gerais state, Brazil | Leaf (HD) | (E)-β-Ocimene (13.4%), valencene (6.9%), (E)-nerolidol (5.9%) | --- | [25] |
| P. aduncum L. | Topes de Collantes Nature Reserve, Escambray Mountains, Cuba | Leaf (HD) | Camphene (10.9%), 1,8-cineole (8.7%), camphor (17.1%), piperitone (34.0%), viridiflorol (7.4%) | Antioxidant (DPPH radical scavenging assay, IC50 30.1 μg/mL) | [50] |
| P. aduncum L. | Gallery Forest, Angico River, Minas Gerais state, Brazil | Leaf (HD) | 1,8-Cineole (55.8%), α-terpineol (5.9%) | Egg hatch inhibition (Haemonchus contortus, IC50 2.6 mg/mL) | [102] |
| P. aduncum L. | Santo Antonio do Tauá, Pará state, Brazil | Aerial parts (HD) | Dillapiole (85.9%) | Antifungal activity against dermatophytes (Trichophyton mentagrophytes, MIC 500 μg/mL; Epidermophyton floccosum, MIC 500 μg/mL; Microsporum canis, MIC 250 μg/mL; Microsporum gypseum, MIC 250 μg/mL; Aspergillus fumigatus, MIC 3.9 μg/mL) | [30] |
| P. aduncum L. | Cultivated, Federal University of Lavras, Brazil | Leaf (HD) | Linalool (9.3–13.4%), β-caryophyllene (5.1–6.7%), α-humulene (8.5–10.6%), (E)-nerolidol (14.3–16.7%), spathulenol (0–5.6%), cis-cadin-4-en-7-ol (7.5–12.2%) | --- | [103] |
| P. aduncum L. | Cultivated, Federal University of Lavras, Brazil | Root (HD) | α-Selinene (14.1–16.5%), geranyl 2-methylbutyrate (8.9–13.6%), bulnesol (4.6–6.1%), elemicin (4.6–5.9%), dillapiole (13.0–18.4%), apiole (16.3–29.5%) | --- | [103] |
| P. aduncum L. | Monte Alegre do Sul, São Paulo state, Brazil | Leaf (HD) | α-Pinene (6.4%), safrole (13.3%), valencene (9.7%), spathulenol (10.6%), asaricin (14.9%) | --- | [31] |
| P. aduncum L. | Votuporanga, São Paulo state, Brazil | Leaf (HD) | Safrole (10.8%), asaricin (80.1%) | --- | [31] |
| P. aduncum L. | Votuporanga, São Paulo state, Brazil | Leaf (HD) | Safrole (10.5%), asaricin (73.4%) | --- | [31] |
| P. aduncum L. | Belém, Pará state, Brazil | Aerial parts (HD) | Dillapiole (73.0%) | --- | [62] |
| P. aduncum L. | Cerro Azul, Paraná state, Brazil | Leaf (HD) | (Z)-β-Ocimene (7.0%), (E)-β-ocimene (13.9%), safrole (6.2%), bicyclogermacrene (20.9%), γ-cadinene (5.5%), spathulenol (5.3%) | Antileishmanial (L. amazonensis promastigotes, IC50 25.9 μg/mL; L. amazonensis axenic amastigotes, IC50 36.2 μg/mL) | [70] |
| P. aduncum L. | Universidade Federal de Lavras, Matto Grosso state, Brazil | Leaf (HD) | Linalool (13.4%), (E)-nerolidol (25.2%), spathulenol (6.3%) | Antitrypanosomal (T. cruzi trypomastigotes, IC50 2.8 μg/mL; linalool is the active agent, IC50 0.31 μg/mL) Antileishmanial (L. braziliensis promatigotes, IC50 77.9 μg/mL; (E)-nerolidol is the active agent, IC50 74.3 μg/mL) | [67,68] |
| P. aduncum L. | Institute of Pharmacy and Food, Havana, Cuba | Aerial parts (HD) | Camphene (5.9%), camphor (17.1%) piperitone (23.7%), viridiflorol (14.5%) | Antiprotozoal (Plasmodium falciparum, IC50 1.3 μg/mL; Trypanosoma brucei, IC50 2.0 μg/mL; Trypanosoma cruzi, IC50 2.1 μg/mL; Leishmania amazonensis, IC50 23.8 μg/mL; Leishmania donovani, IC50 7.7 μg/mL; Leishmania infantum, IC50 8.1 μg/mL) | [32] |
| P. aduncum subsp. ossanum (C. DC.) Saralegui [syn. P. ossanum (C. DC.) Trel.] | Pinar del Río, Cuba | Leaf (HD) | Camphene (6.1%), camphor (8.3%), piperitone (12.9%), β-caryophyllene (6.7%), germacrene D (8.2%), 1-epi-cubenol (6.2%) | --- | [104] |
| P. aduncum subsp. ossanum (C. DC.) Saralegui [syn. P. ossanum (C. DC.) Trel.] | Artemisa Province, Cuba | Leaf (HD) | Camphene (5.4–7.4%), camphor (9.4–13.9%), piperitone (19.0–20.1%), viridiflorol (13.0–18.8%) | Antiprotozoal (Plasmodium falciparum, IC50 1.5 μg/mL; Trypanosoma brucei, IC50 8.1 μg/mL; Trypanosoma cruzi, IC50 8.0 μg/mL; Leishmania amazonensis, IC50 19.3 μg/mL; Leishmania infantum, IC50 32.5 μg/mL), antibacterial (Staphylococcus aureus, IC50 39.5 μg/mL) | [53] |
| P. aequale Vahl | Monteverde, Costa Rica | Leaf (HD) | α-Pinene (39.3%), sabinene (18.4%), limonene (6.7%) | Antibacterial (Bacillus cereus, MIC 156 μg/mL) | [34] |
| P. aequale Vahl | Carajás National Forest, Parauapebas, Pará state, Brazil | Aerial parts (HD) | α-Pinene (12.6%), β-pinene (15.6%), δ-elemene (19.0%), bicyclogermacrene (5.5%), cubebol (7.2%), β-atlantol (5.9%) | Cytotoxic (HCT-116 human colorectal carcinoma, IC50 8.69 μg/mL; ACP03 human gastric adenocarcinoma, IC50 1.54 μg/mL; essential oil induced apoptosis in ACP03 cells) | [95] |
| P. sp. aff. aereum | Monteverde, Costa Rica | Leaf (HD) | β-Caryophyllene (6.6%), δ-cadinene (7.3%), guaiol (41.2%), α-muurolol (5.8%), α-cadinol (9.2%) | Antibacterial (Bacillus cereus, MIC 78 μg/mL; Staphylococcus aureus, MIC 78 μg/mL), cytotoxic (MCF-7 human breast adenocarcinoma) | [34] |
| P. aleyreanum C. DC. | Porto Velho, Rondônia state, Brazil | Leaf (HD) | α-Pinene (7.0%), β-pinene (14.4%), α-phellandrene (8.6%), (Z)-caryophyllene (17.5%), β-caryophyllene (18.6%), δ-cadinene (6.2%) | --- | [105] |
| P. aleyreanum C. DC. | Porto Velho, Rondônia state, Brazil | Aerial parts (HD) | Camphene (5.2%), β-pinene (9.0%), spathulenol (6.7%), caryophyllene oxide (11.5%) | Antinociceptive, anti-inflammatory (mouse model) | [90] |
| P. aleyreanum C. DC. | Carajás National Forest, Parauapebas, Pará state, Brazil | Aerial parts (HD) | δ-Elemene (8.2%), β-elemene (16.3%), β-caryophyllene (6.2%), germacrene D (6.9%), bicyclogermacrene (9.2%), spathulenol (5.2%) | Antifungal, TLC bioautography (Cladosporium cladosporioides, Cladosporium sphaerospermum), cytotoxic (SKMel19 human melanoma, IC50 7.4 μg/mL) | [61] |
| P. amalago L. | Fazenda Sucupira, Embrapa, Brasília, Brazil | Leaf (HD) | α-Pinene (30.5%), camphene (8.9%), limonene (6.8%), borneol (5.7%) | --- | [33] |
| P. amalago L. | Monteverde, Costa Rica | Leaf (HD) | α-Phellandrene (1.7–8.1%), β-elemene (11.5–24.6%), β-caryophyllene (15.9–23.3%), germacrene D (28.9–29.4%), germacrene A (6.5–9.7%) | --- | [34] |
| P. amalago L. | Morro Reuter, Rio Grande do Sul state, Brazil | Aerial parts (HD) | α-Pinene (5.2%), limonene (20.5%), δ-elemene (6.8%), zingiberene (11.2%) | --- | [106] |
| P. amalago L. | Universidade de São Paulo, Brazil | Leaf (HD) | γ-Muurolene (7.3%), germacrene D (9.9%), bicyclogermacrene (27.9%), spathulenol (19.2%), α-cadinol (7.6%) | --- | [35] |
| P. amalago L. | Dourados, Mato Grosso do Sul, Brazil | Leaf (HD) | p-Cymene (9.4%), methyl geranate (7.8%), α-amorphene (25.7%), cubenol (6.2%) | --- | [107] |
| P. amalago L. | Dourados, Mato Grosso do Sul, Brazil | Stem (HD) | Longifolene (6.6%), α-amorphene (23.3%), α-muurolol (9.3%) | --- | [107] |
| P. amalago L. | Dourados, Mato Grosso do Sul, Brazil | Root (HD) | α-Amorphene (14.4%) | --- | [107] |
| P. amalago L. | Dourados, Mato Grosso do Sul, Brazil | Floral (HD) a | p-Cymene (9.3%), limonene (10.5%), silphiperfol-6-ene (13.5%), allo-aromadendrene (18.5%), α-muurolol (5.0%) | --- | [107] |
| P. amalago L. | Campinas, São Paulo state, Brazil | Leaf (HD) | α-Pinene (14.8%), β-phellandrene (39.3%), germacrene D (11.7%) | --- | [31] |
| P. amalago L. | Campinas, São Paulo state, Brazil | Leaf (HD) | α-Pinene (6.7%), sabinene (6.7%), β-phellandrene (15.9%), bicyclogermacrene (20.8%), spathulenol (9.1%) | --- | [31] |
| P. amalago L. | Campinas, São Paulo state, Brazil | Leaf (HD) | α-Pinene (11.7%), β-phellandrene (33.1%), bicyclogermacrene (15.0%) | --- | [31] |
| P. amalago L. | Adamantina, São Paulo state, Brazil | Leaf (HD) | Sabinene (8.2%), myrcene (6.8%), β-phellandrene (12.3%), bicyclogermacrene (19.4%), γ-muurolene (5.9%), spathulenol (5.6%) | --- | [31] |
| P. amalago var. medium (Jacq.) Yunck. | Fênix, Paraná state, Brazil | Floral (HD) a | β-Phellandrene (7.3–8.2%), bicyclogermacrene (3.0–9.1%), δ-cadinene (2.3–6.6%), (E)-nerolidol (14.2–19.9%), germacrene D-4-ol (10.3–12.7%), τ-cadinol (4.9–6.1%), α-cadinol (8.2–11.1%) | --- | [108] |
| P. amplum Kunth | Pariquera-Açu, São Paulo state, Brazil | Leaf (HD) | α-Pinene (18.1%), (Z)-β-ocimene (10.5%), limonene (8.6%), β-caryophyllene (8.8%), germacrene D (5.5%) | --- | [31] |
| P. angustifolium Lam. | Cuzco, Peru | Aerial parts (HD) | Camphene (22.4%), camphor (25.3%), isoborneol (12.8%) | Antibacterial, broth dilution assay (Pseudomonas aeruginosa, MIC 30 μg/mL; Escherichia coli, MIC 100 μg/mL); antifungal, broth dilution assay (Trichophyton mentagrophytes, MIC 10 μg/mL; Candida albicans, MIC 50 μg/mL; Cryptococcus neoformans, MIC 50 μg/mL; Aspergillus flavus, MIC 100 μg/mL) | [109] |
| P. angustifolium Lam. | Abobral Subregion of the Pantanal of Mato Grosso do Sul, Brazil | Leaf (HD) | α-Pinene (5.9%), (E)-nerolidol (5.8%), spathulenol (23.8%), caryophyllene oxide (13.1%) | Antileishmanial (L. infantum amastigotes, IC50 1.43 μg/mL) | [69] |
| P. anonifolium Kunth | Bujaru, Pará state, Brazil | Aerial parts (HD) | α-Pinene (41.1–45.7%), β-pinene (17.2–18.6%), limonene (6.1–8.5%), β-caryophyllene (2.5–6.3%) | --- | [110] |
| P. anonifolium Kunth | Santa Isabel, Pará state, Brazil | Aerial parts (HD) | α-Pinene (53.1%), β-pinene (22.9%) | --- | [110] |
| P. anonifolium Kunth | Ananindeua, Pará state, Brazil | Aerial parts (HD) | α-Pinene (7.3%), limonene (5.9%), ishwarane (19.1%), germacrene D (9.6%), α-eudesmol (33.5%) | --- | [110] |
| P. anonifolium Kunth | Carajás National Forest, Parauapebas, Pará state, Brazil | Aerial parts (HD) | α-Pinene (8.8%), β-selinene (12.7%), α-selinene (11.9%), selin-11-en-4α-ol (20.0%) | Antifungal, TLC bioautography (Cladosporium cladosporioides, Cladosporium sphaerospermum); enzyme inhibitory, TLC bioautography (acetylcholinesterase) | [61] |
| P. arboreum Aubl. | Chepo, Panama | Leaf (HD) | β-Pinene (6.6%), α-copaene (7.4%), germacrene D (5.3%), δ-cadinene (25.8%), (E)-nerolidol (5.2%) | --- | [111] |
| P. arboreum Aubl. | Fazenda Sucupira, Embrapa, Brasília, Brazil | Leaf (HD) | Bicyclogermacrene (12.1%), spathulenol (8.4%), caryophyllene oxide (10.2%) b | --- | [33] |
| P. arboreum Aubl. | Universidade Estadual Paulista, Araraquara, São Paulo state, Brazil | Leaf (HD) | β-Caryophyllene (25.1%), germacrene D (9.6%), bicyclogermacrene (49.5%) | --- | [59] |
| P. arboreum Aubl. | Universidade Estadual Paulista, Araraquara, São Paulo state, Brazil | Floral (HD) a | Limonene (6.3%), linalool (10.4%), β-elemene (5.3%), β-caryophyllene (6.6%), germacrene D (49.3%), germacrene A (8.5%) | --- | [59] |
| P. arboreum Aubl. | Universidade Estadual Paulista, Araraquara, São Paulo state, Brazil | Stem (HD) | δ-3-Carene (18.7%), α-copaene (9.0%), β-caryophyllene (26.5%), bicyclogermacrene (21.1%) | --- | [59] |
| P. arboreum Aubl. | Antonina, Paraná state, Brazil | Leaf (HD) | α-Copaene (5.6%), β-caryophyllene (12.6%), trans-cadina-1(6),4-diene (9.6%), spathulenol (7.9%), caryophyllene oxide (5.9%), 1-epi-cubenol (10.4%), α-cadinol (5.4%) | Antileishmanial (L. amazonensis promastigotes, IC50 15.2 μg/mL; L. amazonensis axenic amastigotes, IC50 > 200 μg/mL) | [70] |
| P. arboreum var. latifolium (C. DC.) Yunck. | Rondônia state, Brazil | Leaf (SD) | Octanal (5.5%), germacrene D (72.9%), γ-elemene (6.8%) c | --- | [112] |
| P. artanthe C. DC. | San Migues, Santander, Colombia | Aerial parts (HD) | δ-Elemene (11.7%), β-caryophyllene (10.2%), epi-cubebol (8.9%), cubebol (6.3%), myristicin (6.4%), apiole (14.5%) | --- | [113] |
| P. augustum Rudge | Reserva Biológica Alberto Manuel Brenes, Costa Rica | Leaf (HD) | α-Pinene (10.5%), α-phellandrene (14.7%), limonene (13.0%), β-phellandrene (5.6%), linalool (10.3%), β-caryophyllene (13.5%) | --- | [114] |
| P. augustum Rudge | Valle de Anton, Cerro Caracoral, Cocle, Panama | Leaf (HD) | α-Pinene (6.0%), β-elemene (12.3%), cembrene (11.7%), cembratrienol 1 (25.4%), cembratrienol 2 (8.6%) | --- | [47] |
| P. auritum Kunth | Boca de Uracillo, Colon Province, Panama | Leaf (HD) | Safrole (70%) | --- | [115] |
| P. auritum Kunth | Güira de Melena, Cuba | Leaf (HD) | Safrole (64.5%) | --- | [116] |
| P. auritum Kunth | Monteverde, Costa Rica | Floral (HD) a | Safrole (93.2%) | --- | [49] |
| P. auritum Kunth | Universidad de La Habana, Cuba | Aerial parts (HD) | Safrole (86.9%) | Antileishmanial (promastigotes of L. major, IC50 29.1 μg/mL; L. mexicana, IC50 63.3 μg/mL; L. braziliensis, IC50 52.1 μg/mL; L. donovani, IC50 12.8 μg/mL; amastigotes of L. donovani, IC50 22.3 μg/mL) | [117] |
| P. auritum Kunth | Cali, Valle del Cauca, Colombia | Aerial parts (MWHD) | Safrole (91.3%) | --- | [118] |
| P. auritum Kunth | Topes de Collantes Nature Reserve, Escambray Mountains, Cuba | Leaf (HD) | Camphene (5.5%), safrole (71.8%) | Antioxidant (DPPH radical scavenging assay, IC50 14.8 μg/mL) | [50] |
| P. barbatum Kunth | Amazonas region, Peru | Aerial parts (HD) | Crocatone (10.9%), (E)-asarone (14.1%), apiole (8.0%), 2′-methoxy-4′,5′-methylenedioxy-propiophenone (29.5%) | --- | [119] |
| P. biasperatum Trel. | Monteverde, Costa Rica | Leaf (HD) | β-Elemene (46.4%), germacrene D (9.5%), bicyclogermacrene (14.1%), germacrene A (13.2%) | Cytotoxic (MCF-7 human breast adenocarcinoma) | [34] |
| P. bogotense C. DC. | Ipiales, Nariño, Colombia | Aerial parts (MWHD) | α-Pinene (8.7%), α-phellandrene (13.7%), limonene (5.3%), trans-sabinene hydrate (14.2%) | Antitrypanosomal (T. cruzi epimastigotes, IC50 10.1 μg/mL); cytotoxic (Vero cells, IC50 90.1 μg/mL), Antifungal, broth dilution assay (Trichophyton rubrum, MIC 79 μg/mL; Trichophyton mentagrophytes, MIC 500 μg/mL); cytotoxic (Vero cells, IC50 25.8 μg/mL) | [118,120,121] |
| P. brachypodon (Benth.) C. DC. | Quibdó, Chocó, Colombia | Aerial parts (MWHD) | β-Caryophyllene (20.2%), 9-epi-β-caryophyllene (5.8%), germacrene D (5.9%), bicyclogermacrene (8.1%), spathulenol (5.7%), caryophyllene oxide (10.8%) | --- | [120] |
| P. brachypodon (Benth.) C. DC. | Tutunendo, Chocó, Colombia | Aerial parts (MWHD) | β-Caryophyllene (20.2%), 9-epi-(E)-caryophyllene (5.8%), germacrene D (5.9%), bicyclogermacrene (8.1%), spathulenol (5.7%), caryophyllene oxide (10.8%) | Antiprotozoal (Trypanosoma cruzi epimastigotes, IC50 0.34 μg/mL; Leishmania infantum promastigotes, IC50 23.4 μg/mL); cytotoxic (Vero cells, IC50 30.5 μg/mL; THP-1 human monocytic leukemia, IC50 66.3 μg/mL) | [118] |
