Hormetic Response to Low-Dose Radiation: Focus on the Immune System and Its Clinical Implications
Abstract
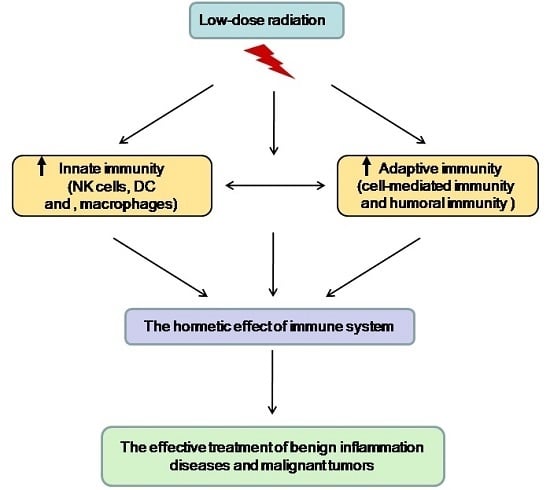
:1. Introduction
2. The Hormetic Effect of LDR on the Immune System
2.1. The Hormetic Effect of LDR on Innate Immunity
2.1.1. The Effect of LDR on NK Cells
2.1.2. The Effect of LDR on Macrophages
2.1.3. The Effect of LDR on Dendritic Cells
2.2. The Hormetic Effect of LDR on Adaptive Immunity
2.2.1. The Effect of LDR on T Cells
2.2.2. The Effect of LDR on B Cells
3. Clinical Implications of an LDR Effect on the Immune System
3.1. The Application of LDR on Autoimmune Diseases
3.2. The Application of LDR on Malignant Tumors
4. Conclusions and Perspectives
Acknowledgments
Conflicts of Interest
References
- Luckey, T.D. Physiological benefits from low levels of ionizing radiation. Health Phys. 1982, 43, 771–789. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.W.; Jiang, H.; Liang, X.; Zhao, Y.; Yu, D.; Zhou, L.; Wang, G.; Tian, H.; Han, F.; Cai, L.; et al. Low-dose radiation may be a novel approach to enhance the effectiveness of cancer therapeutics. Int. J. Cancer 2016, 139, 2157–2168. [Google Scholar] [CrossRef] [PubMed]
- Macklis, R.M. Radithor and the era of mild radium therapy. JAMA 1990, 264, 614–618. [Google Scholar] [CrossRef] [PubMed]
- Anderson, R.E.; Lefkovits, I. In vitro evaluation of radiation-induced augmentation of the immune response. Am. J. Pathol. 1979, 97, 456–472. [Google Scholar] [PubMed]
- Kojima, S.; Matsumori, S.; Ishida, H.; Yamaoka, K. Possible role of elevation of glutathione in the acquisition of enhanced proliferation of mouse splenocytes exposed to small-dose gamma-rays. Int. J. Radiat. Biol. 2000, 76, 1641–1647. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.Z.; Jin, S.Z.; Liu, X.D.; Sun, Y.M. Role of CD28/B7 costimulation and IL-12/IL-10 interaction in the radiation-induced immune changes. BMC Immunol. 2001, 2, 8. [Google Scholar] [CrossRef]
- Berk, L.B.; Hodes, P.J. Roentgen therapy for infections: An historical review. Yale J. Biol. Med. 1991, 64, 155–165. [Google Scholar] [PubMed]
- Scott, S.G. Method of Treating Asthma by Radiation. Br. Med. J. 1926, 1, 939–941. [Google Scholar] [CrossRef] [PubMed]
- Nowosielska, E.M.; Wrembel-Wargocka, J.; Cheda, A.; Lisiak, E.; Janiak, M.K. Enhanced cytotoxic activity of macrophages and suppressed tumor metastases in mice irradiated with low doses of X-rays. J. Radiat. Res. 2006, 47, 229–236. [Google Scholar] [CrossRef] [PubMed]
- Seong, K.M.; Kim, C.S.; Lee, B.S.; Nam, S.Y.; Yang, K.H.; Kim, J.Y.; Park, J.J.; Min, K.J.; Jin, Y.W. Low-dose radiation induces Drosophila innate immunity through Toll pathway activation. J. Radiat. Res. 2012, 53, 242–249. [Google Scholar] [CrossRef] [PubMed]
- Nakatsukasa, H.; Tsukimoto, M.; Tokunaga, A.; Kojima, S. Repeated gamma irradiation attenuates collagen-induced arthritis via up-regulation of regulatory T cells but not by damaging lymphocytes directly. Radiat. Res. 2010, 174, 313–324. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, T.; Tago, F.; Fang, S.P.; Shimura, N.; Kojima, S. Repeated 0.5-Gy gamma-ray irradiation attenuates autoimmune manifestations in MRL-lpr/lpr mice. Int. J. Radiat. Biol. 2005, 81, 731–740. [Google Scholar] [CrossRef] [PubMed]
- Tsukimoto, M.; Nakatsukasa, H.; Sugawara, K.; Yamashita, K.; Kojima, S. Repeated 0.5-Gy gamma irradiation attenuates experimental autoimmune encephalomyelitis with up-regulation of regulatory T cells and suppression of IL17 production. Radiat. Res. 2008, 170, 429–436. [Google Scholar] [CrossRef] [PubMed]
- Cheda, A.; Nowosielska, E.M.; Wrembel-Wargocka, J.; Janiak, M.K. Production of cytokines by peritoneal macrophages and splenocytes after exposures of mice to low doses of X-rays. Radiat. Environ. Biophys. 2008, 47, 275–283. [Google Scholar] [CrossRef] [PubMed]
