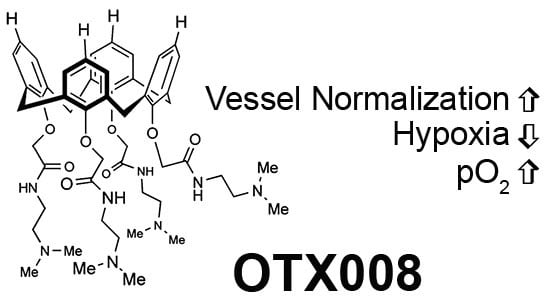
Galectin-1 Inhibitor OTX008 Induces Tumor Vessel Normalization and Tumor Growth Inhibition in Human Head and Neck Squamous Cell Carcinoma Models
Abstract
:1. Introduction
2. Results and Discussion
2.1. OTX008 Increases Overall Tumor Oxygenation
2.2. OTX008 Inhibits Tumor Growth without Apparent Toxicity
2.3. OTX008 Reduces Tumor Hypoxia and Improves Pericyte Coverage
3. Materials and Methods
3.1. Test Article and Control Reagents
3.2. Tumor Models
3.3. Tumor Growth
3.4. Tumor Oxygenation Studies
3.5. Tumor Vessel Density and Pericyte Staining
3.6. Tumor Hypoxia
3.7. Body Weight
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Ferrara, N.; Davis-Smyth, T. The biology of vascular endothelial growth factor. Endocr. Rev. 1997, 18, 4–25. [Google Scholar]
- Horsman, M.R.; Siemann, D.W. Pathophysiologic effects of vascular-targeting agents and the implications for combination with conventional therapies. Cancer Res. 2006, 66, 11520–11539. [Google Scholar] [CrossRef]
- Potente, M.; Gerhardt, H.; Carmeliet, P. Basic and therapeutic aspects of angiogenesis. Cell 2011, 146, 873–887. [Google Scholar] [PubMed]
- Lordick, F.; Geinitz, H.; Theisen, J.; Sendler, A.; Sarbia, M. Increased risk of ischemic bowel complications during treatment with bevacizumab after pelvic irradiation: Report of three cases. Int. J. Radiat. Oncol. Biol. Phys. 2006, 64, 1295–1298. [Google Scholar] [CrossRef]
- Thijssen, V.L.; Postel, R.; Brandwijk, R.J.; Dings, R.P.; Nesmelova, I.; Satijn, S.; Verhofstad, N.; Nakabeppu, Y.; Baum, L.G.; Bakkers, J.; et al. Galectin-1 is essential in tumor angiogenesis and is a target for antiangiogenesis therapy. Proc. Natl. Acad. Sci. USA 2006, 103, 15975–15980. [Google Scholar] [CrossRef] [PubMed]
- Thijssen, V.L.; Barkan, B.; Shoji, H.; Aries, I.M.; Mathieu, V.; Deltour, L.; Hackeng, T.M.; Kiss, R.; Kloog, Y.; Poirier, F.; et al. Tumor cells secrete galectin-1 to enhance endothelial cell activity. Cancer Res. 2010, 70, 6216–6224. [Google Scholar] [PubMed]
- Van den Brule, F.; Califice, S.; Castronovo, V. Expression of galectins in cancer: A critical review. Glycoconj. J. 2004, 19, 537–542. [Google Scholar]
- Camby, I.; Le Mercier, M.; Lefranc, F.; Kiss, R. Galectin-1: A small protein with major functions. Glycobiology 2006, 16, 137R–157R. [Google Scholar] [PubMed]
- Liu, F.T.; Rabinovich, G.A. Galectins as modulators of tumour progression. Nat. Rev. Cancer 2005, 5, 29–41. [Google Scholar] [CrossRef] [PubMed]
- Lefranc, F.; Mathieu, V.; Kiss, R. Galectin-1-mediated biochemical controls of melanoma and glioma aggressive behavior. World J. Biol. Chem. 2011, 2, 193–201. [Google Scholar] [PubMed]
- Dings, R.P.; Loren, M.; Heun, H.; McNiel, E.; Griffioen, A.W.; Mayo, K.H.; Griffin, R.J. Scheduling of radiation with angiogenesis inhibitors anginex and avastin improves therapeutic outcome via vessel normalization. Clin. Cancer Res. 2007, 13, 3395–3402. [Google Scholar] [CrossRef] [PubMed]
- Sirois, S.; Giguere, D.; Roy, R. A first QSAR model for galectin-3 glycomimetic inhibitors based on 3D docked structures. Med. Chem. 2006, 2, 481–489. [Google Scholar] [CrossRef] [PubMed]