| P. brachypodon var. hirsuticaule Yunck. | Samurindó, Chocó, Colombia | Aerial parts (MWHD) | β-Elemene (6.4%), β-caryophyllene (9.8%), α-guaiene (5.9%), germacrene D (16.7%), bicyclogermacrene (6.2%) | Antiprotozoal (Trypanosoma cruzi epimastigotes, IC50 32.5 μg/mL; Leishmania infantum promastigotes, IC50 93.6 μg/mL); cytotoxic (Vero cells, IC50 86.4 μg/mL) | [118] |
| P. bredemeyeri Jacq. | Monteverde, Costa Rica | Leaf (HD) | β-Elemene (34.0%), β-caryophyllene (24.2%), germacrene D (21.7%), bicyclogermacrene (14.1%), germacrene A (11.4%) | Antibacterial, broth dilution assay (Bacillus cereus, MIC 78 μg/mL), enzyme inhibitory (cruzain, IC50 0.96 μg/mL) | [34] |
| P. bredemeyeri Jacq. | Pueblo Bello, Cesar, Colombia | Aerial parts (MWHD) | α-Pinene (20.3%), β-pinene (32.3%), β-caryophyllene (6.3%) | Antifungal, broth dilution assay (Trichophyton rubrum, MIC 157 μg/mL; Trichophyton mentagrophytes, MIC 125 μg/mL); cytotoxic (Vero cells, IC50 15.2 μg/mL) | [121] |
| P. caldense C. DC. | Recife, Pernambuco state, Brazil | Leaf (HD) | δ-Cadinene (5.6%), thujopsan-2β-ol (7.4%), α-muurolol (9.0%), α-cadinol (19.0%) | Antibacterial, agar diffusion assay (Bacillus subtilis, Staphylococcus aureus, Klebsiella pneumoniae) | [54] |
| P. caldense C. DC. | Recife, Pernambuco state, Brazil | Root (HD) | Valencene (10.5%), pentadecane (35.7%), selina-3,7(11)-diene (5.4%) | Antibacterial, agar diffusion assay (Bacillus subtilis, Staphylococcus aureus, Escherichia coli, Klebsiella pneumoniae, Pseudomonas aeruginosa) | [54] |
| P. caldense C. DC. | Recife, Pernambuco state, Brazil | Stem (HD) | Terpinen-4-ol (18.5%), α-terpineol (15.3%), caryophyllene oxide (6.2%), α-cadinol (9.8%) | Antibacterial, agar diffusion assay (Bacillus subtilis, Pseudomonas aeruginosa) | [54] |
| P. callosum Ruiz & Pav. | Marituba, Pará state, Brazil | Aerial parts (HD) | Safrole (69.2%), methyleugenol (8.6%) | Insecticidal (Solenopsis saevissima, IC50 > 500 μg/mL) | [41] |
| P. callosum Ruiz & Pav. | Barcarena, Pará state, Brazil | Aerial parts (HD) | Safrole (66.0%), methyl eugenol (10.2%) | Enzyme inhibitory (acetylcholinesterase) | [62] |
| P. carniconnectivum C. DC. | Porto Velho, Rondônia state, Brazil | Leaf (HD) | β-Pinene (6.3%), caryophyllene oxide (21.3%) | --- | [122] |
| P. carniconnectivum C. DC. | Porto Velho, Rondônia state, Brazil | Stem (HD) | α-Pinene (8.0%), β-pinene (19.0%), spathulenol (23.7%), caryophyllene oxide (7.8%) | --- | [122] |
| P. carpunya Ruiz & Pav. | Cajamarca region, Peru | Leaf (HD) | α-Terpinene (12.1%), p-cymene (10.9%), 1,8-cineole (13.0%), safrole (14.9%), bicyclogermacrene (6.7%), spathulenol (9.8%) | --- | [123] |
| P. carpunya Ruiz & Pav. | Cajamarca region, Peru | Floral (HD) a | α-Pinene (6.2%), α-terpinene (9.8%), p-cymene (7.7%), 1,8-cineole (30.2%), safrole (32.0%) | --- | [123] |
| P. cernuum Vell. | Universidade de São Paulo, Brazil | Leaf (HD) | α-Pinene (7.2%), β-pinene (6.2%), β-caryophyllene (20.7%), germacrene D (6.7%), bicyclogermacrene (21.9%) | Antimicrobial, agar diffusion assay (Staphylococcus aureus, Candida albicans) | [38] |
| P. cernuum Vell. | São Francisco de Assis Natural Reserve, Blumenau, Santa Catarina state, Brazil | Aerial parts (HD) | trans-Dihydroagarofuran (31.0%), elemol (12.0%), 10-epi-γ-eudesmol (13.0%) | --- | [124] |
| P. cernuum Vell. | Universidade de São Paulo, Brazil | Leaf (HD) | β-Elemene (7.2%), β-caryophyllene (22.2%), germacrene D (9.3%), bicyclogermacrene (25.1%), (Z)-α-bisabolene (5.7%), spathulenol (7.2%) | --- | [35,60] |
| P. cernuum Vell. | Universidade de São Paulo, Brazil | Floral (HD) a | α-Copaene (6.5%), β-caryophyllene (9.8%), germacrene D (14.3%), bicyclogermacrene (6.5%), spathulenol (9.7%) | Antifungal, TLC bioautography (Cladosporium cladosporioides, C. sphaerospermum) | [60] |
| P. cernuum Vell. | Reserva da Matinha, Ilhéus, Bahia state, Brazil | Leaf (HD) | β-Elemene (11.6%), β-caryophyllene (8.3%), cis-β-guaiene (8.2%), γ-muurolene (7.6%), epi-cubebol (13.1%), spathulenol (9.6%), caryophyllene oxide (7.7%), valeranone (9.1%) | --- | [125] |
| P. cernuum Vell. | Parque Ecológico do Pereque, Cubatão, São Paulo state, Brazil | Leaf (HD) | β-Elemene (30.0%), β-caryophyllene (16.3%), germacrene D (12.7%), bicyclogermacrene (19.9%) | Cytotoxic (B16F10-Nex2 murine melanoma, IC50 30 μg/mL; A2058 human melanoma, IC50 24 μg/mL; U87-MG human glioblastoma, IC50 19.1 μg/mL; HeLa human cervical tumor, IC50 23 μg/mL; HL-60 humal myloid leukemia, IC50 16 μg/mL) | [39] |
| P. cernuum Vell. | Parque Ecológico do Pereque, Cubatão, São Paulo state, Brazil | Branches (HD) | Camphene (46.4%), p-cymene (5.8%), linalool (8.7%), α-terpineol (11.6%), carvacrol (11.6%) d | Cytotoxic (B16F10-Nex2 murine melanoma, IC50 39.0 μg/mL; A2058 human melanoma, IC50 24.6 μg/mL; U87-MG human glioblastoma, IC50 19.0 μg/mL; HeLa human cervical tumor, IC50 23.6 μg/mL; HL-60 humal myloid leukemia, IC50 15.5 μg/mL) | [96] |
| P. cernuum Vell. | Ubatuba, São Paulo state, Brazil | Leaf (HD) | α-Pinene (10.0%), camphene (6.3%), trans-dihydroagarofuran (28.7%), 10-epi-γ-eudesmol (13.5%), 4-epi-cis-dihydroagarofuran (10.8%) | --- | [31] |
| P. cernuum Vell. | Pariquera-Açu, São Paulo state, Brazil | Leaf (HD) | α-Pinene (11.8%), camphene (8.7%), trans-dihydroagarofuran (33.8%), 10-epi-γ-eudesmol (12.2%) | --- | [31] |
| P. cernuum Vell. | Antonina, Paraná state, Brazil | Leaf (HD) | α-Pinene (11.4%), β-pinene (7.9%), β-elemene (10.1%), β-caryophyllene (6.9%), spathulenol (11.5%), caryophyllene oxide (5.1%), τ-muurolol (6.2%), α-muurolol (5.8%) | Antileishmanial (L. amazonensis promastigotes, IC50 27.1 μg/mL; L. amazonensis axenic amastigotes, IC50 > 200 μg/mL), anti-Mycobacterium tuberculosis (MIC 125 μg/mL) | [70] |
| P. cernuum Vell. | Blumenau, Santa Catarina state, Brazil | Leaf (HD) | α-Pinene (2.6–5.4%), β-caryophyllene (5.9–8.7%), 4-epi-cis-dihydroagarofuran (11.2–13.4%), trans-dihydroagarofuran (30.0–36.7%), elemol (5.9–9.2%), γ-eudesmol (8.3–13.3%) | Antibacterial, agar dilution assay (Bacillus subtilis, MIC 48 μg/mL; Staphylococcus aureus, MIC 780 μg/mL; Streptococcus pyogenes, MIC 780 μg/mL); antifungal, agar dilution assay (Microsporum canis, Microsporum gypseum, Trichophyton mentagrophytes, Trichophyton rubrum, Epidermophyton flocosum, Cryptococcus neoformans, MIC 48 μg/mL) | [37] |
| P. cernuum Vell. var. cernuum | Tijuca Forest, Rio de Janeiro state, Brazil | Leaf (HD) | α-Pinene (10.2%), camphene (5.3%), β-pinene (7.4%), cis-dihydroagarofuran (32.3%), elemol (6.7%) | --- | [36] |
| P. claussenianum (Miq.) C. DC. | São Manoel, Castelo, Espírito Santo, Brazil | Leaf (HD) | Linalool (2.1–5.2%), (E)-nerolidol (81.4–83.3%) | Antileishmanial (promastigotes of L. amazonensis, IC50 30.24 μg/mL) Anticandidal (C. albicans, MIC 0.2–1.26%) | [71,126] |
| P. claussenianum (Miq.) C. DC. | São Manoel, Castelo, Espírito Santo, Brazil | Floral (HD) a | Linalool (50.2–54.5%), (E)-nerolidol (22.7–24.3%) | Antileishmanial (promastigotes of L. amazonensis, IC50 1328 μg/mL) Anticandidal (C. albicans, MIC 0.04–0.1%) Antiparasitic (Plasmodium falciparum W2, IC50 7.9 μg/mL) | [71,73,126] |
| P. corcovadense (Miq.) C. DC. | Jardim Botânico de Recife, Pernambuco, Brazil | Leaf (HD) | α-Pinene (5.9%), terpinolene (17.4%), 4-butyl-1,2-methylenedioxybenzene (30.6%), β-caryophyllene (6.3%) | Mosquito larvicidal activity (Aedes aegypti, LC50 30.5 μg/mL) | [127] |
| P. corrugatum Kuntze | Valle de Anton, Cerro Caracoral, Cocle, Panama | Leaf (HD) | α-Pinene (12.2%), β-pinene (26.6%), limonene (8.2%), p-cymene (8.6%), 1,8-cineole (5.9%), (E)-nerolidol (12.8%), caryophyllene oxide (8.5%) | --- | [47] |
| P. crassinervium Kunth | Universidade de São Paulo, Brazil | Leaf (HD) | β-Caryophyllene (8.1%), germacrene D (14.0%), bicyclogermacrene (9.2%), epi-α-selinene (5.0%), (E)-nerolidol (8.2%), spathulenol (9.8%), guiaol (5.8%), β-eudesmol (10.1%) | Antifungal, TLC bioautography (Cladosporium cladosporioides, Cladosporium sphaerospermum) | [35,60] |
| P. crassinervium Kunth | Mococa, São Paulo state, Brazil | Leaf (HD) | α-Pinene (11.5%), β-pinene (11.6%), β-caryophyllene (7.8%), germacrene D (9.2%), bicyclogermacrene (5.1%), guaiol (5.5%) | --- | [31] |
| P. curtispicum C. DC. | Altos de Campana, Panama | Leaf (HD) | α-Pinene (19.4%), limonene (8.1%), β-caryophyllene (13.9%) | --- | [47] |
| P. cyrtopodon C. DC. | Marituba, Pará state, Brazil | Aerial parts (HD) | α-Cubebene (5.1%), β-caryophyllene (19.2%), germacrene D (10.0%), bicyclogermacrene (13.0%), spathulenol (8.4%) | --- | [128] |
| P. cyrtopodon C. DC. | Santarém, Pará state, Brazil | Aerial parts (HD) | p-Cymene (6.3%), germacrene D (17.9%), bicyclogermacrene (23.3%), (E)-nerolidol (6.6%), spathulenol (6.9%) | --- | [128] |
| P. cyrtopodon C. DC. | Ananindeua, Pará state, Brazil | Aerial parts (HD) | α-Pinene (7.5%), β-pinene (6.0%), β-caryophyllene (34.6%), germacrene D (13.6%), bicyclogermacrene (21.4%), spathulenol (8.4%) | --- | [128] |
| P. cyrtopodon C. DC. | Ananindeua, Pará state, Brazil | Aerial parts (HD) | β-Caryophyllene (18.8%), germacrene D (14.8%), bicyclogermacrene (14.0%), germacrene B (26.8%) | --- | [128] |
| P. cyrtopodon C. DC. | Bujaru, Pará state, Brazil | Aerial parts (HD) | α-Cubebene (6.7%), β-caryophyllene (18.1%), germacrene D (13.6%), bicyclogermacrene (14.9%), germacrene B (10.1%) | --- | [128] |
| P. cyrtopodon C. DC. | Manaus, Amazonas state, Brazil | Aerial parts (HD) | Germacrene D (7.5%), bicyclogermacrene (8.3%), α-cadinol (9.5%), epi-α-bisabolol (26.3%) | --- | [128] |
| P. dactylostigmum Yunck. | Itacoatiara, Amazonas State, Brazil | Aerial parts (HD) | β-Caryophyllene (8.9%), γ-muurolene (5.9%), β-selinene (9.0%), α-selinene (8.0%), caryophyllene oxide (6.0%), τ-muurolol (7.5%), α-cadinol (21.7%) | --- | [129] |
| P. darienense C. DC. | Parque Nacional Chagres, Panama | Leaf (HD) | Limonene (6.3%), (E)-β-farnesene (63.7%) | --- | [47] |
| P. demeraranum (Miq.) C. DC. | Belém, Pará state, Brazil | Aerial parts (HD) | α-Pinene (7.3%), sabinene (12.9%), β-pinene (7.7%), limonene (20.2%) | --- | [130] |
| P. demeraranum (Miq.) C. DC. | Ananindeua, Pará state, Brazil | Aerial parts (HD) | α-Pinene (6.1–12.3%), sabinene (17.0–22.7%), β-pinene (8.2–14.4%), limonene (30.6–40.3%) | --- | [130] |
| P. demeraranum (Miq.) C. DC. | Adolpho Ducke Reserve, Manaus, Amazonas state, Brazil | Leaf (HD) | β-Pinene (6.7%), limonene (19.3%), β-elemene (33.1%), β-caryophyllene (6.0%), germacrene D (5.2%), β-selinene (5.0%), bicyclogermacrene (8.8%) | Antileishmanial (L. amazonensis promastigotes, IC50 86.0 μg/mL; L. amazonensis amastigotes, IC50 78.0 μg/mL; L. guyanensis promastigotes, IC50 22.7 μg/mL) | [72] |
| P. dilatatum Rich. | Fazenda Sucupira, Embrapa, Brasília, Brazil | Leaf (HD) | (E)-β-Ocimene (19.7%), β-caryophyllene (11.4%), germacrene D (8.9%), bicyclogermacrene (8.8%), spathulenol (6.5%), caryophyllene oxide (5.3%) | --- | [33] |
| P. dilatatum Rich. | Alto Alegre, Roraima state, Brazil | Aerial parts (HD) | β-Caryophyllene (11.7%), germacrene D (6.7%), α-selinene (6.1%), δ-cadinene (5.4%), caryophyllene oxide (6.1%), α-cadinol (12.2%) | --- | [131] |
| P. dilatatum Rich. | Alto Alegre, Roraima state, Brazil | Aerial parts (HD) | β-Caryophyllene (15.5%), germacrene D (10.2%), α-selinene (6.9%), δ-cadinene (8.5%), hinesol (6.4%), α-cadinol (7.0%) | --- | [131] |
| P. dilatatum Rich. | Benfica, Pará state, Brazil | Aerial parts (HD) | Germacrene D (12.6%), bicyclogermacrene (7.4%), (E)-nerolidol (10.2%), spathulenol (11.8%), hinesol (6.4%), α-cadinol (5.8%) | --- | [131] |
| P. dilatatum Rich. | Belterra, Pará state, Brazil | Aerial parts (HD) | α-Pinene (9.7%), β-pinene (14.8%), (Z)-β-ocimene (10.0%), β-caryophyllene (7.4%), bicyclogermacrene (27.6%), spathulenol (15.0%) | --- | [131] |
| P. dilatatum Rich. | Marituba, Pará state, Brazil | Aerial parts (HD) | p-Cymene (11.7%), β-selinene (6.4%), curzerene (13.8%), (E)-nerolidol (5.7%), α-eudesmol (8.0%), atractylone (5.1%) | --- | [131] |
| P. dilatatum Rich. | Marituba, Pará state, Brazil | Aerial parts (HD) | Germacrene D (30.2%), bicyclogermacrene (9.4%), spathulenol (40.6%), hinesol (6.4%), α-cadinol (5.8%) | --- | [131] |
| P. dilatatum Rich. | Serra dos Carajás, Pará state, Brazil | Aerial parts (HD) | p-Cymene (5.1%), β-elemene (21.8%), β-caryophyllene (5.1%), germacrene D (18.5%) | --- | [131] |
| P. dilatatum Rich. | Serra dos Carajás, Pará state, Brazil | Aerial parts (HD) | β-Pinene (10.5%), limonene (6.4%), δ-elemene (7.6%), β-elemene (13.8%), bicyclogermacrene (7.9%), spathulenol (9.3%) | --- | [131] |
| P. dilatatum Rich. | Angico, Tocantins state, Brazil | Aerial parts (HD) | (Z)-β-Farnesene (7.0%), germacrene D (24.5%), bicyclogermacrene (6.7%), β-bisabolene (8.1%), (Z)-α-bisabolene (39.3%) | --- | [131] |
| P. dilatatum Rich. | Xambioá, Tocantins state, Brazil | Aerial parts (HD) | Germacrene D (8.5%), bicyclogermacrene (34.7%), spathulenol (35.2%) | --- | [131] |
| P. dilatatum Rich. | Xambioá, Tocantins state, Brazil | Aerial parts (HD) | Germacrene D (15.2%), curzerene (28.7%), β-bisabolene (5.5%), (Z)-α-bisabolene (23.2%) | --- | [131] |
| P. dilatatum Rich. | Carolina, Maranhão state, Brazil | Aerial parts (HD) | Limonene (19.4%), germacrene D (43.0%), bicyclogermacrene (13.2%) | --- | [131] |
| P. diospyrifolium Kunth | Universidade de São Paulo, Brazil | Leaf (HD) | (E)-Nerolidol (18.2%), spathulenol (25.4%), caryophyllene oxide (7.7%), globulol (6.6%), humulene epoxide II (6.9%) | --- | [35,60] |
| P. diospyrifolium Kunth | Universidade de São Paulo, Brazil | Floral (HD) a | α-Copaene (47.7%), β-caryophyllene (12.3%), α-humulene (5.7%) | --- | [60] |
| P. diospyrifolium Kunth | Maringá, Parana state, Brazil | Leaf (HD) | Limonene (8.5%), (E)-β-ocimene (5.8%), β-caryophyllene (16.8%), γ-muurolene (10.6%), cis-eudesma-6,11-diene (21.1%), germacrene B (6.2%) | Antifungal, agar diffusion assay (Candida albicans, Candida parapsilosis, Candida tropicalis) | [55] |
| P. diospyrifolium Kunth | Antonina, Paraná state, Brazil | Leaf (HD) | α-Pinene (6.7%), limonene (6.7%), α-copaene (5.4%), β-caryophyllene (7.4%), γ-gurjunene (6.9%), germacrene B (6.7%), selin-11-en-4α-ol (17.7%) | Antileishmanial (L. amazonensis promastigotes, IC50 13.5 μg/mL; L. amazonensis axenic amastigotes, IC50 76.1 μg/mL; anti-Mycobacterium tuberculosis, MIC 125 μg/mL) | [70] |
