Flavonoids of Kudzu Root Fermented by Eurtotium cristatum Protected Rat Pheochromocytoma Line 12 (PC12) Cells against H2O2-Induced Apoptosis
Abstract
:1. Introduction
2. Results and Discussion
2.1. Special Isoflavonoid Contents of NFK and FK
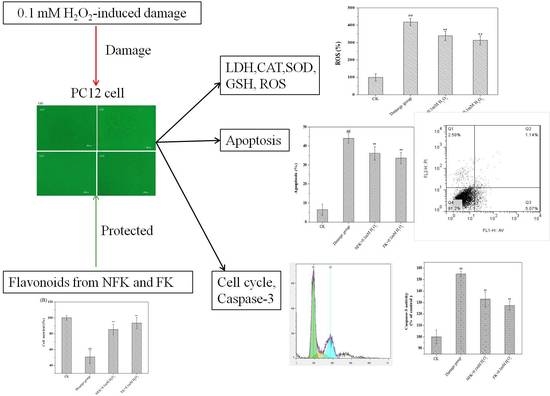
2.2. Protection of PC12 Cell from H2O2-Induced Damage as Revealed by Cell Morphology Analysis through Phase Contrast Microscopy
2.3. LDH, CAT, and SOD Activities, GSH Content, and ROS Levels of PC12 Cell
2.4. NFK and FK against Apoptosis of PC12 Cell as Revealed by Flow Cytometer Analysis
2.5. Cell Cycle Analysis via Flow Cytometry
2.6. Determination of Intracellular Caspase-3 Activity
3. Materials and Methods
3.1. Materials and Reagents
3.2. Preparation of NFK and FK Extraction
3.3. Cell Culture and Treatment
3.4. Cell Survival Assay and LDH Activity
3.5. Determination of SOD, GSH, CAT and ROS Levels
3.6. Flow Cytometry Analysis of Apoptosis
3.7. Cell Cycle Analysis
3.8. Intracellular Caspaes-3 Activity Assay
3.9. Statistical Analysis
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Li, F.; Zhang, X.; Zheng, S.; Lu, K.; Zhao, G.; Ming, J. The composition, antioxidant and antiproliferative capacities of phenolic compounds were extracted from tartary buckwheat bran [Fagopyrum tartaricum (L.) Gaerth]. J. Funct. Foods 2016, 22, 145–155. [Google Scholar] [CrossRef]
- Dey, T.B.; Chakraborty, S.; Jain, K.K.; Sharma, A.; Kuhad, R.C. Antioxidant phenolics and their microbial production by submerged and solid state fermentation process: A review. Trends Food Sci. Technol. 2016, 53, 60–74. [Google Scholar]
- Bebrevska, L.; Foubert, K.; Hermans, N.; Chatterjee, S.; van, M.E.; De, M.G.; Apers, S. In vivo antioxidative activity of a quantified Pueraria lobata root extract. J. Ethnopharmacol. 2010, 127, 112–117. [Google Scholar] [CrossRef] [PubMed]
- Fernandez-Orozco, R.; Frias, J.; Zielinski, H.; Piskula, M.K.; Kozlowska, H.; Vidal-Valverde, C. Kinetic study of the antioxidant compounds and antioxidant capacity during germination of Vigna radiata cv. emmerald, Glycine max cv. jutro and Glycine max cv. merit. Food Chem. 2008, 111, 622–630. [Google Scholar] [CrossRef]
- Siddhuraju, P.; Becker, K. The antioxidant and free radical scavenging activities of processed cowpea (Vigna unguiculata (L.) Walp.) seed extracts. Food Chem. 2007, 101, 10–19. [Google Scholar] [CrossRef]
- Garrido, J.; Gaspar, A.; Garrido, E.M.; Miri, R.; Tavakkoli, M.; Pourali, S.; Firuzi, O. Alkyl esters of hydroxycinnamic acids with improved antioxidant activity and lipophilicity protect PC12 cells against oxidative stress. Biochimie 2012, 94, 961–967. [Google Scholar] [CrossRef] [PubMed]
- Olatunji, O.J.; Chen, H.; Zhou, Y. Lycium chinensis Mill attenuates glutamate induced oxidative toxicity in PC12 cells by increasing antioxidant defense enzymes and down regulating ROS and Ca2+ generation. Neurosci. Lett. 2016, 616, 111–118. [Google Scholar] [CrossRef] [PubMed]