- St-Pierre, C.; Ouellet, M.; Giguere, D.; Ohtake, R.; Roy, R.; Sato, S.; Tremblay, M.J. Galectin-1-specific inhibitors as a new class of compounds to treat hiv-1 infection. Antimicrob. Agents Chemother. 2012, 56, 154–162. [Google Scholar] [CrossRef] [PubMed]
- Salameh, B.A.; Leffler, H.; Nilsson, U.J. 3-(1,2,3-triazol-1-yl)-1-thio-galactosides as small, efficient, and hydrolytically stable inhibitors of galectin-3. Bioorg. Med. Chem. Lett. 2005, 15, 3344–3346. [Google Scholar] [CrossRef] [PubMed]
- Tejler, J.; Leffler, H.; Nilsson, U.J. Synthesis of O-galactosyl aldoximes as potent LacNAc-mimetic galectin-3 inhibitors. Bioorg. Med. Chem. Lett. 2005, 15, 2343–2345. [Google Scholar] [CrossRef] [PubMed]
- Cumpstey, I.; Sundin, A.; Leffler, H.; Nilsson, U.J. C2-symmetrical thiodigalactoside bis-benzamido derivatives as high-affinity inhibitors of galectin-3: Efficient lectin inhibition through double arginine-arene interactions. Angew. Chem. Int. Ed. Engl. 2005, 44, 5110–5112. [Google Scholar] [CrossRef] [PubMed]
- Salameh, B.A.; Sundin, A.; Leffler, H.; Nilsson, U.J. Thioureido N-acetyllactosamine derivatives as potent galectin-7 and 9N inhibitors. Bioorg. Med. Chem. 2006, 14, 1215–1220. [Google Scholar] [CrossRef] [PubMed]
- Collins, P.M.; Oberg, C.T.; Leffler, H.; Nilsson, U.J.; Blanchard, H. Taloside inhibitors of galectin-1 and galectin-3. Chem. Biol. Drug Des. 2012, 79, 339–346. [Google Scholar] [PubMed]
- Rabinovich, G.A.; Cumashi, A.; Bianco, G.A.; Ciavardelli, D.; Iurisci, I.; D’Egidio, M.; Piccolo, E.; Tinari, N.; Nifantiev, N.; Iacobelli, S. Synthetic lactulose amines: Novel class of anticancer agents that induce tumor-cell apoptosis and inhibit galectin-mediated homotypic cell aggregation and endothelial cell morphogenesis. Glycobiology 2006, 16, 210–220. [Google Scholar] [CrossRef] [PubMed]
- Dings, R.P.; van der Schaft, D.W.; Hargittai, B.; Haseman, J.; Griffioen, A.W.; Mayo, K.H. Anti-tumor activity of the novel angiogenesis inhibitor anginex. Cancer Lett. 2003, 194, 55–66. [Google Scholar] [CrossRef]
- Dings, R.P.; Arroyo, M.M.; Lockwood, N.A.; Van Eijk, L.I.; Haseman, J.R.; Griffioen, A.W.; Mayo, K.H. Beta-sheet is the bioactive conformation of the anti-angiogenic anginex peptide. Biochem. J. 2003, 23, 281–288. [Google Scholar] [CrossRef] [PubMed]
- Dings, R.P.; Chen, X.; Hellebrekers, D.M.; van Eijk, L.I.; Zhang, Y.; Hoye, T.R.; Griffioen, A.W.; Mayo, K.H. Design of nonpeptidic topomimetics of antiangiogenic proteins with antitumor activities. J. Natl. Cancer Inst. 2006, 98, 932–936. [Google Scholar] [CrossRef] [PubMed]
- Dings, R.P.; Miller, M.C.; Nesmelova, I.; Astorgues-Xerri, L.; Kumar, N.; Serova, M.; Chen, X.; Raymond, E.; Hoye, T.R.; Mayo, K.H. Antitumor agent calixarene 0118 targets human galectin-1 as an allosteric inhibitor of carbohydrate binding. J. Med. Chem. 2012, 55, 5121–5129. [Google Scholar] [PubMed]