| P. divaricatum G. Mey. | Guaramiranga Mountain, Ceará state, Brazil | Leaf (HD) | α-Pinene (9.0–18.8%), β-pinene (19.9–25.3%), 1,8-cineole (8.9–9.6%), linalool (23.4–29.7%), germacrene D (6.3–6.5%) | --- | [132] |
| P. divaricatum G. Mey. | Guaramiranga Mountain, Ceará state, Brazil | Floral (HD) a | α-Pinene (6.3–17.6%), β-pinene (12.0–18.0%), α-phellandrene (4.6–10.0%), 1,8-cineole (0.7–12.0%), linalool (3.3–8.3%), β-caryophyllene (9.0–11.4%), germacrene D (0.9–6.1%), bicyclogermacrene (5.3–6.9%) | --- | [132] |
| P. divaricatum G. Mey. | Marajó Island, Breves, Pará state, Brazil | Aerial parts (HD) | Eugenol (23.6%), methyleugenol (63.8%) | Antifungal (Cladosporium cladosporioides, MIC 0.5 μg/mL; Cladosporium sphaerospermum, 5.0 μg/mL); antioxidant (DPPH radical scavenging assay, IC50 16.2 μg/mL) | [40] |
| P. divaricatum G. Mey. | Breves, Pará state, Brazil | Aerial parts (HD) | Eugenol (16.2%), methyleugenol (69.2%) | Insecticidal (Solenopsis saevissima, IC50 453 μg/mL) | [41] |
| P. divaricatum G. Mey. | Itabuna, Bahia state, Brazil | Leaf (HD) | Safrole (98.0%) | Antibacterial (Listeria monocytogenes, MIC 156 μg/mL) | [44] |
| P. divaricatum G. Mey. | Rovira, Tolima, Colombia | Aerial parts (MWHD) | α-Pinene (11.4%), β-pinene (5.1%), α-phellandrene (6.1%), 1,8-cineole (18.3%), linalool (15.0%), β-caryophyllene (8.2%) | Antiprotozoal (Trypanosoma cruzi epimastigotes, IC50 13.1 μg/mL; Leishmania infantum promastigotes, IC50 73.3 μg/mL); cytotoxic (Vero cells, IC50 89.8 μg/mL) Antifungal, broth dilution assay (Trichophyton rubrum, MIC 397 μg/mL; Trichophyton mentagrophytes, MIC 500 μg/mL); cytotoxic (Vero cells, IC50 200 μg/mL) | [118,121] |
| P. divaricatum G. Mey. | Marajó Island, Breves, Pará state, Brazil | Aerial parts (HD) | Eugenol (7.9%), methyleugenol (77.1%) | Antifungal (Fusarium solani f. sp. piperis, IC50 698 μg/mL) | [42] |
| P. divaricatum G. Mey. | Breves, Pará state, Brazil | Leaf (HD) | Methyleugenol (80.6–93.3%) | --- | [43] |
| P. dolichotrichum Yunck. | Quibdó, Chocó, Colombia | Aerial parts (MWHD) | β-Elemene (6.4%), β-caryophyllene (9.8%), germacrene D (16.7%), bicyclogermacrene (6.2%), α-guaiene (5.9%) | --- | [120] |
| P. dotanum Trel. | Monteverde, Costa Rica | Leaf (HD) | α-Thujene (5.2%), sabinene (50.4%), germacrene D (15.3%), bicyclogermacrene (7.4%) | --- | [34] |
| P. duckei C. DC. | Adolpho Ducke Reserve, Manaus, Amazonas state, Brazil | Leaf (HD) | 1,8-Cineole (5.8%), β-caryophyllene (27.1%), germacrene D (14.7%), bicyclogermacrene (5.2%), γ-eudesmol (17.9%) | Antileishmanial (L. amazonensis promastigotes, IC50 46.0 μg/mL; L. amazonensis amastigotes, IC50 42.4 μg/mL; L. guyanensis promastigotes, IC50 15.2 μg/mL) | [72] |
| P. dumosum Rudge | Porto Velho, Rondônia state, Brazil | Leaf (HD) | α-Pinene (12.1%), β-pinene (16.0%), α-phellandrene (5.2%), β-caryophyllene (15.9%), aromadendrene (6.9%), bicyclogermacrene (16.2%) | --- | [105] |
| P. eriopodon (Miq.) C. DC. | Pueblo Bello, Cesar, Colombia | Aerial parts (MWHD) | β-Caryophyllene (8.1%), β-selinene (5.0%), dillapiole (38.8%) | Antifungal, broth dilution assay (Trichophyton mentagrophytes, MIC 500 μg/mL); cytotoxic (Vero cells, IC50 15.8 μg/mL) | [121] |
| P. fimbriulatum C. DC. | Altos de Campana National Park, Panama | Leaf (HD) | Linalool (5.3%), linalyl acetate (5.3%), β-caryophyllene (11.3%), germacrene D (12.8%) | --- | [111] |
| P. fimbriulatum C. DC. | Monteverde, Costa Rica | Leaf (HD) | α-Pinene (10.2%), δ-elemene (9.4%), germacrene D (32.9%), bicyclogermacrene (8.1%) | Antibacterial (Bacillus cereus, MIC 39 μg/mL) | [34] |
| P. friedrichsthalii C. DC. | Pacayas, Cartago, Costa Rica | Leaf (HD) | α-Pinene (14.7%), camphene (5.2%), germacrene D (7.1%) | --- | [133] |
| P. friedrichsthalii C. DC. | Pacayas, Cartago, Costa Rica | Floral (HD) a | α-Pinene (13.4%), β-phellandrene (5.2%), trans-p-menth-2-en-1-ol (7.0%), cis-p-menth-2-en-1-ol (5.1%) | --- | [133] |
| P. friedrichsthalii C. DC. | Fortuma, Quebrada Honda, Chiriqui, Panama | Leaf (HD) | Germacrene D (9.6%), α-selinene (12.0%), β-selinene (7.9%), selin-11-en-4α-ol (12.8%) | --- | [133] |
| P. gaudichaudianum Kunth | Sapiranga, Rio Grande do Sul state, Brazil | Leaf (HD) | β-Pinene (5.6%), β-caryophyllene (17.4%), α-humulene (37.5%), allo-aromadendrene (7.7%) | --- | [134] |
| P. gaudichaudianum Kunth | Universidade de São Paulo, Brazil | Aerial parts (HD) | β-Caryophyllene (12.1%), α-humulene (13.3%), β-selinene (15.7%), α-selinene (16.6%) | --- | [135] |
| P. gaudichaudianum Kunth | Universidade de São Paulo, Brazil | Aerial parts (HD) | β-Caryophyllene (19.3%), α-humulene (29.2%), α-selinene (8.9%) | --- | [135] |
| P. gaudichaudianum Kunth | State of Rondônia, Brazil | Leaf (HD) | Aromadendrene (15.6%), ishwarane (10.0%), β-selinene (10.5%), viridiflorol (27.5%), selin-11-en-4α-ol (8.5%) | Mosquito larvicidal (Aedes aegypti, LC50 121 μg/mL) | [136] |
| P. gaudichaudianum Kunth | Riozinho, Rio Grande do Sul state, Brazil | Leaf (HD) | β-Caryophyllene (8.9%), α-humulene (16.5%), bicyclogermacrene (7.4%), (E)-nerolidol (22.4%) | Cytotoxic (V79 Chinese hamster lung cells, IC50 4.0 μg/mL) | [137] |
| P. gaudichaudianum Kunth | Universidade de São Paulo, Brazil | Leaf (HD) | β-Caryophyllene (15.6%), α-humulene (23.4%), β-selinene (6.6%), viridiflorene (8.1%), hinesol (6.4%), α-cadinol (7.0%) | Antifungal, broth dilution assay (Candida krusei, MIC 31.25 μg/mL) | [35] |
| P. gaudichaudianum Kunth | Riozinho, Rio Grande do Sul state, Brazil | Leaf (HD) | β-Caryophyllene (7.5%), α-humulene (21.3%), bicyclogermacrene (13.2%), (E)-nerolidol (22.1%) | Not mutagenic (Saccharomyces cerevisiae); EO and nerolidol generate reactive oxygen species | [138] |
| P. gaudichaudianum Kunth | Pariquera-Açu, São Paulo state, Brazil | Leaf (HD) | α-Pinene (12.2%), β-pinene (7.0%), β-caryophyllene (8.5%), trans-β-guaiene (6.9%), (E)-nerolidol (17.5%), caryophyllene oxide (8.5%) | --- | [31] |
| P. gaudichaudianum Kunth | Antonina, Paraná state, Brazil | Leaf (HD) | δ-3-Carene (5.9%), γ-elemene (5.4%), δ-cadinene (45.3%) | Antileishmanial (L. amazonensis promastigotes, IC50 93.5 μg/mL) | [70] |
| P. glabratum Kunth | Reserva da Matinha, Ilhéus, Bahia state, Brazil | Leaf (HD) | (Z)-Caryophyllene (5.2%), β-caryophyllene (14.6%), δ-cadinene (6.3%), (E)-nerolidol (5.3%), longiborneol (12.0%) | --- | [125] |
| P. glabrescens (Miq.) C. DC. | Monteverde, Costa Rica | Leaf (HD) | α-Pinene (26.0%), limonene (56.6%) | Cytotoxic (MCF-7 human breast adenocarcinoma) | [34] |
| P. grande Vahl | Parque Nacional Camino de Cruces, Panama | Leaf (HD) | α-Pinene (6.3%), β-pinene (14.5%), γ-terpinene (8.0%), p-cymene (43.9%) | --- | [47] |
| P. heterophyllum Ruiz & Pav. | Estancia, Bolivia | Leaf (SD) | α-Pinene (9.3%), β-pinene (6.2%), 1,8-cineole (39.0%), (E)-β-ocimene (6.5%), asaricin (8.8%) | --- | [101] |
| P. hispidinervum C. DC. | Porto Alegre, Rio Grande do Sul state, Brazil | Leaf (HD) | Terpinolene (5.4%), safrole (85.1%) | Amebicidal (Acanthamoeba polyphaga trophozoites, LC50 66 μg/mL) | [139] |
| P. hispidum Sw. | Rondônia state, Brazil | Leaf (SD) | α-Pinene (5.2%), camphene (15.6%), β-phellandrene (9.7%), β-caryophyllene (5.4%), α-guaiene (11.5%), γ-cadinene (25.1%), γ-elemene (10.9%) c | --- | [112] |
| P. hispidum Sw. | Pinar del Río, Cuba | Leaf (HD) | Curzerene (12.9%), elemol (7.6%), γ-eudesmol (9.3%), β-eudesmol (17.5%), α-eudesmol (8.1%), 14-Hydroxy-α-muurolene (5.0%) | --- | [46] |
| P. hispidum Sw. | Fazenda Sucupira, Embrapa, Brasília, Brazil | Leaf (HD) | α-Pinene (9.0%), β-pinene (19.7%), δ-3-carene (7.4%), spathulenol (6.2%), α-cadinol (6.9%) | --- | [33] |
| P. hispidum Sw. | Pacurita, Chocó, Colombia | Leaf (HD) | β-Elemene (5.1%), β-caryophyllene (5.1%), (E)-nerolidol (23.6%), caryophyllene oxide (5.4%) | --- | [48] |
| P. hispidum Sw. | Fênix, Paraná state, Brazil | Floral (HD) a | α-Pinene (7.1–13.9%), β-pinene (7.5–13.3%), α-copaene (28.7–36.2%) | --- | [108] |
| P. hispidum Sw. | Chiguará, Mérida state, Venezuela | Leaf (HD) | α-Pinene (15.3%), β-pinene (14.8%), δ-3-carene (6.9%), β-elemene (8.1%), β-caryophyllene (6.2%), germacrene B (5.2%), spathulenol (5.0%), caryophyllene oxide (7.8%) | Antibacterial (Bacillus subtilis, MIC 12.5 μg/mL; Bacillus cereus, MIC 12.5 μg/mL; Staphylococcus aureus, MIC 12.5 μg/mL; Staphylococcus epidermidis, MIC 12.5 μg/mL; Staphylococcus saprophyticus, MIC 12.5 μg/mL; Enterococcus faecalis, MIC 15.0 μg/mL), antifungal (Candida albicans, MIC 200 μg/mL), cytotoxic (HeLa human cervical carcinoma, IC50 36.6 μg/mL; A-549 human lung carcinoma, IC50 37.5 μg/mL; MCF-7 human breast adenocarcinoma, IC50 34.2 μg/mL) | [97] |
| P. hispidum Sw. | Reserva da Matinha, Ilhéus, Bahia state, Brazil | Leaf (HD) | α-Pinene (6.6%), β-pinene (12.0%), khusimene (12.1%), γ-cadinene (13.2%), δ-cadinene (6.3%), ledol (8.8%) | --- | [125] |
| P. hispidum Sw. | Carajás National Forest, Parauapebas, Pará state, Brazil | Aerial parts (HD) | δ-3-Carene (9.1%), limonene (6.9%), α-copaene (7.3%), β-caryophyllene (10.5%), α-humulene (9.5%), β-selinene (5.1%), caryophyllene oxide (5.9%) | Antifungal, TLC bioautography (Cladosporium cladosporioides, Cladosporium sphaerospermum); enzyme inhibitory, TLC bioautography (acetylcholinesterase) | [61] |
| P. hispidum Sw. | Atrato, Chocó, Colombia | Aerial parts (MWHD) | β-Elemene (5.1%), β-caryophyllene (5.1%), (E)-nerolidol (23.6%), caryophyllene oxide (5.4%) | Antifungal, broth dilution assay (Fusarium oxysporum, MIC 500 μg/mL; Trichophyton rubrum, MIC 99 μg/mL; Trichophyton mentagrophytes, MIC 125 μg/mL); cytotoxic (Vero cells, IC50 51.7 μg/mL) | [121] |
| P. hispidum Sw. | Altos de Campana, Panama | Leaf (HD) | Piperitone (10.0%), dillapiole (57.7%) | Mosquito larvicidal (Aedes aegypti, LC100 250 μg/mL) | [47] |
| P. hostmannianum (Miq.) C. DC. | State of Rondônia, Brazil | Leaf (HD) | Piperitone (5.6%), germacrene D (6.8%), asaricin (27.4%), myristicin (20.3%), dillapiole (7.7%) | Mosquito larvicidal (Aedes aegypti, LC50 54 μg/mL) | [136] |
| P. humaytanum Yunck. | State of Rondônia, Brazil | Leaf (HD) | β-Selinene (15.8%), sesquicineole (5.0%), spathulenol (6.3%), caryophyllene oxide (16.6%), β-oplopenone (6.0%) | Mosquito larvicidal (Aedes aegypti, LC50 156 μg/mL) | [136] |
| P. ilheusense Yunck. | Ilheus, Bahia, Brazil | Leaf (HD) | β-Caryophyllene (11.8%), γ-cadinene (6.9%), germacrene B (7.2%), gleenol (7.5%), patchouli alcohol (11.1%) | Antimicrobial, agar diffusion assay (Bacillus subtilis, Staphylococcus aureus, Candida albicans, Candida crusei, Candida parapsilosis) | [57] |
| P. imperiale (Miq.) C. DC. | Monteverde, Costa Rica | Leaf (HD) | β-Elemene (5.2%), β-caryophyllene (25.5%), α-guaiene (7.6%), germacrene D (5.5%), bicyclogermacrene (19.7%), germacrene A (8.5%), α-bulnesene (10.8%), dillapiole (6.7%) | Antibacterial (Bacillus cereus, MIC 156 μg/mL), cytotoxic (MCF-7 human breast adenocarcinoma) | [34] |
| P. jacquemontianum Kunth | Lachuá, Alta Verapaz, Guatemala | Leaf (HD) | Linalool (69.4%), (E)-nerolidol (8.0%) | --- | [140] |
| P. jacquemontianum Kunth | Parque Nacional Soberania, Panama | Leaf (HD) | α-Pinene (9.6%), β-pinene (10.1%), α-phellandrene (13.8%), limonene (12.2%), p-cymene (7.4%), linalool (14.5%) | --- | [47] |
| P. klotzschianum (Kunth) C. DC. | Vila do Riacho, Gimuna Forest, Aracruz, Espírito Santo, Brazil | Leaf (HD) | 4-Butyl-1,2-methylenedioxybenzene (81.0%), γ-asarone (9.1%) | --- | [141] |
| P. klotzschianum (Kunth) C. DC. | Vila do Riacho, Gimuna Forest, Aracruz, Espírito Santo, Brazil | Root (HD) | 4-Butyl-1,2-methylenedioxybenzene (96.2%) | Mosquito larvicidal activity (Aedes aegypti, LC50 10.0 μg/mL) | [141] |
| P. klotzschianum (Kunth) C. DC. | Vila do Riacho, Gimuna Forest, Aracruz, Espírito Santo, Brazil | Seed (HD) | α-Phellandrene (17.0%), p-cymene (7.4%), limonene (17.8%), 4-Butyl-1,2-methylenedioxybenzene (36.9%), α-trans-bergamotene (8.8%) | Mosquito larvicidal activity (Aedes aegypti, LC50 13.3 μg/mL) | [141] |
| P. klotzschianum (Kunth) C. DC. | Vila do Riacho, Gimuna Forest, Aracruz, Espírito Santo, Brazil | Stem (HD) | 4-Butyl-1,2-methylenedioxybenzene (84.8%), γ-asarone (5.4%) | --- | [141] |
| P. krukoffii Yunck. | Carajás National Forest, Parauapebas, Pará state, Brazil | Aerial parts (HD) | β-Elemene (1.7–8.2%), myristicin (26.7–40.6%), τ-muurolol (0.2–5.7%), apiole (25.3–34.1%) | --- | [142] |
| P. lanceifolium Kunth | San Isidro del Tejar, Costa Rica | Leaf (HD) | β-Caryophyllene (20.6%), germacrene D (12.5%), elemicin (24.4%), apiole (11.7%) | --- | [143] |
| P. lanceifolium Kunth | San Isidro del Tejar, Costa Rica | Floral (HD) a | α-Pinene (13.7%), β-pinene (15.8%), γ-terpinene (6.9%), β-caryophyllene (5.1%), elemicin (16.4%), apiole (9.8%) | --- | [143] |
| P. lanceifolium Kunth | Monteverde, Costa Rica | Leaf (HD) | Dillapiole (74.6%) | --- | [34] |
| P. lanceifolium Kunth | Bagadó, Chocó, Colombia | Aerial parts (MWHD) | β-Pinene (5.4%), β-caryophyllene (11.6%), germacrene D (10.7%), β-selinene (7.8%), δ-cadinene (6.1%), caryophyllene oxide (5.9%) | Antiprotozoal (Trypanosoma cruzi epimastigotes, IC50 7.48 μg/mL; Leishmania infantum promastigotes, IC50 37.8 μg/mL); cytotoxic (Vero cells, IC50 46.0 μg/mL; THP-1 human monocytic leukemia, IC50 55.7 μg/mL) | [118] |
| P. leptorum Kunth | Monte Alegre do Sul, São Paulo state, Brazil | Leaf (HD) | Seychellene (34.7%), caryophyllene oxide (12.5%) | --- | [31] |
| P. longispicum C. DC. | Altos de Campana, Panama | Leaf (HD) | β-Caryophyllene (45.2%), caryophyllene oxide (5.5%) | Mosquito larvicidal (Aedes aegypti, LC100 250 μg/mL) | [47] |
| P. lucaeanum var. grandifolium Yunck. | Rio de Janeiro state, Brazil | Leaf (HD) | α-Pinene (30.0%), β-caryophyllene (5.0%), α-zingiberene (30.4%), β-bisabolene (8.9%), β-sesquiphellandrene (11.1%) | Antiparasitic (Plasmodium falciparum W2, IC50 2.65 μg/mL) | [73] |
| P. madeiranum Yunck. | Reserva da Matinha, Ilhéus, Bahia state, Brazil | Leaf (HD) | β-Caryophyllene (11.2%), germacrene D-4-ol (11.1%), 1,10-di-epi-cubenol (7.0%), α-bisabolol (7.1%), epi-α-bisabolol (5.4%) | --- | [125] |