- Ji, B.S.; Gao, Y. Protective effect of trihexyphenidyl on hydrogen peroxide-induced oxidative damage in PC12 cells. Neurosci. Lett. 2008, 437, 50–54. [Google Scholar] [CrossRef] [PubMed]
- Ma, W.; Yuan, L.; Yu, H.; Ding, B.; Xi, Y.; Feng, J.; Xiao, R. Genistein as a neuroprotective antioxidant attenuates redox imbalance induced by beta-amyloid peptides 25–35 in PC12 cells. Int. J. Dev. Neurosci. 2010, 28, 289–295. [Google Scholar] [CrossRef] [PubMed]
- Fan, L.; Dang, X.; Shi, Z.; Zhang, C.; Wang, K. Hydroxysafflor yellow A protects PC12 cells against the apoptosis induced by oxygen and glucose deprivation. Cell. Mol. Neurobiol. 2011, 31, 1187–1194. [Google Scholar] [CrossRef] [PubMed]
- Xing, G.; Dong, M.; Li, X.; Zou, Y.; Fan, L.; Wang, X.; Niu, Y. Neuroprotective effects of puerarin against beta-amyloid-induced neurotoxicity in PC12 cells via a PI3K-dependent signaling pathway. Brain Res. Bull. 2011, 85, 212–218. [Google Scholar] [CrossRef] [PubMed]
- Han, X.; Zhu, S.; Wang, B.; Chen, L.; Li, R.; Yao, W.; Qu, Z. Antioxidant action of 7,8-dihydroxyflavone protects PC12 cells against 6-hydroxydopamine-induced cytotoxicity. Neurochem. Int. 2014, 64, 18–23. [Google Scholar] [CrossRef] [PubMed]
- Hsieh, H.M.; Wu, W.M.; Hu, M.L. Genistein attenuates D-galactose-induced oxidative damage through decreased reactive oxygen species and NF-κB binding activity in neuronal PC12 cells. Life Sci. 2011, 88, 82–88. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.H.; Su, M.Y.; Huang, H.Y.; Lin, L.; Yuan, C.G. Protective effects of the citrus flavanones to PC12 cells against cytotoxicity induced by hydrogen peroxide. Neurosci. Lett. 2010, 484, 6–11. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.C.; Zhu, Y.G.; Zhu, L.A.; Huang, C.; Chen, Y.; Chen, L.M.; Zhao, C.H. Ginsenoside Rg1 attenuates dopamine-induced apoptosis in PC12 cells by suppressing oxidative stress. Eur. J. Pharmacol. 2003, 473, 1–7. [Google Scholar] [CrossRef]
- Cheng, B.; Lu, H.; Bai, B.; Chen, J. d-β-Hydroxybutyrate inhibited the apoptosis of PC12 cells induced by H2O2 via inhibiting oxidative stress. Neurochem. Int. 2013, 62, 620–625. [Google Scholar] [CrossRef] [PubMed]
- Hwang, S.L.; Yen, G.C. Neuroprotective Effects of the Citrus Flavanones against H2O2-Induced Cytotoxicity in PC12 Cells. J. Agric. Food Chem. 2008, 56, 859–864. [Google Scholar] [CrossRef] [PubMed]
- Olatunji, O.J.; Feng, Y.; Olatunji, O.O.; Tang, J.; Ouyang, Z.; Su, Z. Cordycepin protects PC12 cells against 6-hydroxydopamine induced neurotoxicity via its antioxidant properties. Biomed. Pharmacother. 2016, 81, 7–14. [Google Scholar] [CrossRef] [PubMed]