- Valach, J.; Fik, Z.; Strnad, H.; Chovanec, M.; Plzak, J.; Cada, Z.; Szabo, P.; Sachova, J.; Hroudova, M.; Urbanova, M.; et al. Smooth muscle actin-expressing stromal fibroblasts in head and neck squamous cell carcinoma: Increased expression of galectin-1 and induction of poor prognosis factors. Int. J. Cancer 2012, 131, 2499–2508. [Google Scholar] [PubMed]
- Noda, Y.; Kondo, Y.; Sakai, M.; Sato, S.; Kishino, M. Galectin-1 is a useful marker for detecting neoplastic squamous cells in oral cytology smears. Hum. Pathol. 2016, 52, 101–109. [Google Scholar] [CrossRef] [PubMed]
- Astorgues-Xerri, L.; Serova, M.; Bieche, I.; Vivier, S.; Cvitkovic, E.; Noel, K.; Raymond, E.; Faivre, S. Effects of PTX-008, a non-peptidic topomimetic targeting galectin-1 in human epithelial cancer cells. Mol. Cancer Ther. 2009, 8. [Google Scholar] [CrossRef]
- Willett, C.G.; Boucher, Y.; di Tomaso, E.; Duda, D.G.; Munn, L.L.; Tong, R.T.; Chung, D.C.; Sahani, D.V.; Kalva, S.P.; Kozin, S.V.; et al. Direct evidence that the VEGF-specific antibody bevacizumab has antivascular effects in human rectal cancer. Nat. Med. 2004, 10, 145–147. [Google Scholar] [PubMed]
- Dings, R.P.; Vang, K.B.; Castermans, K.; Popescu, F.; Zhang, Y.; Oude Egbrink, M.G.; Mescher, M.F.; Farrar, M.A.; Griffioen, A.W.; Mayo, K.H. Enhancement of T-cell-mediated antitumor response: Angiostatic adjuvant to immunotherapy against cancer. Clin. Cancer Res. 2011, 17, 3134–3145. [Google Scholar] [PubMed]
- Dings, R.P.; Mayo, K.H. A journey in structure-based drug discovery: From designed peptides to protein surface topomimetics as antibiotic and antiangiogenic agents. Acc. Chem. Res. 2007, 40, 1057–1065. [Google Scholar] [CrossRef] [PubMed]
- Hudis, C.A. Clinical implications of antiangiogenic therapies. Oncology 2005, 19, 26–31. [Google Scholar] [PubMed]
- Bordeaux, J.; Welsh, A.; Agarwal, S.; Killiam, E.; Baquero, M.; Hanna, J.; Anagnostou, V.; Rimm, D. Antibody validation. BioTechniques 2010, 48, 197–209. [Google Scholar] [CrossRef] [PubMed]
- Imai, K.; Takaoka, A. Comparing antibody and small-molecule therapies for cancer. Nat. Rev. Cancer 2006, 6, 714–727. [Google Scholar] [PubMed]
- Zhang, P.F.; Li, K.S.; Shen, Y.H.; Gao, P.T.; Dong, Z.R.; Cai, J.B.; Zhang, C.; Huang, X.Y.; Tian, M.X.; Hu, Z.Q.; et al. Galectin-1 induces hepatocellular carcinoma emt and sorafenib resistance by activating FAK/PI3K/AKT signaling. Cell Death Dis. 2016, 7, e2201. [Google Scholar] [CrossRef] [PubMed]
- Shih, T.-C.; Liu, R.; Fung, G.; Bhardwaj, G.; Ghosh, P.M.; Lam, K.S. A novel galectin-1 inhibitor discovered through one-bead two-compound library potentiates the antitumor effects of paclitaxel in vivo. Mol. Cancer Ther. 2017, 16, 1212–1223. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Bosch, N.; Fernández-Barrena, M.G.; Moreno, M.; Ortiz-Zapater, E.; Munné-Collado, J.; Iglesias, M.; André, S.; Gabius, H.-J.; Hwang, R.F.; Poirier, F.; et al. Galectin-1 drives pancreatic carcinogenesis through stroma remodeling and hedgehog signaling activation. Cancer Res. 2014, 74, 3512–3524. [Google Scholar] [PubMed]
- Dings, R.P.; Yokoyama, Y.; Ramakrishnan, S.; Griffioen, A.W.; Mayo, K.H. The designed angiostatic peptide anginex synergistically improves chemotherapy and antiangiogenesis therapy with angiostatin. Cancer Res. 2003, 63, 382–385. [Google Scholar] [PubMed]