| P. malacophyllum (C. Presl) C. DC. | Florianópolis, Santa Catarina, Brazil | Leaf (HD) | α-Pinene (5.0%), camphene (30.8%), camphor (32.8%) | Antibacterial (Staphylococcus aureus, MIC 3700 μg/mL; Bacillus cereus, MIC 1850 μg/mL; Acinetobacter baumanii, MIC 3700 μg/mL; Escherichia coli, MIC 1850 μg/mL; Pseudomonas aeruginosa, MIC 3700 μg/mL); antifungal (Epidermophyton flocosum, MIC 1000 μg/mL; Microsporum gypseum, MIC 1000 μg/mL; Trichophyton mentagrophytes, MIC 500 μg/mL; Trichophyton rubrum, MIC 1000 μg/mL; Candida albicans, MIC 1000 μg/mL; Cryptococcus neoformans, MIC 500 μg/mL); antiparasitic (Trypanosoma cruzi epimastigotes, IC50 312 μg/mL) | [56] |
| P. manausense Yunck. | Ananindeua, Pará state, Brazil | Aerial parts (HD) | α-Pinene (5.2–6.6%), β-pinene (4.7–6.5%), β-caryophyllene (7.7–8.5%), germacrene D (3.5–6.1%), bicyclogermacrene (32.0–34.0%), δ-cadinene (5.8–7.0%), gleenol (6.8–9.4%) | --- | [144] |
| P. manausense Yunck. | Acará, Pará state, Brazil | Aerial parts (HD) | α-Pinene (9.1%), β-pinene (9.2%), β-caryophyllene (5.9%), bicyclogermacrene (41.0%), δ-cadinene (5.8%) | --- | [144] |
| P. manausense Yunck. | Marituba, Pará state, Brazil | Aerial parts (HD) | β-Caryophyllene (6.0%), aromadendrene (5.0%), bicyclogermacrene (7.8%), spathulenol (15.0%), globulol (9.4%), α-muurolol (7.6%) | --- | [144] |
| P. marginatum Jacq. | Itacoatiara, Amazonas State, Brazil | Leaf (HD) | (E)-β-Ocimene (5.2%), α-copaene (5.6%), β-caryophyllene (9.1%), γ-elemene (8.5%), propiopiperone (18.2%) | --- | [145] |
| P. marginatum Jacq. | Itacoatiara, Amazonas State, Brazil | Stem (HD) | δ-3-Carene (6.9%), β-caryophyllene (11.6%), myristicin (19.3%), propiopiperone (18.6%) | --- | [145] |
| P. marginatum Jacq. | Monteverde, Costa Rica | Aerial parts (HD) | p-Cymene (7.1%), estragole (6.6%), p-anisaldehyde (22.0%), (E)-anethole (45.9%), anisyl methyl ketone (14.2%) | --- | [49] |
| P. marginatum Jacq. | Cultivated (State University of Campinas, São Paulo, Brazil) | Leaf (HD) | --- | Antibacterial (Escherichia coli, MIC 700 μg/mL) | [52] |
| P. marginatum Jacq. | Monte Alegre, Pará state, Brazil | Leaf (HD) | (E)-β-Ocimene (5.6%), safrole (63.9%), methyleugenol (5.9%), propiopiperone (7.3%) | --- | [45] |
| P. marginatum Jacq. | Xambioá, Tocantins state, Brazil | Leaf (HD) | Safrole (52.3–52.5%), myristicin (6.3–9.3%), propiopiperone (11.8–14.1%) | --- | [45] |
| P. marginatum Jacq. | Nazaré, Tocantins state, Brazil | Leaf (HD) | Safrole (41.1%), myristicin (8.2%), propiopiperone (30.4%) | --- | [45] |
| P. marginatum Jacq. | Monte Alegre, Pará state, Brazil | Leaf (HD) | (Z)-β-Ocimene (5.3%), (E)-β-ocimene (13.5%), safrole (23.9%), β-caryophyllene (6.0%), propiopiperone (33.2%) | --- | [45] |
| P. marginatum Jacq. | Belém, Pará state, Brazil | Leaf (HD) | p-Mentha-1(7),8-diene (39.0%), (E)-β-ocimene (9.8%), propiopiperone (19.0%) | --- | [45] |
| P. marginatum Jacq. | Alter do Chão, Pará state, Brazil | Leaf (HD) | α-Pinene (5.0%), p-mentha-1(7),8-diene (34.8%), (E)-β-ocimene (8.7%), propiopiperone (23.1%), elemicin (6.5%) | --- | [45] |
| P. marginatum Jacq. | Belterra, Pará state, Brazil | Leaf (HD) | p-Mentha-1(7),8-diene (22.9%), (E)-β-ocimene (8.2%), propiopiperone (40.7%) | --- | [45] |
| P. marginatum Jacq. | Melgaço, Pará state, Brazil | Leaf (HD) | (E)-β-Ocimene (8.0%), safrole (10.4%), germacrene D (8.1%), bicyclogermacrene (6.4%), myristicin (16.0%), propiopiperone (17.4%) | --- | [45] |
| P. marginatum Jacq. | Xinguara, Pará state, Brazil | Leaf (HD) | (Z)-β-Ocimene (8.6%), (E)-β-ocimene (15.2%), germacrene D (10.4%), myristicin (5.4%), propiopiperone (14.5%), τ-muurolol (5.0%) | --- | [45] |
| P. marginatum Jacq. | Manaus, Amazonas state, Brazil | Leaf (HD) | Safrole (6.4%), α-copaene (7.4%), β-caryophyllene (9.5%), germacrene D (5.5%), propiopiperone (25.0%) | --- | [45] |
| P. marginatum Jacq. | Macapá, Amapá state, Brazil | Leaf (HD) | (E)-β-Ocimene (5.5%), β-caryophyllene (10.6%), myristicin (9.6%), propiopiperone (22.9%) | --- | [45] |
| P. marginatum Jacq. | Monte Alegre, Pará state, Brazil | Leaf (HD) | (Z)-β-Ocimene (5.7%), (E)-β-ocimene (13.5%), β-caryophyllene (9.3%), propiopiperone (40.2%) | --- | [45] |
| P. marginatum Jacq. | Viseu, Pará state, Brazil | Leaf (HD) | γ-Terpinene (14.4%), myristicin (5.0%), propiopiperone (29.6%), spathulenol (6.6%) | --- | [45] |
| P. marginatum Jacq. | Alta Floresta, Mato Grosso state, Brazil | Leaf (HD) | γ-Terpinene (8.6%), myristicin (5.5%), propiopiperone (18.4%) | --- | [45] |
| P. marginatum Jacq. | Manaus, Amazonas state, Brazil | Leaf (HD) | γ-Terpinene (6.5%), safrole (5.7%), β-caryophyllene (13.3%), germacrene D (8.7%), propiopiperone (7.9%) | --- | [45] |
| P. marginatum Jacq. | Manaus, Amazonas state, Brazil | Leaf (HD) | (Z)-β-Ocimene (5.2%), (E)-β-ocimene (8.7%), α-copaene (11.4%), β-caryophyllene (10.2%), germacrene D (7.6%), bicyclogermacrene (8.2%), propiopiperone (10.4%) | --- | [45] |
| P. marginatum Jacq. | Salvaterra, Pará state, Brazil | Leaf (HD) | p-Mentha-1(7),8-diene (5.2%), (Z)-anethole (8.4%), (E)-anethole (16.5%), isoosmorhizole (17.4%), (E)-isoosmorhizole (29.1%) | --- | [45] |
| P. marginatum Jacq. | Manaus, Amazonas state, Brazil | Leaf (HD) | (Z)-Anethole (6.0%), (E)-anethole (26.4%), isoosmorhizole (11.2%), (E)-isoosmorhizole (32.2%) | --- | [45] |
| P. marginatum Jacq. | Óbidos, Pará state, Brazil | Leaf (HD) | (E)-Anethole (13.6%), isoosmorhizole (24.5%), (E)-isoosmorhizole (46.8%) | --- | [45] |
| P. marginatum Jacq. | Medicilândia, Pará state, Brazil | Leaf (HD) | β-Caryophyllene (6.7%), (E)-isoosmorhizole (15.8%), crocatone (21.9%), 2′-methoxy-4′,5′-methylenedioxypropiophenone (26.3%) | --- | [45] |
| P. marginatum Jacq. | Paredão, Roraima state, Brazil | Leaf (HD) | β-Caryophyllene (13.6%), bicyclogermacrene (11.7%), (Z)-asarone (8.8%), exalatacin (7.9%), (E)-asarone (10.8%) | --- | [45] |
| P. marginatum Jacq. | Venadillo, Tolima, Colombia | Aerial parts (HD) | α-Phellandrene (11.1%), limonene (7.5%), β-caryophyllene (11.0%), elemicin (18.0%), isoelemicin (9.2%) | --- | [120] |
| P. marginatum Jacq. | Universidade Federal Rural de Pernambuco, Recife, Brazil | Leaf (HD) | β-Caryophyllene (7.5%), α-acoradiene (5.1%), bicyclogermacrene (9.4%), elemol (9.7%), (Z)-asarone (30.4%), patchouli alcohol (16.0%), (E)-asarone (6.4%) | Mosquito larvicidal (Aedes aegypti, LC50 23.8 μg/mL) | [146] |
| P. marginatum Jacq. | Universidade Federal Rural de Pernambuco, Recife, Brazil | Floral (HD) a | α-Copaene (9.4%), β-caryophyllene (13.1%), α-acoradiene (9.7%), patchouli alcohol (23.4%), (E)-asarone (22.1%) | Mosquito larvicidal (Aedes aegypti, LC50 19.9 μg/mL) | [146] |
| P. marginatum Jacq. | Universidade Federal Rural de Pernambuco, Recife, Brazil | Stem (HD) | β-Caryophyllene (6.8%), seychellene (5.8%), elemicin (6.9%), (Z)-asarone (8.5%), patchouli alcohol (25.7%), (E)-asarone (32.6%) | Mosquito larvicidal (Aedes aegypti, LC50 19.9 μg/mL) | [146] |
| P. marginatum Jacq. | Belém, Pará state, Brazil | Aerial parts (HD) | p-Mentha-1(7),8-diene (39.0%), (E)-β-ocimene (9.8%), propiopiperone (19.0%) | Insecticidal (Solenopsis saevissima, IC50 240 μg/mL) | [41] |
| P. marginatum Jacq. | Manaus, Amazonas state, Brazil | Aerial parts (HD) | (E)-Anethole (26.4%), isoosmorhizole (11.2%), (E)-isoosmorhizole (32.2%) | Insecticidal (Solenopsis saevissima, IC50 439 μg/mL) | [41] |
| P. marginatum Jacq. | Venadillo, Tolima, Colombia | Aerial parts (MWHD) | α-Phellandrene (11.2%), limonene (7.6%), β-caryophyllene (11.1%), elemicin (18.4%), isoelemicin (9.3%) | Antiprotozoal (Trypanosoma cruzi epimastigotes, IC50 16.2 μg/mL; Leishmania infantum promastigotes, IC50 88.7 μg/mL); cytotoxic (Vero cells, IC50 40.2 μg/mL) Antifungal, broth dilution assay (Trichophyton rubrum, MIC 500 μg/mL; Trichophyton mentagrophytes, MIC 250 μg/mL) | [118,121] |
| P. marginatum Jacq. | Belém, Pará state, Brazil | Aerial parts (HD) | β-Caryophyllene (5.0%), propiopiperone (21.8%), elemol (5.9%) | Antifungal, TLC bioautography (Cladosporium cladosporioides, Cladosporium sphareospermum), enzyme inhibitory (acetylcholinesterase) | [62] |
| P. mikanianum (Kunth) Steud. | Sapiranga, Rio Grande do Sul state, Brazil | Leaf (HD) | α-Pinene (6.5%), myrcene (5.6%), limonene (14.8%), β-caryophyllene (10.5%), bicyclogermacrene (14.3%) | --- | [134] |
| P. mikanianum (Kunth) Steud. | Atalanta, Santa Catarina state, Brazil | Leaf (HD) | Safrole (82.0%) | --- | [147] |
| P. mikanianum (Kunth) Steud. | Curitiba, Paraná state, Brazil | Leaf (HD) | Bicyclogermacrene (5.3%), (Z)-isoelemicin (21.5%), (E)-asarone (11.6%), β-vetivone (33.5%) | --- | [148] |
| P. mikanianum (Kunth) Steud. | Picada Café, Rio Grando do Sul state, Brazil | Aerial parts (HD) | Bicyclogermacrene (6.6%), germacrene B (7.8%), α-cadinol (5.1%), apiole (64.9%) | Acaricidal (Rhipicephalus (Boophilus) microplus, LC50 2.33 μL/mL) | [106] |
| P. mikanianum (Kunth) Steud. | Atalanta, Santa Catarina state, Brazil | Leaf (HD) | α-Thujene (6.0%), safrole (72.4%) | --- | [70] |
| P. mollicomum Kunth | Cultivated (State University of Campinas, São Paulo, Brazil) | Leaf (HD) | --- | Antibacterial (Escherichia coli, MIC 1000 μg/mL) | [52] |
| P. mollicomum Kunth | Cultivated, FIOCRUZ, Rio de Janeiro, Brazil | Leaf (HD) | (Z)-β-Ocimene (14.1%), (E)-β-ocimene (12.1%), germacrene D (10.8%), germacrene B (13.4%), myrtenic acid (7.5%), α-bisabolol (9.9%), (E)-nerolidol (9.6%) | Antinociceptive (mouse model, 1 mg/kg) | [91] |
| P. mosenii C. DC. | Antonina, Paraná state, Brazil | Leaf (HD) | β-Caryophyllene (8.6%), α-humulene (11.3%), bicyclogermacrene (7.4%), caryophyllene oxide (12.1%), viridiflorol (5.8%), humulene epoxide II (6.3%) | Antileishmanial (L. amazonensis promastigotes, IC50 17.4 μg/mL; L. amazonensis axenic amastigotes, IC50 >200 μg/mL), anti-Mycobacterium tuberculosis (MIC 250 μg/mL) | [70] |
| P. multiplinervium C. DC. | Parque Nacional Soberania, Panama | Leaf (HD) | α-Pinene (7.1%), β-pinene (7.9%), α-phellandrene (11.8%), limonene (11.4%), p-cymene (9.0%), linalool (16.5%), (E)-nerolidol (5.5%) | --- | [47] |
| P. nemorense C. DC. | Monteverde, Costa Rica | Leaf (HD) | α-Phellandrene (8.8%), limonene (6.3%), α-copaene (5.7%), β-bourbonene (14.0%), β-caryophyllene (5.6%), β-copaene (15.0%), γ-elemene (6.8%), germacrene D (8.4%), bicyclogermacrene (7.5%) | --- | [34] |
| P. oblanceolatum Trel. | Monteverde, Costa Rica | Leaf (HD) | α-Pinene (6.2%), linalool (11.3%), β-caryophyllene (6.8%), germacrene D (8.9%), δ-amorphene (9.0%) | Antibacterial (Bacillus cereus, MIC 78 μg/mL), cytotoxic (MCF-7 human breast adenocarcinoma) | [34] |
| P. obliquum Ruiz & Pav. | Altos de Campana National Park, Panama | Leaf (HD) | β-Caryophyllene (27.6%), spathulenol (10.6%), caryophyllene oxide (8.3%) | --- | [111] |
| P. obliquum Ruiz & Pav. | Wasak'entsa reserve, Ecuador | Aerial parts (HD) | γ-Terpinene (17.1%), terpinolene (11.5%), safrole (45.9%) | --- | [65] |
| P. obrutum Trel. & Yunck. | Samurindó, Chocó, Colombia | Aerial parts (MWHD) | Linalool (15.8%), β-elemene (7.6%), α-humulene (6.4%), (E)-nerolidol (5.8%) | Antiprotozoal (Trypanosoma cruzi epimastigotes, IC50 29.3 μg/mL; Leishmania infantum promastigotes, IC50 35.9 μg/mL; L. infantum amastigotes, IC50 89.0 μg/mL); cytotoxic (Vero cells, IC50 45.3 μg/mL) | [118] |
| P. ovatum Vahl | Fazenda Sucupira, Embrapa, Brasília, Brazil | Leaf (HD) | α-Pinene (23.1%), β-pinene (14.2%), β-caryophyllene (5.3%), germacrene D (10.3%), epi-cubebol (10.7%) | --- | [33] |
| P. peltatum L. [syn. Pothomorphe peltata (L.) Miq.] | Pinar del Río, Cuba | Leaf (HD) | α-Copaene (5.2%), trans-calamenene (5.4%), spathulenol (9.0%), caryophyllene oxide (22.9%) | --- | [27] |
| P. permucronatum Yunck. | Tijuca Forest, Rio de Janeiro state, Brazil | Leaf (HD) | β-Caryophyllene (6.8%), δ-cadinene (12.7%), α-cadinol (6.9%) | --- | [149] |
| P. permucronatum Yunck. | State of Rondônia, Brazil | Leaf (HD) | Asaricin (8.6%), myristicin (25.6%), elemicin (9.9%), dillapiole (54.7%) | Mosquito larvicidal (Aedes aegypti, LC50 36 μg/mL) | [136] |
| P. plurinervosum Yunck. | Egler Reserva, Amazonas, Brazil | Aerial parts (HD) | 1,8-Cineole (31.6%), β-caryophyllene (6.6%), (E)-nerolidol (6.4%), caryophyllene oxide (5.7%), guaiol (6.2%), α-cadinol (8.5%) | --- | [129] |
| P. pseudolindenii C. DC. | Turrialba, Cartago, Costa Rica | Leaf (HD) | β-Pinene (6.7%), β-elemene (15.0%), β-caryophyllene (11.8%), α-humulene (7.0%), germacrene D (9.0%), germacrene B (5.4%) | --- | [133] |
| P. regnellii (Miq.) C. DC. | Universidade de São Paulo, Brazil | Aerial parts (HD) | β-Caryophyllene (23.4%), (E)-nerolidol (13.7%), spathulenol (11.1%), globulol (6.1%) | --- | [135] |
| P. regnellii (Miq.) C. DC. | Universidade de São Paulo, Brazil | Leaf (HD) | Myrcene (52.6%), linalool (15.9%), β-caryophyllene (8.5%) | Antimicrobial, agar diffusion assay (Staphylococcus aureus, Candida albicans) | [38] |
| P. regnellii (Miq.) C. DC. | Cultivated (State University of Campinas, São Paulo, Brazil) | Leaf (HD) | --- | Antibacterial (Escherichia coli, MIC 300 μg/mL) | [52] |
| P. regnellii (Miq.) C. DC. | Universidade de São Paulo, Brazil | Leaf (HD) | β-Pinene (13.3%), myrcene (15.5%), β-caryophyllene (7.2%), aromadendrene (8.3%), bicyclogermacrene (9.7%), (E)-nerolidol (8.4%), spathulenol (7.8%) | --- | [35] |
| P. regnellii (Miq) C. DC. var. regnellii (C. DC.) Yunck | Universidade de São Paulo, Brazil | Leaf (HD) | β-caryophyllene (8.2–9.5%), germacrene D (45.6–51.4%) and α-chamigrene (8.9–11.3%) | Cytotoxic (B16F10-Nex2 murine melanoma, IC50 66 μg/mL; A2058 human melanoma, IC50 57 μg/mL; HeLa human cervical carcinoma, IC50 13 μg/mL; SiHa human cervical IC50 71 μg/mL; HCT human colon carcinoma, IC50 61 μg/mL; SKBR3 breast cancer, IC50 79 μg/mL; U87 human glioblastoma, IC50 71 μg/mL; β-caryophyllene, germacrene D, α-chamigrene cytotoxic to HeLa cells: IC50 11, 7, 32 μg/mL, respectively) | [98] |
| P. renitens (Miq.) Yunck. | Mirante da Serra, Rondonia, Brazil | Aerial parts (HD) | α-Pinene (12.5%), camphene (5.6%), β-pinene (12.4%), (Z)-caryophyllene (6.9%), germacrene D (13.8%), bicyclogermacrene (6.6%), guaiol (13.9%), eudesm-7(11)-en-4-ol (9.3%) | --- | [150] |