- Lin, C.M.; Lin, R.D.; Chen, S.T.; Lin, Y.P.; Chiu, W.T.; Lin, J.W.; Lee, M.H. Neurocytoprotective effects of the bioactive constituents of Pueraria thomsonii in 6-hydroxydopamine (6-OHDA)-treated nerve growth factor (NGF)-differentiated PC12 cells. Phytochemistry 2010, 71, 2147–2156. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Xing, D.; Zhu, D.; Chen, Q. Low-power laser irradiation inhibiting Aβ25–35-induced PC12 cell apoptosis via PKC activation. Cell. Physiol. Biochem. 2008, 22, 215–222. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.B.; Liu, H.P.; Tian, R.T.; Yang, D.J.; Chen, S.L.; Xu, H.X.; Xie, P.S. High-performance thin-layer chromatographic fingerprints of isoflavonoids for distinguishing between Radix Puerariae Lobate and Radix Puerariae Thomsonii. J. Chromatogr. A 2006, 1121, 114–119. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Yang, B.; Du, X.; Yi, C. Optimisation of supercritical fluid extraction of flavonoids from Pueraria lobata. Food Chem. 2008, 108, 737–741. [Google Scholar] [CrossRef] [PubMed]
- Cao, X.; Yu, T.; Zhang, T.; Xin, L.; Ito, Y. Separation and purification of isoflavones from Pueraria lobata by high-speed counter-current chromatography. J. Chromatogr. A 1999, 855, 709–713. [Google Scholar] [CrossRef]
- Chuankhayan, P.; Rimlumduan, T.; Svasti, J.; Cairns, J.R. Hydrolysis of soybean isoflavonoid glycosides by dalbergia β-glucosidases. J. Agric. Food Chem. 2007, 55, 2407–2412. [Google Scholar] [CrossRef] [PubMed]
- Pyo, Y.H.; Lee, T.C.; Lee, Y.C. Enrichment of bioactive isoflavones in soymilk fermented with β-glucosidase-producing lactic acid bacteria. Food Res. Int. 2005, 38, 551–559. [Google Scholar] [CrossRef]
- He, Z.; Hu, M.; Zha, Y.H.; Li, Z.C.; Zhao, B.; Yu, L.L.; Qian, Y. Piracetam Ameliorated Oxygen and Glucose Deprivation-Induced Injury in Rat Cortical Neurons Via Inhibition of Oxidative Stress, Excitatory Amino Acids Release and P53/Bax. Cell. Mol. Neurobiol. 2014, 34, 539–547. [Google Scholar] [CrossRef] [PubMed]
- Li, X.L.; Zhou, H.B.; Cheng, W.D.; Meng, X.H.; Zhang, Q.J.; Wang, L.X. Effect of phytoestrogen isoflavone on MPP+-induced apoptosis in PC12 cells. Biomed. Prev. Nutr. 2011, 1, 67–69. [Google Scholar] [CrossRef]
- Crispo, J.A.; Piche, M.; Ansell, D.R.; Eibl, J.K.; Tai, I.T.; Kumar, A.; Tai, T.C. Protective effects of methyl gallate on H2O2-induced apoptosis in PC12 cells. Biochem. Biophys. Res. Commun. 2010, 393, 773–778. [Google Scholar] [CrossRef] [PubMed]
- Chang, R.; Zhou, R.; Qi, X.; Wang, J.; Wu, F.; Yang, W.; Yu, J. Protective effects of aloinon oxygen and glucose deprivation-induced injury in PC12 cells. Brain Res. Bull. 2016, 121, 75–83. [Google Scholar] [CrossRef] [PubMed]
- Xiao, X.; Liu, J.; Hu, J.; Zhu, X.; Yang, H.; Wang, C.; Zhang, Y. Protective effects of protopine on hydrogen peroxide-induced oxidative injury of PC12 cells via Ca2+ antagonism and antioxidant mechanisms. Eur. J. Pharmacol. 2008, 591, 21–27. [Google Scholar] [CrossRef] [PubMed]