- Astorgues-Xerri, L.; De Groot, D.; Serova, M.; Huet, E.; Menashi, S.; Riveiro, M.E.; Cvitkovic, E.; Noel, K.; Raymond, E.; Faivre, S. Targeting galectin-1 with PTX-008 inhibits invasion, tumor growth and metastasis in human carcinoma models. Cancer Res. 2011, 71. [Google Scholar] [CrossRef]
- Astorgues-Xerri, L.; Tijeras-Raballand, A.; Riveiro, M.E.; Serova, M.; Martinet, M.; Huet, E.; Menashi, S.; Herait, P.; Cvitkovic, E.; Noel, K.; et al. Antitumor and antiangiogenic effects of OTX-008, a novel calyx[4]arene, are mediated by galectin-1 inhibition in human tumor xenograft models. Cancer Res. 2012, 72. [Google Scholar] [CrossRef]
- Dings, R.P.; Levine, J.I.; Brown, S.G.; Astorgues-Xerri, L.; MacDonald, J.R.; Hoye, T.R.; Raymond, E.; Mayo, K.H. Polycationic calixarene PTX013, a potent cytotoxic agent against tumors and drug resistant cancer. Investig. New Drugs 2013, 31, 1142–1150. [Google Scholar] [CrossRef] [PubMed]
- Astorgues-Xerri, L.; Riveiro, M.E.; Tijeras-Raballand, A.; Serova, M.; Rabinovich, G.A.; Bieche, I.; Vidaud, M.; de Gramont, A.; Martinet, M.; Cvitkovic, E.; et al. OTX008, a selective small-molecule inhibitor of galectin-1, downregulates cancer cell proliferation, invasion and tumour angiogenesis. Eur. J. Cancer 2014, 50, 2463–2477. [Google Scholar] [PubMed]
- Pagan, J.; Przybyla, B.; Jamshidi-Parsian, A.; Gupta, K.; Griffin, R.J. Blood outgrowth endothelial cells increase tumor growth rates and modify tumor physiology: Relevance for therapeutic targeting. Cancers 2013, 5, 205–217. [Google Scholar] [CrossRef] [PubMed]
- Koonce, N.A.; Levy, J.; Hardee, M.E.; Jamshidi-Parsian, A.; Vang, K.B.; Sharma, S.; Raleigh, J.A.; Dings, R.P.; Griffin, R.J. Targeting artificial tumor stromal targets for molecular imaging of tumor vascular hypoxia. PLoS ONE 2015, 10, e0135607. [Google Scholar]





© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Koonce, N.A.; Griffin, R.J.; Dings, R.P.M. Galectin-1 Inhibitor OTX008 Induces Tumor Vessel Normalization and Tumor Growth Inhibition in Human Head and Neck Squamous Cell Carcinoma Models. Int. J. Mol. Sci. 2017, 18, 2671. https://doi.org/10.3390/ijms18122671
Koonce NA, Griffin RJ, Dings RPM. Galectin-1 Inhibitor OTX008 Induces Tumor Vessel Normalization and Tumor Growth Inhibition in Human Head and Neck Squamous Cell Carcinoma Models. International Journal of Molecular Sciences. 2017; 18(12):2671. https://doi.org/10.3390/ijms18122671
Chicago/Turabian StyleKoonce, Nathan A., Robert J. Griffin, and Ruud P. M. Dings. 2017. "Galectin-1 Inhibitor OTX008 Induces Tumor Vessel Normalization and Tumor Growth Inhibition in Human Head and Neck Squamous Cell Carcinoma Models" International Journal of Molecular Sciences 18, no. 12: 2671. https://doi.org/10.3390/ijms18122671
APA StyleKoonce, N. A., Griffin, R. J., & Dings, R. P. M. (2017). Galectin-1 Inhibitor OTX008 Induces Tumor Vessel Normalization and Tumor Growth Inhibition in Human Head and Neck Squamous Cell Carcinoma Models. International Journal of Molecular Sciences, 18(12), 2671. https://doi.org/10.3390/ijms18122671