| P. reticulatum L. | Costa Arriba, Rio Cascajal, Colon, Panama | Leaf (HD) | β-Elemene (16.1%), β-selinene (19.0%), α-selinene (15.5%), spathulenol (6.1%) | --- | [47] |
| P. rivinoides Kunth | Cultivated, FIOCRUZ, Rio de Janeiro, Brazil | Leaf (HD) | α-Pinene (32.9%), β-pinene (20.7%), β-caryophyllene (7.6%), germacrene B (6.7%) | Antinociceptive (mouse model, 1 mg/kg) | [91] |
| P. rivinoides Kunth | Ubatuba, São Paulo state, Brazil | Leaf (HD) | α-Pinene (73.2%), β-pinene (5.2%) | --- | [31] |
| P. rivinoides Kunth | Antonina, Paraná state, Brazil | Leaf (HD) | β-Caryophyllene (6.6%), α-humulene (10.0%), dehydroaromadendrane (7.8%), bicyclogermacrene (11.8%), (Z)-α-bisabolene (10.9%), spathulenol (5.1%) | Antileishmanial (L. amazonensis promastigotes, IC50 10.9 μg/mL; L. amazonensis axenic amastigotes, IC50 > 200 μg/mL), anti-Mycobacterium tuberculosis (MIC 125 μg/mL) | [70] |
| P. septuplinervium (Miq.) C. DC. | Pandó, Chocó, Colombia | Aerial parts (MWHD) | β-Caryophyllene (5.0%), epi-cubebol (9.0%), δ-cadinene (10.9%), germacrene D-4-ol (5.6%), viridiflorol (7.9%) | Antiprotozoal (Trypanosoma cruzi epimastigotes, IC50 14.0 μg/mL; Leishmania infantum promastigotes, IC50 30.1 μg/mL; L. infantum amastigotes, IC50 64.8 μg/mL); cytotoxic (Vero cells, IC50 42.7 μg/mL; THP-1 human monocytic leukemia, IC50 48.8 μg/mL) | [118] |
| P. solmsianum C. DC. | Teresópolis, Rio de Janeiro state, Brazil | Leaf (HD) | δ-3-Carene (23.3%), asaricin (39.2%) | The essential oil and the major component asaricin cause depressant and ataxia effects in mice. | [151] |
| P. solmsianum C. DC. | Universidade de São Paulo, Brazil | Leaf (HD) | Spathulenol (5.2%), isoelemecin (53.5%) | Antifungal, broth dilution assay (Cryptococcus neoformans, MIC 62.5 μg/mL) Antifungal, TLC bioautography (Cladosporium cladosporioides, C. sphareospermum) | [35,60] |
| P. solmsianum C. DC. | Ubatuba, São Paulo state, Brazil | Leaf (HD) | α-Pinene (22.7%), myrcene (26.1%), δ-3-carene (66.9%), α-selinene (5.5%) | --- | [31] |
| P. tectoniaefolium (Kunth) Kunth ex C. DC. | Fazenda Sucupira, Embrapa, Brasília, Brazil | Leaf (HD) | α-Pinene (12.9%), β-pinene (8.8%), caryophyllene oxide (10.9%) e | --- | [33] |
| P. trigonum C. DC. | Altos de Campana, Panama | Leaf (HD) | α-Copaene (6.0%), β-elemene (8.4%), β-caryophyllene (7.1%), germacrene D (19.7%), δ-cadinene (7.2%), α-cadinol (5.8%) | --- | [47] |
| P. tuberculatum Jacq. var. tuberculatum | Rondônia state, Brazil | Leaf (HD) | α-Pinene (8.4%), β-pinene (7.0%), limonene (6.7%), (E)-β-ocimene (9.0%), β-caryophyllene (26.3%), (E)-β-farnesene (6.1%), α-cadinol (13.7%) | --- | [152] |
| P. tuberculatum Jacq. | Universidade Estadual Paulista, Araraquara, São Paulo state, Brazil | Leaf (HD) | α-Pinene (10.4%), β-pinene (12.5%), (E)-β-ocimene (8.6%), β-caryophyllene (40.2%), (E)-β-farnesene (8.3%), germacrene D (5.5%) | --- | [59] |
| P. tuberculatum Jacq. | Universidade Estadual Paulista, Araraquara, São Paulo state, Brazil | Floral (HD) a | α-Pinene (28.7%), β-pinene (38.2%), (E)-β-ocimene (9.8%), β-caryophyllene (14.0%) | --- | [59] |
| P. tuberculatum Jacq. | Universidade Estadual Paulista, Araraquara, São Paulo state, Brazil | Stem (HD) | α-Pinene (17.3%), β-pinene (27.0%), (E)-β-ocimene (14.5%), β-caryophyllene (32.1%) | Antifungal, TLC bioautography (Cladosporium cladosporioides, Cladosporium sphareospermum) | [59] |
| P. tuberculatum Jacq. | Universidade de São Paulo, Brazil | Leaf (HD) | (E)-Nerolidol (12.7%), spathulenol (15.8%), viridiflorol (13.5%), τ-cadinol (6.3%) | --- | [35] |
| P. umbellatum L. | Monteverde, Costa Rica | Aerial parts (HD) | β-Elemene (6.9%), β-caryophyllene (28.3%), germacrene D (16.7%), bicyclogermacrene (6.6%), (E,E)-α-farnesene (14.5%) | --- | [49] |
| P. umbellatum L. | Araraquara, São Paulo state, Brazil | Leaf (HD) | γ-Muurolene (8.9%), germacrene D (34.2%), bicyclogermacrene (9.0%), γ-cadinene (5.9%), δ-cadinene (15.0%) | --- | [35,60] |
| P. umbellatum L. | Topes de Collantes Nature Reserve, Escambray Mountains, Cuba | Leaf (HD) | Camphor (9.6%), safrole (26.4%), β-caryophyllene (6.6%) | Antioxidant (DPPH radical scavenging assay, IC50 32.3 μg/mL) | [50] |
| P. umbellatum L. | Campinas, São Paulo state, Brazil | Leaf (HD) | β-Caryophyllene (6.3%), germacrene D (55.8%), bicyclogermacrene (11.8%) | --- | [31] |
| P. variabile C. DC. | Lachuá, Alta Verapaz, Guatemala | Leaf (HD) | Camphene (16.6%), p-cymene (6.3%), limonene (13.9%), camphor (28.4%), guaiol (6.3%) | --- | [140] |
| P. vicosanum Yunck. | Parque Estabdual do Rio Doce, Minas Gerais state, Brazil | Aerial parts (HD) | α-Pinene (6.1%), 1,8-cineole (10.4%), limonene (45.5%) | --- | [153] |
| P. vicosanum Yunck. | Universidade Federal de Minas Gerais, Belo Horizonte, Minas Gerais state, Brazil | Aerial parts (HD) | α-Pinene (7.2%), 1,8-cineole (15.0%), limonene (40.0%), terpinolene (10.1%) | --- | [153] |
| P. vicosanum Yunck. | Dourados, Mato Grosso do Sul, Brazil | Leaf (HD) | Limonene (9.1%), γ-elemene (14.2%), α-alaskene (13.4%) | Anti-inflammatory (rat paw edema, 100–300 mg/kg) | [89] |
| P. vitaceum Yunck. | Manaus-Caracaraí, Amazonas, Brazil | Aerial parts (HD) | p-Cymene (12.8%), limonene (33.2%), (E)-nerolidol (20.6%), caryophyllene oxide (5.2%) | --- | [129] |
| P. xylosteoides (Kunth) Steud. | Fazenda Sucupira, Embrapa, Brasília, Brazil | Leaf (HD) | Myrcene (31.0%), α-terpinene (11.3%), p-cymene (12.4%), γ-terpinene (26.1%) | --- | [17,33] |
| P. xylosteoides (Kunth) Steud. | São Francisco de Paula, Rio Grande do Sul state, Brazil | Aerial parts (HD) | α-Pinene (6.0%), limonene (5.1%), zingiberene (9.3%), safrole (47.8%) | Acaricidal (Rhipicephalus (Boophilus) microplus, LC50 6.15 μL/mL) | [106] |
| P. xylosteoides (Kunth) Steud. | Orleans, Santa Catarina state, Brazil | Leaf (HD) | α-Pinene (7.7%), safrole (84.1%) | Antibacterial, broth dilution assay (Bacillus cereus, MIC 2091 μg/mL; Staphylococcus aureus, MIC 2091 μg/mL) | [154] |
| P. xylosteoides (Kunth) Steud. | São Bonifácio, Santa Catarina state, Brazil | Leaf (HD) | α-Pinene (15.3%), safrole (75.8%) | Antibacterial, broth dilution assay (Bacillus cereus, MIC 2091 μg/mL; Staphylococcus aureus, MIC 2091 μg/mL) | [154] |
| P. xylosteoides (Kunth) Steud. | Ubatuba, São Paulo state, Brazil | Leaf (HD) | Germacrene B (10.6%), trans-β-guaiene (7.8%), (E)-nerolidol (8.2%), spathulenol (12.3%), β-copaen-4α-ol (9.4%) | --- | [31] |
| P. xylosteoides (Kunth) Steud. | Cerro Azul, Paraná state, Brazil | Leaf (HD) | α-Thujene (7.9%), β-phellandrene (22.6%), δ-elemene (6.6%), β-caryophyllene (7.0%), bicyclogermacrene (7.2%), (E)-nerolidol (8.5%) | --- | [70] |
Appendix B






Appendix C
| Piper Species | Classes (%) | Biological activity | Ref. | |||||
|---|---|---|---|---|---|---|---|---|
| PP | MH | OM | SH | OS | Total | |||
| P. aducum | 0.0 | 13.7 | 40.8 | 16.8 | 24.3 | 95.6 | Antiprotozoal | [53] |
| P. aduncum | 0.0 | 20.9 | 7.5 | 42.2 | 18.3 | 88.9 | Antiprotozoal | [70] |
| P. aduncum | 87.8 | 0.9 | 0.0 | 6.5 | 2.4 | 97.6 | Antimicrobial | [30] |
| P. aduncum | 0.0 | 14.1 | 31.8 | 26.0 | 11.6 | 83.5 | Antimicrobial | [35] |
| P. aduncum | 0.9 | 9.7 | 13.4 | 19.3 | 43.9 | 87.2 | Antiprotozoal | [68] |
| P. aduncum | 0.0 | 9.7 | 50.3 | 8.3 | 29.3 | 97.6 | Antiprotozoal | [32] |
| P. aduncum | 0.0 | 13.7 | 31.7 | 39.8 | 11.8 | 97.0 | Antimicrobial | [59] |
| P. aduncum | 0.0 | 55.1 | 11.8 | 13.9 | 10.6 | 91.4 | Antimicrobial | [59] |
| P. aduncum | 46.8 | 25.1 | 15.7 | 6.3 | 1.0 | 94.8 | Antimicrobial | [65] |
| P. aduncum | 89.2 | 1.1 | 2.2 | 3.8 | 2.6 | 98.9 | Insecticidal | [16] |
| P. aduncum | 95.2 | 0.0 | 0.0 | 0.0 | 3.9 | 99.2 | Acaricidal | [28] |
| P. aequale | 0.0 | 29.2 | 0.0 | 42.9 | 20.9 | 93.0 | Citotoxic | [95] |
| P. aequale | 3.7 | 70.2 | 0.2 | 12.4 | 13.5 | 100.0 | Antimicrobial | [34] |
| P. angustifolium | 0.0 | 13.4 | 4.7 | 21.9 | 53.0 | 93.0 | Antiprotozoal | [69] |
| P. anonifolium | 0.0 | 11.7 | 0.0 | 38.6 | 38.9 | 89.2 | Antimicrobial/Enzimaitc | [61] |
| P. aleyreanum | 0.0 | 10.1 | 0.0 | 56.7 | 23.1 | 89.9 | Antimicrobial/Citotoxic | [61] |
| P. aleyreanum | 0.0 | 16.6 | 16.4 | 16.2 | 28.3 | 77.5 | Antinociceptive/Anti-inflammatory | [90] |
| P. arboreum | 0.0 | 0.0 | 1.7 | 46.7 | 41.0 | 89.4 | Antiprotozoal | [70] |
| P. auritum | 88.5 | 3.4 | 0.7 | 3.2 | 0.6 | 96.4 | Antiprotozoal | [117] |
| P. biasperatum | 0.0 | 4.5 | 0.0 | 94.9 | 0.0 | 99.4 | Cytotoxic | [34] |
| P. bredemeyeri | 0.0 | 0.2 | 0.0 | 99.8 | 0.0 | 100.0 | Antimicrobial | [34] |
| P. caldense | 0.0 | 0.0 | 47.1 | 7.2 | 24.3 | 78.6 | Antimicrobial | [54] |
| P. caldense | 0.0 | 0.0 | 6.5 | 17.2 | 59.7 | 83.4 | Antimicrobial | [54] |
| P. caldense | 0.0 | 0.4 | 0.0 | 63.5 | 20.1 | 84.0 | Antimicrobial | [54] |
| P. callosun | 80.1 | 6.9 | 4.2 | 4.9 | 2.1 | 98.2 | Enzyme inhibitory | [62] |
| P. cernuum | 0.0 | 18.9 | 0.0 | 62.4 | 16.7 | 97.9 | Antimicrobial | [38] |
| P. cernuum | 0.0 | 3.1 | 0.0 | 81.3 | 15.0 | 99.4 | Antimicrobial | [60] |
| P. cernuum | 0.0 | 0.0 | 0.0 | 78.9 | 0.0 | 78.9 | Cytotoxic | [39] |
| P. cernuum | 0.0 | 52.2 | 23.2 | 0.0 | 0.0 | 75.4 | Cytotoxic | [96] |
| P. cernuum | 0.0 | 20.5 | 0.0 | 31.0 | 35.0 | 86.5 | Antiprotozoal/Antimicrobial | [70] |
| P. cernuum | 0.0 | 12.3 | 1.2 | 10.4 | 75.5 | 99.4 | Antimicrobial | [37] |
| P. claussenianum | 0.0 | 0.3 | 0.0 | 9.1 | 86.2 | 95.6 | Antiprotozoal/Antimicrobial | [71,126] |
| P. claussenianum | 0.0 | 1.5 | 51.4 | 7.5 | 28.3 | 88.7 | Antiprotozoal/Antimicrobial | [71,126] |
| P. corcovadensis | 30.6 | 35.1 | 0.2 | 20.4 | 6.4 | 92.7 | Insecticidal | [127] |
| P. crassinervium | 0.0 | 7.8 | 0.0 | 54.8 | 37.1 | 99.7 | Antimicrobial | [60] |
| P. demeraranum | 0.0 | 29.9 | 0.0 | 63.0 | 0.0 | 92.9 | Antiprotozoal | [72] |
| P. diospyrifolium | 0.0 | 19.5 | 1.1 | 68.2 | 11.2 | 100.0 | Antimicrobial | [55] |
| P. diospyrifolium | 0.0 | 16.1 | 0.0 | 46.5 | 28.5 | 91.1 | Antiprotozoal | [70] |
| P. divaricatum | 89.6 | 3.3 | 0.0 | 5.6 | 0.6 | 99.1 | Antimicrobial | [40] |
| P. divaricatum | 98.0 | 0.0 | 0.0 | 0.0 | 0.0 | 98.0 | Antimicrobial | [44] |
| P. divaricatum | 89.1 | 7.2 | 0.1 | 1.9 | 0.2 | 98.5 | Antimicrobial | [42] |
| P. duckei | 0.0 | 1.1 | 5.8 | 60.2 | 23.0 | 90.1 | Antiprotozoal | [72] |
| P. fimbriulatum | 0.0 | 19.5 | 0.0 | 76.4 | 4.1 | 100.0 | Antimicrobial | [34] |
| P. gaudichaudianum | 0.0 | 2.4 | 0.0 | 44.3 | 44.0 | 90.8 | Insecticidal | [136] |
| P. gaudichaudianum | 0.0 | 0.1 | 0.1 | 65.4 | 28.3 | 93.8 | Cytotoxic | [138] |
| P. gaudichaudianum | 0.1 | 4.2 | 0.4 | 56.0 | 29.5 | 90.2 | Cytotoxic | [137] |
| P. gaudichaudianum | 0.0 | 0.9 | 0.0 | 72.6 | 14.4 | 87.9 | Antimicrobial | [35] |
| P. gaudichaudianum | 0.0 | 7.1 | 0.0 | 76.0 | 9.9 | 93.0 | Antiprotozoal | [70] |
| P. glabratum | 0.2 | 25.8 | 1.0 | 50.4 | 21.2 | 98.6 | Anti-inflammatory | [88] |
| P. glabrescens | 0.0 | 83.7 | 0.0 | 15.3 | 1.0 | 100.0 | Cytotoxic | [34] |
| P. hispidinervum | 85.5 | 9.3 | 0.0 | 2.5 | 0.8 | 98.0 | Antimicrobial | [139] |
| P. hispidum | 0.0 | 18.5 | 1.0 | 52.2 | 16.6 | 88.3 | Antimicrobial/Enzyme inhibitory | [61] |
| P. hispidum | 0.0 | 43.9 | 1.7 | 27.8 | 15.4 | 88.8 | Antimicrobial/Cytotoxic | [97] |
| P. hispidum | 58.3 | 5.0 | 12.6 | 14.2 | 5.0 | 95.1 | Insecticidal | [47] |
| P. hostmannianum | 57.0 | 1.0 | 5.6 | 20.1 | 10.6 | 94.3 | Insecticidal | [136] |
| P. humaytanum | 0.0 | 1.5 | 0.0 | 34.0 | 45.4 | 80.9 | Insecticidal | [136] |
| P. ilheuense | 0.0 | 0.0 | 0.0 | 46.5 | 34.1 | 80.6 | Antimicrobial | [57] |
| P. imperiale | 6.7 | 2.7 | 0.0 | 89.4 | 1.2 | 100.0 | Antimicrobial/Cytotoxic | [34] |
| P. klotzschianum | 98.5 | 0.0 | 0.0 | 0.6 | 0.5 | 99.6 | Insecticidal | [141] |
| P. klotzschianum | 39.4 | 44.3 | 0.0 | 14.5 | 0.0 | 98.2 | Insecticidal | [141] |
| P. longispicum | 1.1 | 1.4 | 0.2 | 66.0 | 11.9 | 80.6 | Insecticidal | [47] |
| P. marginatum | 0.0 | 1.4 | 0.7 | 2.2 | 26.2 | 30.5 | Insecticidal | [146] |
| P. marginatum | 28.4 | 0.0 | 0.0 | 44.6 | 26.2 | 99.2 | Insecticidal | [146] |
| P. marginatum | 51.6 | 0.0 | 0.0 | 21.7 | 26.4 | 99.7 | Insecticidal | [146] |
| P. marginatum | 42.0 | 10.3 | 1.6 | 17.6 | 17.5 | 89.0 | Antimicrobial/Enzyme inhibitory | [62] |
| P. mikanianum | 67.9 | 0.5 | 0.0 | 23.4 | 8.6 | 100.4 | Acaricidal | [106] |
| P. mollicomum | 0.6 | 24.2 | 9.8 | 33.2 | 25.1 | 92.9 | Antinociceptive | [91] |
| P. mosenii | 0.0 | 7.2 | 0.0 | 41.5 | 37.7 | 86.4 | Antiprotozoal/Antimicrobial | [70] |
| P. oblanceolatum | 0.0 | 16.6 | 12.2 | 61.4 | 9.8 | 100.0 | Antimicrobial/Cytotoxic | [34] |
| P. permucronatum | 98.8 | 0.8 | 0.0 | 0.0 | 0.0 | 99.6 | Insecticidal | [136] |
| P. regnellii | 0.0 | 60.8 | 17.8 | 13.8 | 6.1 | 98.5 | Antimicrobial | [38] |
| P. regnellii | 0.3 | 0.0 | 0.4 | 82.0 | 10.8 | 93.5 | Cytotoxic | [98] |
| P. rivinoides | 0.0 | 65.9 | 0.8 | 21.8 | 4.8 | 93.2 | Antinociceptive | [91] |
| P. rivinoides | 0.0 | 10.4 | 0.0 | 54.7 | 20.1 | 85.2 | Antiprotozoal | [70] |
| P. solmsianum | 40.3 | 30.3 | 0.0 | 5.8 | 4.2 | 80.7 | Depressant/Ataxia | [151] |
| P. solmsianum | 53.5 | 0.0 | 0.0 | 12.4 | 12.3 | 78.2 | Antimicrobial | [35] |
| P. tuberculatum | 0.0 | 35.7 | 0.3 | 60.2 | 2.9 | 99.1 | Antimicrobial | [59] |
| P. vicosanum | 0.0 | 16.4 | 0.0 | 62.6 | 20.8 | 99.8 | Anti-inflammatory | [89] |
| P. xylosteoides | 48.5 | 17.0 | 0.4 | 23.7 | 10.4 | 100.0 | Acaricidal | [106] |

References
- Quijano-Abril, M.A.; Callejas-Posada, R.; Miranda-Esquivel, D.R. Areas of endemism and distribution patterns for Neotropical Piper species (Piperaceae). J. Biogeogr. 2006, 33, 1266–1278. [Google Scholar] [CrossRef]
- Ramírez Amezcua, J.M. Piper commutatum (Piperaceae), the correct name for a widespread species in Mexico and Mesoamerica. Acta Botanica Mexicana 2016, 116, 9–19. [Google Scholar] [CrossRef]
- Angiosperm Phylogeny Group. An update of the Angiosperm Phylogeny Group classification for the orders and families of flowering plants: APG IV. Bot. J. Linn. Soc. 2016, 181, 1–20. [Google Scholar]
- Jaramillo, M.A.; Manos, P.S. Phylogeny and patterns of floral diversity in the genus Piper (Piperaceae). Am. J. Bot. 2001, 88, 706–716. [Google Scholar] [CrossRef] [PubMed]
- Guimarães, E.F.; Monteiro, D. Neotropical Piperaceae. Available online: https://www.kew.org/science/tropamerica/neotropikey/families/Piperaceae.htm (accessed on 12 October 2017).