- Korenić, A.; Boltze, J.; Deten, A.; Peters, M.; Andjus, P.; Radenović, L. Astrocytic mitochondrial membrane hyperpolarization following extended oxygen and glucose deprivation. PLoS ONE 2014, 9, e90697. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, W.; Wei, M.M.; Wu, J.J.; Rui, X.; Dong, M.S. Novel fermented chickpea milk with enhanced level of -aminobutyric acid and neuroprotective effect on PC12 cells. Peer J. 2016, 4, e2292. [Google Scholar] [CrossRef] [PubMed]
- Shulyakova, N.; Sidorova-Darmos, E.; Fong, J.; Zhang, G.; Mills, L.R.; Eubanks, J.H. Over-expression of the Sirt3 sirtuin Protects neuronally differentiated PC12 Cells from degeneration induced by oxidative stress and trophic withdrawal. Brain Res. 2014, 1587, 40–53. [Google Scholar] [CrossRef] [PubMed]
- Suganthy, N.; Devi, K.P. Protective effect of catechin rich extract of Rhizophora mucronata against β-amyloid-induced toxicity in PC12 cells. J. Appl. Biomed. 2016, 14, 137–146. [Google Scholar] [CrossRef]
- Benedí, J.; Arroyo, R.; Romero, C.; Martín-Aragón, S.; Villar, A.M. Antioxidant properties and protective effects of a standardized extract of hypericum perforatum on hydrogen peroxide-induced oxidative damage in PC12 cells. Life Sci. 2004, 75, 1263–1276. [Google Scholar] [CrossRef] [PubMed]
- Hong, H.; Liu, G.Q. Protection against hydrogen peroxide-induced cytotoxicity in PC12 cells by scutellarin. Life Sci. 2004, 74, 2959–2973. [Google Scholar] [CrossRef] [PubMed]
- Liu, E.; Du, X.; Ge, R.; Liang, T.; Niu, Q.; Li, Q. Comparative toxicity and apoptosis induced by diorganotins in rat pheochromocytoma (PC12) cells. Food Chem. Toxicol. 2013, 60, 302–308. [Google Scholar] [CrossRef] [PubMed]
- Pavlica, S.; Gebhardt, R. Protective effects of flavonoids and two metabolites against oxidative stress in neuronal PC12 cells. Life Sci. 2010, 86, 79–86. [Google Scholar] [CrossRef] [PubMed]
- Fujita, Y.; Izawa, Y.; Ali, N.; Kanematsu, Y.; Tsuchiya, K.; Hamano, S. Pramipexole protects against ho-induced pc12 cell death. Naunyn-Schmiedeberg's Arch. Pharmacol. 2006, 372, 257–266. [Google Scholar] [CrossRef] [PubMed]
- Fang, S.Q.; Wang, Y.T.; Wei, J.X.; Shu, Y.H.; Xiao, L.; Lu, X.M. Beneficial effects of chlorogenic acid on alcohol-induced damage in PC12 cells. Biomed. Pharmacother. 2016, 79, 254–262. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Liu, Q.; Wang, W.; Li, X.; Zhang, J. A polysaccharide isolated from Cynomorium songaricum Rupr. protects PC12 cells against H2O2-induced injury. Int. J. Biol. Macromol. 2016, 87, 222–228. [Google Scholar] [CrossRef] [PubMed]
- Eslami, H.; Sharifi, A.M.; Rahimi, H.; Rahati, M. Protective effect of telmisartan against oxidative damage induced by high glucose in neuronal PC12 cell. Neurosci. Lett. 2014, 558, 31–36. [Google Scholar] [CrossRef] [PubMed]