- Di Stasi, L.C.; Hiruma-Lima, C.A. Plantas Medicinais na Amazônia e na Mata Atlântica; Editora UNESP: São Paulo, Brazil, 2002. [Google Scholar]
- Brú, J.; Guzman, J.D. Folk medicine, phytochemistry and pharmacological application of Piper marginatum. Rev. Bras. Farmacogn. 2016, 26, 767–779. [Google Scholar] [CrossRef]
- Duke, J.A.; Bogenschutz-Godwin, M.J.; Ottesen, A.R. Duke’s Handbook of Medicinal Plants of Latin America; CRC Press: Boca Raton, FL, USA, 2009. [Google Scholar]
- Cáceres, A.; Kato, M.J. Importance of a multidisciplinary evaluation of Piper genus for development of new natural products in Latin America. Int. J. Phytocosmetics Nat. Ingred. 2014, 1, 4. [Google Scholar]
- Takeara, R.; Gonçalves, R.; Ayres, V.F.S.; Guimarães, A.C. Biological properties of essential oils from the Piper species of Brazil: A review. In Aromatic and Medicinal Plants—Back to Nature; El-Shemy, H., Ed.; InTech: Rijeka, Croatia, 2017; pp. 81–93. [Google Scholar]
- Dyer, L.A.; Palmer, A.D.N. Piper: A Model Genus for Studies of Phytochemistry, Ecology, and Evolution; Kluwer Academic/Plenum Publishers: New York, NY, USA, 2004. [Google Scholar]
- Nascimento, J.C.; de Paula, V.F.; David, J.M.; David, J.P. Occurrence, biological activities and 13C NMR data of amides from Piper (Piperaceae). Quim. Nova 2012, 35, 2288–2311. [Google Scholar] [CrossRef]
- Paz, R.F.; Guimarães, E.F.; Ramos, C.S. The occurrence of phenylpropanoids in the saps of six Piper species (Piperaceae) from Brazil. Gayana Bot. 2017, 74, 236–239. [Google Scholar] [CrossRef]
- Maia, J.G.S.; Andrade, E.H.A. Database of the Amazon aromatic plants and their essential oils. Quim. Nova 2009, 32, 595–622. [Google Scholar] [CrossRef]
- Maia, J.G.S.; Zoghbi, M.G.B.; Andrade, E.H.A.; Santos, A.S.; da Silva, M.H.; Luz, A.I.R.; Bastos, C.N. Constituents of the essential oil of Piper aduncum L. growing wild in the Amazon region. Flavour Fragr. J. 1998, 13, 269–272. [Google Scholar] [CrossRef]
- De Almeida, R.R.P.; Souto, R.N.P.; Bastos, C.M.; da Silva, M.H.L.; Maia, J.G.S. Chemical variation in Piper aduncum and biological properties of its dillapiole-rich essential oil. Chem. Biodivers. 2009, 6, 1427–1434. [Google Scholar] [CrossRef] [PubMed]
- Potzernheim, M.; Costa, A.F.; Bizzo, H.R.; Carvalho-Silva, M.; Vieira, R.F. Essential oil of Piper xylosteoides (Kunth) Steud. from Federal District, Brazil. J. Essent. Oil Res. 2006, 18, 523–524. [Google Scholar] [CrossRef]
- Silva, J.P.L.; Queiroz, D.M.; Azevedo, L.H.; Leal, L.C.; Rodrigues, J.L.; Lima, A.F.; Marchi, G.M.; Brito-Júnior, M.; Faria-e-Silva, A.L. Effect of eugenol exposure time and post-removal delay on the bond strength of a self-etching adhesive to dentin. Oper. Dent. 2011, 36, 66–71. [Google Scholar] [CrossRef] [PubMed]
- Ribeiro, A.S.; Batista, E.D.; Dairiki, J.K.; Chaves, F.C.; Inoue, L.A. Anesthetic properties of Ocimum gratissimum essential oil for juvenile matrinxã. Acta Sci. Anim. Sci. 2016, 38, 1–7. [Google Scholar] [CrossRef]
- Ravindran, P.N. Black Pepper: Piper nigrum; CRC Press: Boca Raton, FL, USA, 2000. [Google Scholar]
- Shivashankar, M. Genetic diversity and relationships of Piper species using molecular marker. Int. J. Curr. Microbiol. Appl. Sci. 2014, 3, 1101–1109. [Google Scholar]
- Sen, S.; Skaria, R.; Muneer, P.M.A. Genetic diversity analysis in Piper species (Piperaceae) Using RAPD markers. Mol. Biotechnol. 2010, 46, 72–79. [Google Scholar] [CrossRef] [PubMed]
- Chaveerach, A.; Tanee, T.; Sanubol, A.; Monkheang, P.; Sudmoon, R. Efficient DNA barcode regions for classifying Piper species (Piperaceae). PhytoKeys 2016, 70, 1–10. [Google Scholar]
- Singh, K.; Das, G.; Jadhao, K.R.; Rout, G.R. Molecular diversity and phytochemical characterization of Piper species. J. Appl. Hortic. 2016, 18, 187–194. [Google Scholar]
- Oliveira, G.L.; Moreira, D.D.; Mendes, A.D.; Guimarães, E.F.; Figueiredo, L.S.; Kaplan, M.A.; Martins, E.R. Growth study and essential oil analysis of Piper aduncum from two sites of Cerrado biome of Minas Gerais State, Brazil. Rev. Bras. Farmacogn. 2013, 23, 743–753. [Google Scholar] [CrossRef]
- De Oliveira, J.C.S.; Dias, I.J.M.; da Camara, C.A.G.; Schwartz, M.O.E. Volatile constituents of the leaf oils of Piper aduncum L. from different regions of Pernambuco (northeast of Brazil). J. Essent. Oil Res. 2006, 18, 557–559. [Google Scholar] [CrossRef]
- Pino, J.A.; Marbot, R.; Bello, A.; Urquiola, A. Essential oils of Piper peltata (L.) Miq. and Piper aduncum L. from Cuba. J. Essent. Oil Res. 2004, 16, 124–126. [Google Scholar] [CrossRef]
- Silva, W.C.; de Souza Martins, J.R.; de Souza, H.E.M.; Heinzen, H.; Cesio, M.V.; Mato, M.; Albrecht, F.; de Azevedo, J.L.; de Barros, N.M. Toxicity of Piper aduncum L. (Piperales: Piperaceae) from the Amazon forest for the cattle tick Rhipicephalus (Boophilus) microplus (Acari: Ixodidae). Vet. Parasitol. 2009, 164, 267–274. [Google Scholar] [CrossRef] [PubMed]
- Araújo, M.J.C.; Câmara, C.A.G.; Born, F.S.; Moraes, M.M.; Badji, C.A. Acaricidal activity and repellency of essential oil from Piper aduncum and its components against Tetranychus urticae. Exp. Appl. Acarol. 2012, 57, 139–155. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, R.G.; Monteiro, M.C.; da Silva, J.K.R.; Maia, J.G.S. Antifungal action of the dillapiole-rich oil of Piper aduncum against dermatomycoses caused by filamentous fungi. Br. J. Med. Med. Res. 2016, 15, 1–10. [Google Scholar] [CrossRef]
- Perigo, C.V.; Torres, R.B.; Bernacci, L.C.; Guimarães, E.F.; Haber, L.L.; Facanali, R.; Vieira, M.A.R.; Quecini, V.; Marques, M.O.M. The chemical composition and antibacterial activity of eleven Piper species from distinct rainforest areas in Southeastern Brazil. Ind. Crops Prod. 2016, 94, 528–539. [Google Scholar] [CrossRef]
- Monzote, L.; Scull, R.; Cos, P.; Setzer, W.N. Essential oil from Piper aduncum: Chemical analysis, antimicrobial assessment, and literature review. Medicines 2017, 4, 49. [Google Scholar] [CrossRef] [PubMed]
- Potzernheim, M.; Bizzo, H.R.; Agostini-Costa, T.S.; Vieira, R.F.; Carvalho-Cilva, M.; Gracindo, L.A.M.B. Chemical characterization of seven Piper species (Piperaceae) from Federal District, Brazil, based on volatile oil constituents. Rev. Bras. Plantas Med. 2006, 8, 10–12. [Google Scholar]
- Setzer, W.N.; Park, G.; Agius, B.R.; Stokes, S.L.; Walker, T.M.; Haber, W.A. Chemical compositions and biological activities of leaf essential oils of twelve species of Piper from Monteverde, Costa Rica. Nat. Prod. Commun. 2008, 3, 1367–1374. [Google Scholar]
- Morandim-Giannetti, A.A.; Pin, A.R.; Pietro, N.A.S.; de Oliveira, H.C.; Mendes-Giannini, M.J.S.; Alecio, A.C.; Kato, M.J.; de Oliveira, J.E.; Furlan, M. Composition and antifungal activity against Candida albicans, Candida parapsilosis, Candida krusei and Cryptococcus neoformans of essential oils from leaves of Piper and Peperomia species. J. Med. Plants Res. 2010, 4, 1810–1814. [Google Scholar]
- Torquilho, H.S.; Pinto, A.C.; de Godoy, R.L.O.; Guimarães, E.F. Essential oil of Piper cernum Vell. var. cernum Yuncker from Rio de Janeiro, Brazil. J. Essent. Oil Res. 2000, 12, 443–444. [Google Scholar] [CrossRef]
- Gasparetto, A.; Cruz, A.B.; Wagner, T.M.; Bonomini, T.J.; Correa, R.; Malheiros, A. Seasonal variation in the chemical composition, antimicrobial and mutagenic potential of essential oils from Piper cernuum. Ind. Crops Prod. 2017, 95, 256–263. [Google Scholar] [CrossRef]
- Costantin, M.B.; Sartorelli, P.; Limberger, R.; Henriques, A.T.; Steppe, M.; Ferreira, M.J.P.; Ohara, M.T.; Emerenciano, V.P.; Kato, M.J. Essential oils from Piper cernuum and Piper regnellii: Antimicrobial activities and analysis by GC/MS and 13C-NMR. Planta Med. 2001, 67, 771–773. [Google Scholar] [CrossRef] [PubMed]
- Capello, T.M.; Martins, E.G.A.; de Farias, C.F.; Figueiredo, C.R.; Matsuo, A.L.; Passero, L.F.D.; Oliveira-Silva, D.; Sartorelli, P.; Lago, J.H.G. Chemical composition and in vitro cytotoxic and antileishmanial activities of extract and essential oil from leaves of Piper cernuum. Nat. Prod. Commun. 2015, 10, 285–288. [Google Scholar] [PubMed]
- Da Silva, J.K.R.; Andrade, E.H.A.; Guimarães, E.F.; Maia, J.G.S. Essential oil composition, antioxidant capacity and antifungal activity of Piper divaricatum. Nat. Prod. Commun. 2010, 5, 477–480. [Google Scholar] [PubMed]
- Souto, R.N.P.; Harada, A.Y.; Andrade, E.H.A.; Maia, J.G.S. Insecticidal activity of Piper essential oils from the Amazon against the fire ant Solenopsis saevissima (Smith) (Hymenoptera: Formicidae). Neotrop. Entomol. 2012, 41, 510–517. [Google Scholar] [CrossRef] [PubMed]
- Da Silva, J.K.R.; Silva, J.R.A.; Nascimento, S.B.; Luz, S.F.M.; Meireles, E.N.; Alves, C.N.; Ramos, A.R.; Maia, J.G.S. Antifungal activity and computational study of constituents from Piper divaricatum essential oil against Fusarium infection in black pepper. Molecules 2014, 19, 17926–17942. [Google Scholar] [CrossRef] [PubMed]
- Meireles, E.N.; Xavier, L.P.; Ramos, A.R.; Maia, J.G.S.; Setzer, W.N.; da Silva, J.K.R. Phenylpropanoids produced by Piper divaricatum, a resistant species to infection by Fusarium solani f. sp. piperis, the pathogenic agent of fusariosis in black pepper. J. Plant Pathol. Microbiol. 2016, 7, 333. [Google Scholar]
- Barbosa, Q.P.S.; da Câmara, C.A.G.; Ramos, C.S.; Nascimento, D.C.O.; Lima-Filho, J.V.; Guimarães, E.F. Chemical composition, circadian rhythm and antibacterial activity of essential oils of Piper divericatum: A new source of safrole. Quim. Nova 2012, 35, 1806–1808. [Google Scholar] [CrossRef]
- Andrade, E.H.A.; Carreira, L.M.M.; da Silva, M.H.L.; da Silva, J.D.; Bastos, C.N.; Sousa, P.J.C.; Guimarães, E.F.; Maia, J.G.S. Variability in essential-oil composition of Piper marginatum sensu lato. Chem. Biodivers. 2008, 5, 197–208. [Google Scholar] [CrossRef] [PubMed]
- Pino, J.A.; Marbot, R.; Bello, A.; Urquiola, A. Composition of the essential oil of Piper hispidum Sw. from Cuba. J. Essent. Oil Res. 2004, 16, 459–460. [Google Scholar] [CrossRef]
- Santana, A.I.; Vila, R.; Cañigueral, S.; Gupta, M.P. Chemical composition and biological activity of essential oils from different species of Piper from Panama. Planta Med. 2016, 82, 986–991. [Google Scholar] [CrossRef] [PubMed]
- Benitez, N.P.; León, E.M.M.; Stashenko, E.E. Essential oil composition from two species of Piperaceae family grown in Colombia. J. Chromatogr. Sci. 2009, 47, 804–807. [Google Scholar] [CrossRef]
- Vogler, B.; Noletto, J.A.; Haber, W.A.; Setzer, W.N. Chemical constituents of the essential oils of three Piper species from Monteverde, Costa Rica. J. Essent. Oil Bear. Plants 2006, 9, 230–238. [Google Scholar] [CrossRef]
- Rodriguez, E.J.; Saucedo-Hernández, Y.; Vander Heyden, Y.; Simó-Alfonso, E.F.; Ramis-Ramos, G.; Lerma-García, M.J.; Monteagudo, U.; Bravo, L.; Medinilla, M.; de Armas, Y.; et al. Chemical analysis and antioxidant activity of the essential oils of three Piperaceae species growing in the central region of Cuba. Nat. Prod. Commun. 2013, 8, 1325–1328. [Google Scholar] [PubMed]
- Roca, I.; Akova, M.; Baquero, F.; Carlet, J.; Cavaleri, M.; Coenen, S.; Cohen, J.; Findlay, D.; Gyssens, I.; Heure, O.E.; et al. The global threat of antimicrobial resistance: Science for intervention. New Microbes New Infect. 2015, 6, 22–29. [Google Scholar] [CrossRef] [PubMed]
- Duarte, M.C.T.; Leme, E.E.; Delarmelina, C.; Soares, A.A.; Figueira, G.M.; Sartoratto, A. Activity of essential oils from Brazilian medicinal plants on Escherichia coli. J. Ethnopharmacol. 2007, 111, 197–201. [Google Scholar] [CrossRef] [PubMed]
- Gutiérrez, Y.; Montes, R.; Scull, R.; Sánchez, A.; Cos, P.; Monzote, L.; Setzer, W.N. Chemodiversity associated with cytotoxicity and antimicrobial activity of Piper aduncum var. ossanum. Chem. Biodivers. 2016, 13, 1715–1719. [Google Scholar] [CrossRef] [PubMed]
- Silva Rocha, D.; da Silva, J.M.; do Amaral Ferraz Navarro, D.M.; Gomes Camara, C.A.; de Lira, C.S.; Souza Ramos, C. Potential antimicrobial and chemical composition of essential oils from Piper caldense tissues. J. Mex. Chem. Soc. 2016, 60, 148–151. [Google Scholar]
- Vieira, S.C.; Paulo, L.F.; Svidzinski, T.I.; Dias Filho, B.P.; Nakamura, C.V.; Souza, A.D.; Young, M.C.; Cortez, D.A. Antifungal activity of Piper diospyrifolium Kunth (Piperaceae) essential oil. Brazilian J. Microbiol. 2011, 42, 1001–1005. [Google Scholar] [CrossRef]
- Santos, T.G.; Rebeleo, R.A.; Dalmarco, E.M.; Guedes, A.; de Gasper, A.L.; Bella Cruz, A.; Schmit, A.P.; Bella Cruz, R.C.; Steindel, M.; Nunes, R.K. Composição química e avaliação da atividade antimicrobiana do oleo essencial das folhas de Piper malacophyllum (C. Presl.) C. DC. Quim. Nova 2012, 35, 477–481. [Google Scholar] [CrossRef]
- De Oliveira, R.A.; de Assis, A.M.A.D.; da Silva, L.A.M.; Andrioli, J.L.; de Oliveira, F.F. Chemical profile and antimicrobial activity of essential oil of Piper ilheusense. Chem. Nat. Compd. 2016, 52, 331–333. [Google Scholar] [CrossRef]
- Osorio, J.R.; Mora, L.E.; Dulcey, A.J.C.; Andica, R.S. Extraction, chemical composition and antimicrobial activity of the essential oils of pipilongo (Piper tuberculatum) using supercritical carbon dioxide. Rev. Ciencias 2013, 17, 45–56. [Google Scholar]