- Sun, L.; Chen, W.; Meng, Y.; Yang, X.; Yuan, L.; Guo, Y. Interactions between polyphenols in thinned young apples and porcine pancreatic alpha-amylase: Inhibition, detailed kinetics and fluorescence quenching. Food Chem. 2016, 208, 51–60. [Google Scholar] [CrossRef] [PubMed]
- Xiong, C.; Li, Q.; Chen, C.; Chen, Z.; Huang, W. Neuroprotective effect of crude polysaccharide isolated from the fruiting bodies of Morchella importuna against H2O2-induced PC12 cell cytotoxicity by reducing oxidative stress. Biomed. Pharmacother. 2016, 83, 569–576. [Google Scholar] [CrossRef] [PubMed]
- Hati, S.; Vij, S.; Singh, B.P.; Mandal, S. β-Glucosidase activity and bioconversion of isoflavones during fermentation of soymilk. J. Sci. Food Agric. 2015, 95, 216–220. [Google Scholar] [CrossRef] [PubMed]









| Special Compounds | NFK | FK |
|---|---|---|
| One: Puerarin | 1903.56 ± 62.88 A | 2395.26 ± 78.65 B |
| Two: Daidzin | 147.13 ± 3.39 A | 69.82 ± 4.22 B |
| Three: Glycitin | 129.04 ± 1.62 A | 91.45 ± 9.40 B |
| Four: Genistin | 16.02 ± 0.35 A | 11.42 ± 0.60 B |
| Five: Shikimic acid | 141.18 ± 3.82 A | 149.55 ± 10.21 B |
| Six: Daidzein | 145.62 ± 23.91 A | 188.77 ± 12.47 B |
| Seven: Glycitein | 43.87 ± 1.79 A | 46.40 ± 2.80 B |
| Eight: Genistein | 2.16 ± 0.25 A | 2.78 ± 0.20 B |
| LDH, CAT, SOD Activities, GSH Content | Control Group | Damage Group | Sample Group | |
|---|---|---|---|---|
| NFK | FK | |||
| LDH (U/L) | 292.05 ± 53.42 | 566.45 ± 79.43 ## | 468.45 ± 19.20 a ** | 443.80 ± 33.26 b ** |
| CAT (U/mg pro.) | 9.25 ± 1.48 | 1.61 ± 0.30 ## | 2.67 ± 0.27 a ** | 3.63 ± 0.61 b ** |
| SOD (U/mg pro.) | 116.26 ± 5.82 | 21.07 ± 2.81 ## | 27.88 ± 1.716 a ** | 29.98 ± 4.87 a ** |
| GSH (mmol/mg pro.) | 29.13 ± 5.31 | 6.17 ± 0.39 ## | 14.28 ± 0.54 a ** | 18.99 ± 0.75 b ** |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, B.; Li, W.; Dong, M. Flavonoids of Kudzu Root Fermented by Eurtotium cristatum Protected Rat Pheochromocytoma Line 12 (PC12) Cells against H2O2-Induced Apoptosis. Int. J. Mol. Sci. 2017, 18, 2754. https://doi.org/10.3390/ijms18122754
Zhang B, Li W, Dong M. Flavonoids of Kudzu Root Fermented by Eurtotium cristatum Protected Rat Pheochromocytoma Line 12 (PC12) Cells against H2O2-Induced Apoptosis. International Journal of Molecular Sciences. 2017; 18(12):2754. https://doi.org/10.3390/ijms18122754
Chicago/Turabian StyleZhang, Bo, Wen Li, and Mingsheng Dong. 2017. "Flavonoids of Kudzu Root Fermented by Eurtotium cristatum Protected Rat Pheochromocytoma Line 12 (PC12) Cells against H2O2-Induced Apoptosis" International Journal of Molecular Sciences 18, no. 12: 2754. https://doi.org/10.3390/ijms18122754
APA StyleZhang, B., Li, W., & Dong, M. (2017). Flavonoids of Kudzu Root Fermented by Eurtotium cristatum Protected Rat Pheochromocytoma Line 12 (PC12) Cells against H2O2-Induced Apoptosis. International Journal of Molecular Sciences, 18(12), 2754. https://doi.org/10.3390/ijms18122754