- Navickiene, H.M.; Morandim, A.D.; Alécio, A.C.; Regasini, L.O.; Bergamo, D.C.; Telascrea, M.; Cavalheiro, A.J.; Lopes, M.N.; Bolzani, V.D.; Furlan, M.; et al. Composition and antifungal activity of essential oils from Piper aduncum, Piper arboreum and Piper tuberculatum. Quim. Nova 2006, 29, 467–470. [Google Scholar] [CrossRef]
- Morandim, A.D.; Pin, A.R.; Pietro, N.A.; Alecio, A.C.; Kato, M.J.; Young, C.M.; de Oliveira, J.E.; Furlan, M. Composition and screening of antifungal activity against Cladosporium sphaerospermum and Cladosporium cladosporioides of essential oils of leaves and fruits of Piper species. Afr. J. Biotechnol. 2010, 9, 6135–6139. [Google Scholar]
- Da Silva, J.K.; Pinto, L.C.; Burbano, R.M.; Montenegro, R.C.; Guimarães, E.F.; Andrade, E.H.; Maia, J.G. Essential oils of Amazon Piper species and their cytotoxic, antifungal, antioxidant and anti-cholinesterase activities. Ind. Crops Prod. 2014, 58, 55–60. [Google Scholar] [CrossRef]
- Da Silva, J.K.; Silva, N.N.; Santana, J.F.; Andrade, E.H.; Maia, J.G.; Setzer, W.N. Phenylpropanoid-rich essential oils of Piper species from the Amazon and their antifungal and anti-cholinesterase activities. Nat. Prod. Commun. 2016, 11, 1907–1911. [Google Scholar]
- Tasić, S.; Miladinović-Tasić, N. Cladosporium spp.: Cause of opportunistic mycoses. Acta Fac. Medicae Naissensis 2007, 24, 15–19. [Google Scholar]
- Ng, K.P.; Yew, S.M.; Chan, C.L.; Soo-Hoo, T.S.; Na, S.L.; Hassan, H.; Ngeow, Y.F.; Hoh, C.-C.; Lee, K.-W.; Yee, W.-Y. Sequencing of Cladosporium sphaerospermum, a dematiaceous fungus isolated from blood culture. Eukaryot. Cell 2012, 11, 705–706. [Google Scholar] [CrossRef] [PubMed]
- Guerrini, A.; Sacchetti, G.; Rossi, D.; Paganetto, G.; Muzzoli, M.; Andreotti, E.; Tognolini, M.; Maldonado, M.E.; Bruni, R. Bioactivities of Piper aduncum L. and Piper obliquum Ruiz & Pavon (Piperaceae) essential oils from Eastern Ecuador. Environ. Toxicol. Pharmacol. 2009, 27, 39–48. [Google Scholar] [PubMed]
- Pink, R.; Hudson, A.; Mouriès, M.-A.; Bendig, M. Opportunities and challenges in antiparasitic drug discovery. Nat. Rev. Drug Discov. 2005, 4, 727–741. [Google Scholar] [CrossRef] [PubMed]
- Ceole, L.F.; Cardoso, M.D.; Soares, M.J. Nerolidol, the main constituent of Piper aduncum essential oil, has anti-Leishmania braziliensis activity. Parasitology 2017, 144, 1179–1190. [Google Scholar] [CrossRef] [PubMed]
- Villamizar, L.H.; Cardoso, M.D.; de Andrade, J.; Teixeira, M.L.; Soares, M.J. Linalool, a Piper aduncum essential oil component, has selective activity against Trypanosoma cruzi trypomastigote forms at 4 °C. Memórias do Instituto Oswaldo Cruz 2017, 112, 131–139. [Google Scholar] [CrossRef] [PubMed]
- Bosquiroli, L.S.S.; Demarque, D.P.; Rizk, Y.S.; Cunha, M.C.; Marques, M.C.S.; de Matos, M.F.C.; Kadri, M.C.T.; Carollo, C.A.; Arruda, C.C.P. In vitro anti-Leishmania infantum activity of essential oil from Piper angustifolium. Rev. Bras. Farmacogn. 2015, 25, 124–128. [Google Scholar] [CrossRef]
- Bernuci, K.Z.; Iwanaga, C.C.; Fernadez-Andrade, C.M.; Lorenzetti, F.B.; Torres-Santos, E.C.; Faiões, V.D.; Gonçalves, J.E.; do Amaral, W.; Deschamps, C.; Scodro, R.B.; Cardoso, R.F. Evaluation of chemical composition and antileishmanial and antituberculosis activities of essential oils of Piper species. Molecules 2016, 21, 1698. [Google Scholar] [CrossRef] [PubMed]
- Marques, A.M.; Barreto, A.L.S.; Batista, E.M.; da Curvelo, J.A.R.; Velozo, L.S.M.; de Moreira, D.L.; Guimarães, E.F.; Soares, R.M.A.; Kaplan, M.A.C. Chemistry and biological activity of essential oils from Piper claussenianum (Piperaceae). Nat. Prod. Commun. 2010, 5, 1837–1840. [Google Scholar] [PubMed]
- Do Carmo, D.F.M.; Amaral, A.C.F.; Machado, G.M.C.; Leon, L.L.; de Silva, J.R.A. Chemical and biological analyses of the essential oils and main constituents of Piper species. Molecules 2012, 17, 1819–1829. [Google Scholar] [CrossRef] [PubMed]
- Marques, A.M.; Peixoto, A.C.C.; de Paula, R.C.; Nascimento, M.F.A.; Soares, L.F.; Velozo, L.S.M.; Guimarães, E.F.; Kaplan, M.A.C. Phytochemical investigation of anti-plasmodial metabolites from Brazilian native Piper species. J. Essent. Oil Bear. Plants 2015, 18, 74–81. [Google Scholar] [CrossRef]
- Monzote, L.; Alarcón, O.; Setzer, W.N. Antiprotozoal activity of essential oils. Agric. Conspec. Sci. 2012, 77, 167–175. [Google Scholar]
- Setzer, W.N.; Stokes, S.L.; Penton, A.F.; Takaku, S.; Haber, W.A.; Hansell, E.; Caffrey, C.R.; McKerrow, J.H. Cruzain inhibitory activity of leaf essential oils of neotropical lauraceae and essential oil components. Nat. Prod. Commun. 2007, 2, 1203–1210. [Google Scholar]
- Colovic, M.B.; Krstic, D.Z.; Lazarevic-Pasti, T.D.; Bondzic, A.M.; Vasic, V.M. Acetylcholinesterase inhibitors: Pharmacology and toxicology. Curr. Neuropharmacol. 2013, 11, 315–335. [Google Scholar] [CrossRef] [PubMed]
- Ingkaninan, K.; Temkitthawon, P.; Chuenchom, K.; Yuyaem, T.; Thongnoi, W. Screening for acetylcholinesterase inhibitory activity in plants used in Thai traditional rejuvenating and neurotonic remedies. J. Ethnopharmacol. 2003, 89, 261–264. [Google Scholar] [CrossRef] [PubMed]
- Adewusi, E.A.; Moodley, N.; Steenkamp, V. Antioxidant and acetylcholinesterase inhibitory activity of selected southern African medicinal plants. S. Afr. J. Bot. 2011, 77, 638–644. [Google Scholar] [CrossRef]
- Ferreres, F.; Oliveira, A.P.; Gil-Izquierdo, A.; Valentão, P.; Andrade, P.B. Piper betle leaves: Profiling phenolic compounds by HPLC/DAD–ESI/MSn and anti-cholinesterase activity. Phytochem. Anal. 2014, 25, 453–460. [Google Scholar] [CrossRef] [PubMed]
- Salleh, W.M.; Hashim, N.A.; Ahmad, F.; Yen, K.H. Anticholinesterase and antityrosinase activities of ten Piper species from Malaysia. Adv. Pharm. Bull. 2014, 4, 527–531. [Google Scholar] [PubMed]
- Xiang, C.-P.; Han, J.-X.; Li, X.-C.; Li, Y.-H.; Zhang, Y.; Chen, L.; Qu, Y.; Hao, C.-Y.; Li, H.-Z.; Yang, C.-R.; et al. Chemical composition and acetylcholinesterase inhibitory activity of essential oils from Piper species. J. Agric. Food Chem. 2017, 65, 3702–3710. [Google Scholar] [CrossRef] [PubMed]
- Wani, T.A.; Chandrashekara, H.H.; Kumar, D.; Prasad, R.; Sardar, K.K.; Kumar, D.; Tandan, S.K. Anti-inflammatory and antipyretic activities of the ethanolic extract of Shorea robusta Gaertn. f. resin. Indian J. Biochem. Biophys. 2012, 49, 463–467. [Google Scholar] [PubMed]
- Adorjan, B.; Buchbauer, G. Biological properties of essential oils: An updated review. Flavour Fragr. J. 2010, 25, 407–426. [Google Scholar] [CrossRef]
- Miguel, M.G. Antioxidant and anti-inflammatory activities of essential oils: A short review. Molecules 2010, 15, 9252–9287. [Google Scholar] [CrossRef] [PubMed]
- De Sousa, D.P. Analgesic-like activity of essential oils constituents. Molecules 2011, 16, 2233–2252. [Google Scholar] [CrossRef] [PubMed]
- De Cássia da Silveira e Sá, R.; Andrade, L.N.; de Sousa, D.P. A review on anti-inflammatory activity of monoterpenes. Molecules 2013, 18, 1227–1254. [Google Scholar] [CrossRef] [PubMed]
- De Cássia da Silveira e Sá, R.; Andrade, L.N.; dos Reis Barreto de Oliveira, R.; de Sousa, D.P. A review on anti-inflammatory activity of phenylpropanoids found in essential oils. Molecules 2014, 19, 1459–1480. [Google Scholar] [CrossRef] [PubMed]
- Branquinho, L.S.; Santos, J.A.; Cardoso, C.A.; da Silva Mota, J.; Junior, U.L.; Kassuya, C.A.; Arena, A.C. Anti-inflammatory and toxicological evaluation of essential oil from Piper glabratum leaves. J. Ethnopharmacol. 2017, 198, 372–378. [Google Scholar] [CrossRef] [PubMed]
- Brait, D.R.; Vaz, M.S.; da Silva Arrigo, J.; de Carvalho, L.N.; de Araújo, F.H.; Vani, J.M.; da Silva Mota, J.; Cardoso, C.A.; Oliveira, R.J.; Negrão, F.J.; et al. Toxicological analysis and anti-inflammatory effects of essential oil from Piper vicosanum leaves. Regul. Toxicol. Pharmacol. 2015, 73, 699–705. [Google Scholar] [CrossRef] [PubMed]
- Lima, D.K.; Ballico, L.J.; Lapa, F.R.; Gonçalves, H.P.; de Souza, L.M.; Iacomini, M.; de Paula Werner, M.F.; Baggio, C.H.; Pereira, I.T.; da Silva, L.M.; et al. Evaluation of the antinociceptive, anti-inflammatory and gastric antiulcer activities of the essential oil from Piper aleyreanum C DC in rodents. J. Ethnopharmacol. 2012, 142, 274–282. [Google Scholar] [CrossRef] [PubMed]
- De Souza, S.P.; Valverde, S.S.; Costa, N.F.; Calheiros, A.S.; Lima, K.S.C.; Frutuoso, V.S.; Lima, A.L.S. Chemical composition and antinociceptive activity of the essential oil of Piper mollicomum and Piper rivinoides. J. Med. Plants Res. 2014, 8, 788–793. [Google Scholar]
- Yu, J.-Q.; Lei, J.-C.; Zhang, X.-Q.; Yu, H.-D.; Tian, D.-Z.; Liao, Z.-X.; Zou, G. Anticancer, antioxidant and antimicrobial activities of the essential oil of Lycopus lucidus Turcz. var. hirtus Regel. Food Chem. 2011, 126, 1593–1598. [Google Scholar] [CrossRef] [PubMed]
- Mitoshi, M.; Kuriyama, I.; Nakayama, H.; Miyazato, H.; Sugimoto, K.; Kobayashi, Y.; Jippo, T.; Kanazawa, K.; Yoshida, H.; Mizushina, Y. Effects of essential oils from herbal plants and citrus fruits on DNA. J. Agric. Food Chem. 2012, 60, 11343–11350. [Google Scholar] [CrossRef] [PubMed]
- Gautam, N.; Mantha, A.K.; Mittal, S. Essential oils and their constituents as anticancer agents: A mechanistic view. BioMed Res. Int. 2014, 2014, 154106. [Google Scholar] [CrossRef] [PubMed]
- Da Silva, J.K.R.; Pinto, L.C.; Burbano, R.M.R.; Montenegro, R.C.; Andrade, E.H.A.; Maia, J.G.S. Composition and cytotoxic and antioxidant activities of the oil of Piper aequale Vahl. Lipids Health Dis. 2016, 15, 174. [Google Scholar] [CrossRef] [PubMed]
- Girola, N.; Figueiredo, C.R.; Farias, C.F.; Azevedo, R.A.; Ferreira, A.K.; Teixeira, S.F.; Capello, T.M.; Martins, E.G.A.; Matsuo, A.L.; Travassos, L.R.; et al. Camphene isolated from essential oil of Piper cernuum (Piperaceae) induces intrinsic apoptosis in melanoma cells and displays antitumor activity in vivo. Biochem. Biophys. Res. Commun. 2015, 467, 928–934. [Google Scholar] [CrossRef] [PubMed]
- Morales, A.; Rojas, J.; Moujir, L.M.; Araujo, L.; Rondón, M. Chemical composition, antimicrobial and cytotoxic activities of Piper hispidum Sw. essential oil collected in Venezuela. J. Appl. Pharm. Sci. 2013, 3, 16–20. [Google Scholar]
- Anderson, R.R.; Girola, N.; Figueiredo, C.R.; Londero, V.S.; Lago, J.H.G. Circadian variation and in vitro cytotoxic activity evaluation of volatile compounds from leaves of Piper regnellii (Miq) C. DC. var. regnellii (C. DC.) Yunck (Piperaceae). Nat. Prod. Res. 2017. [Google Scholar] [CrossRef] [PubMed]
- Lognay, G.C.; Bouxin, P.; Marlier, M.; Haubruge, E.; Gaspar, C.; Rodriguez, A. Composition of the essential oil of Piper acutifolium Ruiz. and Pav. from Peru. J. Essent. Oil Res. 1996, 8, 689–691. [Google Scholar] [CrossRef]
- Vila, R.; Tomi, F.; Mundina, M.; Santana, A.I.; Solís, P.N.; López Arce, J.B.; Balderrama Iclina, J.L.; Iglesias, J.; Gupta, M.P.; Casanova, J.; et al. Unusual composition of the essential oils from the leaves of Piper aduncum. Flavour Fragr. J. 2005, 20, 67–69. [Google Scholar] [CrossRef]
- Lopez Arze, J.B.; Collin, G.; Garneau, F.-X.; Jean, F.-I.; Gagnon, H. Essential oils from Bolivia. VIII. Piperaceae: Piper heterophyllum Ruiz et Pavón, P. aduncum L. J. Essent. Oil Bear. Plants 2008, 11, 53–57. [Google Scholar] [CrossRef]
- Oliveira, G.L.; Vieira, T.M.; Nunes, V.F.; Ruas, M.D.; Duarte, E.R.; Moreira, D.D.; Kaplan, M.A.; Martins, E.R. Chemical composition and efficacy in the egg-hatching inhibition of essential oil of Piper aduncum against Haemonchus contortus from sheep. Rev. Bras. Farmacogn. 2014, 24, 288–292. [Google Scholar] [CrossRef]
- Pacheco, F.V.; de Paula Avelar, R.; Alvarenga, I.C.; Bertolucci, S.K.; de Alvarenga, A.A.; Pinto, J.E. Essential oil of monkey-pepper (Piper aduncum L.) cultivated under different light environments. Ind. Crops Prod. 2016, 85, 251–257. [Google Scholar] [CrossRef]
- Pino, J.A.; Bello, A.; Urquiola, A. The leaf oil of Piper ossanum Trel. from Cuba. J. Essent. Oil Res. 2002, 14, 375. [Google Scholar] [CrossRef]
- Facundo, V.A.; Ferreira, S.A.; de Morais, S.M. Essential oils of Piper dumosum Rudge and Piper aleyreanum C.DC (Piperaceae) from Brazilian Amazonian forest. J. Essent. Oil Res. 2007, 19, 165–166. [Google Scholar] [CrossRef]
- De BF Ferraz, A.; Balbino, J.M.; Zini, C.A.; Ribeiro, V.L.; Bordignon, S.A.; von Poser, G. Acaricidal activity and chemical composition of the essential oil from three Piper species. Parasitol. Res. 2010, 107, 243–248. [Google Scholar] [CrossRef] [PubMed]
- Da Silva Mota, J.; de Souza, D.S.; Boone, C.V.; Lima Cardoso, C.A.; Bastos Caramão, E. Identification of the volatile compounds of leaf, flower, root and stem oils of Piper amalago (Piperaceae). J. Essent. Oil Bear. Plants 2013, 16, 11–16. [Google Scholar] [CrossRef]
- Simeone, M.L.F.; Mikich, S.B.; Côcco, L.C.; Hansel, F.A.; Bianconi, G.V. Chemical composition of essential oils from ripe and unripe fruits of Piper amalago L. var. medium (Jacq.)Yunck and Piper hispidum Sw. J. Essent. Oil Res. 2011, 23, 54–58. [Google Scholar] [CrossRef]
- Tirillini, B.; Velasquez, E.R.; Pellegrino, R. Chemical composition and antimicrobial activity of essential oil of Piper angustifolium. Planta Med. 1996, 62, 372–373. [Google Scholar] [CrossRef] [PubMed]
- Andrade, E.H.A.; Ribeiro, A.F.; Guimarães, E.F.; Maia, J.G.S. Essential oil composition of Piper anonofolium (Kunth) C. DC. J. Essent. Oil Bear. Plants 2005, 8, 289–294. [Google Scholar] [CrossRef]
- Mundina, M.; Vila, R.; Tomi, F.; Gupta, M.P.; Adzet, T.; Casanova, J.; Igueral, S. Leaf essential oils of three Panamanian Piper species. Phytochemistry 1998, 47, 1277–1282. [Google Scholar] [CrossRef]
- Machado, S.M.F.; Militão, J.S.L.T.; Facundo, V.A.; Ribeiro, A.; Morais, S.M.; Machado, M.I.L. Leaf oils of two Brazilian Piper species: Piper arboreum Aublet var. latifolium (C.DC) Yuncker and Piper hispidum Sw. J. Essent. Oil Res. 1994, 6, 643–644. [Google Scholar] [CrossRef]
- Avella, E.; Rios-Motta, J. Main constituents and cytotoxic activity of the essential oil of Piper artanthe. Chem. Nat. Compd. 2010, 46, 547–549. [Google Scholar] [CrossRef]
- Cicció, J.F. Essential oil from the leaves of Piper augustum from “Alberto M. Brenes” Biological Preserve, Costa Rica. J. Essent. Oil Res. 2005, 17, 251–253. [Google Scholar] [CrossRef]
- Gupta, M.P.; Arias, T.D.; Williams, N.H.; Bos, R.; Tattje, D.H.E. Safrole, the main component of the essential oil from Piper auritum of Panama. J. Nat. Prod. 1985, 48, 330. [Google Scholar] [CrossRef]
- Pino, J.A.; Rosado, A.; Rodriguez, M.; Garcia, D. Composition of leaf oil of Piper auritum H.B.K. grown in Cuba. J. Essent. Oil Res. 1998, 10, 333–334. [Google Scholar] [CrossRef]
- Monzote, L.; García, M.; Montalvo, A.M.; Scull, R.; Miranda, M. Chemistry, cytotoxicity and antileishmanial activity of the essential oil from Piper auritum. Memorias do Instituto Oswaldo Cruz 2010, 105, 168–173. [Google Scholar] [CrossRef] [PubMed]
- Leal, S.M.; Pino, N.; Stashenko, E.E.; Martinez, J.R.; Escobar, P. Antiprotozoal activity of essential oils derived from Piper spp. grown in Colombia. J. Essent. Oil Res. 2013, 25, 512–519. [Google Scholar] [CrossRef]
- Vargas, L.; Pérez-Alonso, M.J.; Velasco-Negueruela, A.; Palá-Paúl, J.; García Vallejo, M.C. Leaf essential oil of Piper barbatum H.B.K. (Piperaceae) from Peru. J. Essent. Oil Res. 2003, 15, 163–164. [Google Scholar] [CrossRef]
- Olivero-Verbel, J.; Güette-Fernandez, J.; Stashenko, E. Acute toxicity against Artemia franciscana of essential oils isolated from plants of the genus Lippia and Piper collected in Colombia. Boletin Latinoamericano y del Caribe de Plantas Medicinales y Aromaticas 2009, 8, 419–427. [Google Scholar]
- Tangarife-Castaño, V.; Correa-Royero, J.B.; Roa-Linares, V.C.; Pino-Benitez, N.; Betancur-Gavis, L.A.; Durán, D.C.; Stashenko, E.E.; Mesa-Arango, A.C. Anti-dermatophyte, anti-Fusarium and cytotoxic activity of essential oils and plant extracts of Piper genus. J. Essent. Oil Res. 2014, 26, 221–227. [Google Scholar] [CrossRef]
- Facundo, V.A.; Rezende, C.M.; Pinto, Â.C. Essential oil of Piper carniconnectivum C. CD. leaves and stems. J. Essent. Oil Res. 2006, 18, 296–297. [Google Scholar] [CrossRef]
- Vargas, L.; Velasco-Negueruela, A.; Pérez-Alonso, M.J.; Palá-Paúl, J.; García Vallejo, M.C. Essential oil composition of the leaves and spikes of Piper carpunya Ruíz et Pavón (Piperaceae) from Peru. J. Essent. Oil Res. 2004, 16, 122–123. [Google Scholar] [CrossRef]
- De Abreu, A.M.; Brighente, I.M.C.; Aguilar, E.M.; Rebelo, R.A. Volatile constituents of Piperaceae from Santa Catarina, Brazil—Essential oil composition of Piper cernuum Vell. and Peperomia emarginella (Sw.) C. DC. J. Essent. Oil Res. 2005, 17, 286–288. [Google Scholar] [CrossRef]
- Assis, A.; Brito, V.; Bittencourt, M.; Silva, L.; Oliveira, F.; Oliveira, R. Essential oils composition of four Piper species from Brazil. J. Essent. Oil Res. 2013, 25, 203–209. [Google Scholar] [CrossRef]
- Curvelo, J.A.R.; Marques, A.M.; Barreto, A.L.S.; Romanos, M.T.V.; Portela, M.B.; Kaplan, M.A.C.; Soares, R.M.A. A novel nerolidol-rich essential oil from Piper claussenianum modulates Candida albicans biofilm. J. Med. Microbiol. 2014, 63, 697–702. [Google Scholar] [CrossRef] [PubMed]
- Da Silva, M.F.; Bezerra-Silva, P.C.; de Lira, C.S.; de Lima Albuquerque, B.N.; Neto, A.C.; Pontual, E.V.; Maciel, J.R.; Paiva, P.M.; Navarro, D.M. Composition and biological activities of the essential oil of Piper corcovadensis (Miq.) C. DC (Piperaceae). Exp. Parasitol. 2016, 165, 64–70. [Google Scholar] [CrossRef] [PubMed]
- Andrade, E.H.A.; Guimarães, E.F.; da Silva, M.H.L.; Pereira, R.A.; Bastos, C.N.; Maia, J.G.S. Essential oil composition of Piper cyrtopodon (Miq.) C. DC. J. Essent. Oil Bear. Plants 2006, 9, 53–59. [Google Scholar] [CrossRef]
- Luz, A.I.R.; da Silva, J.D.; Zoghbi, M.G.B.; Andrade, E.H.A.; da Silva, M.H.L.; Maia, J.G.S. Volatile constituents of Brazilian Piperaceae. Part 4. Essential oil composition of Piper dactylostigmum, P. plurinervosum and P. vitaceum. J. Essent. Oil Res. 2000, 12, 94–96. [Google Scholar] [CrossRef]
- Andrade, E.H.A.; Guimarães, E.F.; Maia, J.G.S. Essential oil composition of Piper demeraranum (Miq.) C. DC. J. Essent. Oil Bear. Plants 2006, 9, 47–52. [Google Scholar] [CrossRef]
- Andrade, E.H.A.; Alves, C.N.; Guimarães, E.F.; Carreira, L.M.M.; Maia, J.G.S. Variability in essential oil composition of Piper dilatatum L.C. Rich. Biochem. Syst. Ecol. 2011, 39, 669–675. [Google Scholar] [CrossRef]
- De Almeida, J.G.L.; Silveira, E.R.; Pessoa, O.D.L.; Nunes, E.P. Essential oil composition from leaves and fruits of Piper divaricatum G. Mey. J. Essent. Oil Res. 2009, 21, 228–230. [Google Scholar] [CrossRef]
- Vila, R.; Mundina, M.; Tomi, F.; Cicció, J.F.; Gupta, M.P.; Iglesias, J.; Casanova, J.; Cañigueral, S. Constituents of the essential oils from Piper friedrichsthalii C.DC. and P. pseudolindenii C.DC. from Central America. Flavour Fragr. J. 2003, 18, 198–201. [Google Scholar] [CrossRef]
- Von Poser, G.L.; Rörig, L.R.; Henriques, A.T.; Lamaty, G.; Menut, C.; Bessière, J.M. Aromatic plants from Brazil. III. The chemical composition of Piper gaudichaudianum Kunth and P. mikanianum (Kunth) Steudel essential oils. J. Essent. Oil Res. 1994, 6, 337–340. [Google Scholar] [CrossRef]
- Andrade, E.H.; Zoghbi, M.D.; Santos, A.S.; Maia, J.G. Essential oils of Piper gaudichaudianum Kunth and P. regnellii (Miq.) C.DC. J. Essent. Oil Res. 1998, 10, 465–467. [Google Scholar] [CrossRef]
- De Morais, S.M.; Facundo, V.A.; Bertini, L.M.; Cavalcanti, E.S.; dos Anjos Júnior, J.F.; Ferreira, S.A.; de Brito, E.S.; de Souza Neto, M.A. Chemical composition and larvicidal activity of essential oils from Piper species. Biochem. Syst. Ecol. 2007, 35, 670–675. [Google Scholar] [CrossRef]
- Péres, V.F.; Moura, D.J.; Sperotto, A.R.M.; Damasceno, F.C.; Caramão, E.B.; Zini, C.A.; Saffi, J. Chemical composition and cytotoxic, mutagenic and genotoxic activities of the essential oil from Piper gaudichaudianum Kunth leaves. Food Chem. Toxicol. 2009, 47, 2389–2395. [Google Scholar] [CrossRef] [PubMed]
- Sperotto, A.R.M.; Moura, D.J.; Péres, V.F.; Damasceno, F.C.; Caramão, E.B.; Henriques, J.A.P.; Saffi, J. Cytotoxic mechanism of Piper gaudichaudianum Kunth essential oil and its major compound nerolidol. Food Chem. Toxicol. 2013, 57, 57–68. [Google Scholar] [CrossRef] [PubMed]
- Sauter, I.P.; Rossa, G.E.; Lucas, A.M.; Cibulski, S.P.; Roehe, P.M.; da Silva, L.A.A.; Rott, M.B.; Mário, R.; Vargas, R.M.F.; Cassel, E.; et al. Chemical composition and amoebicidal activity of Piper hispidinervum (Piperaceae) essential oil. Ind. Crop. Prod. 2012, 40, 292–295. [Google Scholar] [CrossRef]
- Cruz, S.M.; Cáceres, A.; Álvarez, L.; Morales, J.; Apel, M.A.; Henriques, A.T.; Salamanca, E.; Giménez, A.; Vásquez, Y.; Gupta, M.P. Piper jacquemontianum and Piper variabile from Guatemala and bioactivity of the dichloromethane and methanol extracts. Rev. Bras. Farmacogn. 2011, 21, 587–593. [Google Scholar] [CrossRef]
- Do Nascimento, J.C.; David, J.M.; Barbosa, L.C.A.; de Paula, V.F.; Demuner, A.J.; David, J.P.; Conserva, L.M.; Ferreira, J.C.; Guimarães, E.F. Larvicidal activities and chemical composition of essential oils from Piper klotzschianum (Kunth) C. DC. (Piperaceae). Pest Manag. Sci. 2013, 69, 1267–1271. [Google Scholar] [PubMed]
- Da Silva, J.K.R.; Andrade, E.H.A.; Kato, M.J.; Carreira, L.M.M.; Guimarães, E.F.; Maia, J.G.S. Antioxidant capacity and larvicidal and antifungal activities of essential oils and extracts from Piper krukoffii. Nat. Prod. Commun. 2011, 6, 1361–1366. [Google Scholar] [PubMed]
- Mundina, M.; Vila, R.; Tomi, F.; Tomàs, X.; Cicció, J.F.; Adzet, T.; Casanova, J.; Cañigueral, S. Composition and chemical polymorphism of the essential oils from Piper lanceaefolium. Biochem. Syst. Ecol. 2001, 29, 739–748. [Google Scholar] [CrossRef]
- Andrade, E.H.A.; Ribeiro, A.F.; Guimarães, E.F.; Maia, J.G.S. Essential oil composition of Piper manausense Yuncker. J. Essent. Oil Bear. Plants 2005, 8, 295–299. [Google Scholar] [CrossRef]
- Ramos, L.S.; da Silva, M.L.; Luz, A.I.R.; Zoghbi, M.G.B.; Maia, J.G.S. Essential oil of Piper marginatum. J. Nat. Prod. 1986, 49, 712–713. [Google Scholar] [CrossRef]
- Autran, E.S.; Neves, I.A.; Silva, C.S.B.; Santos, G.K.N.; Câmara, C.A.G.; Navarro, D.M.A.F. Chemical composition, oviposition deterrent and larvicidal activities against Aedes aegypti of essential oils from Piper marginatum Jacq. (Piperaceae). Bioresour. Technol. 2009, 100, 2284–2288. [Google Scholar] [CrossRef] [PubMed]
- De Abreu, A.M.; Sevegnani, L.; Machicado, A.R.; Zimermann, D.; Rebelo, R.A. Piper mikanianum (Kunth) Steudel from Santa Catarina, Brazil—A new source of safrole. J. Essent. Oil Res. 2002, 14, 361–363. [Google Scholar] [CrossRef]
- Leal, L.F.; Miguel, O.G.; Silva, R.Z.; Yunes, R.A.; Santos, A.S.; Cechinel-Filho, V. Chemical composition of Piper mikanianum essential oil. J. Essent. Oil Res. 2005, 17, 316–317. [Google Scholar] [CrossRef]
- Torquilho, H.S.; Pinto, A.C.; Godoy, R.L.O.; Guimarães, E.F. Essential oil of Piper permucronatum Yuncker (Piperaceae) from Rio de Janeiro, Brazil. J. Essent. Oil Res. 1999, 11, 429–430. [Google Scholar] [CrossRef]
- Soleane, H.; De Azevedo, M.S.; Facundo, V.A.; Rover, M.; Santos, O.D.; Slana, G.B.; Barreto, A.S. Essential oil of Piper renitens (Miq.) Yunck leaves and stems (Piperaceae) from Brazilian Amazonian forest. J. Essent. Oil Res. 2007, 19, 557–558. [Google Scholar] [CrossRef]
- Moreira, D.L.; Souza, P.O.; Kaplan, M.A.C.; Pereira, N.A.; Cardoso, G.L.; Guimarães, E.F. Effect of leaf essential oil from Piper solmsianum C. DC. in mice behaviour. Anais da Academia Brasileira de Ciências 2001, 73, 33–57. [Google Scholar] [CrossRef] [PubMed]
- Facundo, V.A.; de Morais, S.M. Essential oil of Piper tuberculatum var. tuberculatum (Micq.) CDC leaves. J. Essent. Oil Res. 2005, 17, 304–305. [Google Scholar] [CrossRef]
- Mesquita, J.M.O.; Oliveira, A.B.; Braga, F.C.; Lombardi, J.A.; da Cunha, A.P.; Salgueiro, L.; Cavaleiro, C. Essential oil constituents of Piper vicosanum Yunker from the Brazilian Atlantic forest. J. Essent. Oil Res. 2006, 18, 392–395. [Google Scholar] [CrossRef]
- Dognini, J.; Meneghetti, E.K.; Teske, M.N.; Begnini, I.M.; Rebelo, R.A.; Dalmarco, E.M.; Verdi, M.; de Gasper, A.L. Antibacterial activity of high safrole contain essential oils from Piper xylosteoides (Kunth) Steudel. J. Essent. Oil Res. 2012, 24, 241–244. [Google Scholar] [CrossRef]
| Activity | Classes (%) | ||||
|---|---|---|---|---|---|
| MH | OM | SH | OS | PP | |
| Antimicrobial | 0–70.2 | 0–51.4 | 0–99.8 | 0–86.2 | 0–98.0 |
| Cytotoxic | 0–83.7 | 0–23.2 | 0–94.9 | 0–29.5 | 0–6.7 |
| Antiprotozoal | 0–29.9 | 0–50.3 | 3.3–76.0 | 0–86.2 | 0–88.5 |
| Insecticidal | 0–44.3 | 0–12.6 | 0–66.0 | 0–45.4 | 0–98.8 |
| Enzymatic | 6.9–18.5 | 1–4.2 | 4.9–52.2 | 2.1–17.5 | 0–80.8 |
| Anti-inflammatory | 16.4–25.8 | 0–16.4 | 16.2–62.6 | 20.8–28.3 | 0–0.2 |
| Antinociceptive | 16.6–65.9 | 0.8–16.4 | 16.2–33.2 | 4.8–28.3 | 0–0.6 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Da Silva, J.K.; Da Trindade, R.; Alves, N.S.; Figueiredo, P.L.; Maia, J.G.S.; Setzer, W.N. Essential Oils from Neotropical Piper Species and Their Biological Activities. Int. J. Mol. Sci. 2017, 18, 2571. https://doi.org/10.3390/ijms18122571
Da Silva JK, Da Trindade R, Alves NS, Figueiredo PL, Maia JGS, Setzer WN. Essential Oils from Neotropical Piper Species and Their Biological Activities. International Journal of Molecular Sciences. 2017; 18(12):2571. https://doi.org/10.3390/ijms18122571
Chicago/Turabian StyleDa Silva, Joyce Kelly, Rafaela Da Trindade, Nayara Sabrina Alves, Pablo Luís Figueiredo, José Guilherme S. Maia, and William N. Setzer. 2017. "Essential Oils from Neotropical Piper Species and Their Biological Activities" International Journal of Molecular Sciences 18, no. 12: 2571. https://doi.org/10.3390/ijms18122571
APA StyleDa Silva, J. K., Da Trindade, R., Alves, N. S., Figueiredo, P. L., Maia, J. G. S., & Setzer, W. N. (2017). Essential Oils from Neotropical Piper Species and Their Biological Activities. International Journal of Molecular Sciences, 18(12), 2571. https://doi.org/10.3390/ijms18122571