- Ina, Y.; Sakai, K. Activation of immunological network by chronic low-dose-rate irradiation in wild-type mouse strains: Analysis of immune cell populations and surface molecules. Int. J. Radiat. Biol. 2005, 81, 721–729. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.D.; Ma, S.M.; Liu, S.Z. Effects of 0.075 Gy X-ray irradiation on the expression of IL-10 and IL-12 in mice. Phys. Med. Biol. 2003, 48, 2041–2049. [Google Scholar] [CrossRef] [PubMed]
- Pandey, R.; Shankar, B.S.; Sharma, D.; Sainis, K.B. Low dose radiation induced immunomodulation: Effect on macrophages and CD8+ T cells. Int. J. Radiat. Biol. 2005, 81, 801–812. [Google Scholar] [CrossRef] [PubMed]
- Lodoen, M.B.; Lanier, L.L. Natural killer cells as an initial defense against pathogens. Curr. Opin. Immunol. 2006, 18, 391–398. [Google Scholar] [CrossRef] [PubMed]
- Cheda, A.; Wrembel-Wargocka, J.; Lisiak, E.; Nowosielska, E.M.; Marciniak, M.; Janiak, M.K. Single low doses of X rays inhibit the development of experimental tumor metastases and trigger the activities of NK cells in mice. Radiat. Res. 2004, 161, 335–340. [Google Scholar] [CrossRef] [PubMed]
- Hashimoto, S.; Shirato, H.; Hosokawa, M.; Nishioka, T.; Kuramitsu, Y.; Matushita, K.; Kobayashi, M.; Miyasaka, K. The suppression of metastases and the change in host immune response after low-dose total-body irradiation in tumor-bearing rats. Radiat. Res. 1999, 151, 717–724. [Google Scholar] [CrossRef] [PubMed]
- Yang, G.; Kong, Q.; Wang, G.; Jin, H.; Zhou, L.; Yu, D.; Niu, C.; Han, W.; Li, W.; Cui, J. Low-dose ionizing radiation induces direct activation of natural killer cells and provides a novel approach for adoptive cellular immunotherapy. Cancer Biother. Radiopharm. 2014, 29, 428–434. [Google Scholar] [CrossRef] [PubMed]
- Sonn, C.H.; Choi, J.R.; Kim, T.J.; Yu, Y.B.; Kim, K.; Shin, S.C.; Park, G.H.; Shirakawa, T.; Kim, H.S.; Lee, K.M. Augmentation of natural cytotoxicity by chronic low-dose ionizing radiation in murine natural killer cells primed by IL-2. J. Radiat. Res. 2012, 53, 823–829. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.Z.; Jin, S.Z.; Liu, X.D. Radiation-induced bystander effect in immune response. Biomed. Environ. Sci. 2004, 17, 40–46. [Google Scholar] [PubMed]
- Bogdandi, E.N.; Balogh, A.; Felgyinszki, N.; Szatmari, T.; Persa, E.; Hildebrandt, G.; Safrany, G.; Lumniczky, K. Effects of low-dose radiation on the immune system of mice after total-body irradiation. Radiat. Res. 2010, 174, 480–489. [Google Scholar] [CrossRef] [PubMed]
- Kojima, S.; Nakayama, K.; Ishida, H. Low dose gamma-rays activate immune functions via induction of glutathione and delay tumor growth. J. Radiat. Res. 2004, 45, 33–39. [Google Scholar] [CrossRef] [PubMed]
- Gordon, S.; Pluddemann, A. Tissue macrophage heterogeneity: Issues and prospects. Semin. Immunopathol. 2013, 35, 533–540. [Google Scholar] [CrossRef] [PubMed]
- Gordon, S.; Pluddemann, A.; Martinez Estrada, F. Macrophage heterogeneity in tissues: Phenotypic diversity and functions. Immunol. Rev. 2014, 262, 36–55. [Google Scholar] [CrossRef] [PubMed]
- Gordon, S.; Taylor, P.R. Monocyte and macrophage heterogeneity. Nat. Rev. Immunol. 2005, 5, 953–964. [Google Scholar] [CrossRef] [PubMed]
- Joshi, S.; Singh, A.R.; Zulcic, M.; Bao, L.; Messer, K.; Ideker, T.; Dutkowski, J.; Durden, D.L. Rac2 controls tumor growth, metastasis and M1-M2 macrophage differentiation in vivo. PLoS ONE 2014, 9, 95893. [Google Scholar] [CrossRef] [PubMed]
- Martinez, F.O.; Gordon, S. The M1 and M2 paradigm of macrophage activation: time for reassessment. F1000Prime Rep. 2014, 6, 13. [Google Scholar] [CrossRef] [PubMed]
- Biswas, S.K.; Mantovani, A. Macrophage plasticity and interaction with lymphocyte subsets: Cancer as a paradigm. Nat. Immunol. 2010, 11, 889–896. [Google Scholar] [CrossRef] [PubMed]
- Gordon, S.; Martinez, F.O. Alternative activation of macrophages: mechanism and functions. Immunity 2010, 32, 593–604. [Google Scholar] [CrossRef] [PubMed]
- Qian, B.Z.; Pollard, J.W. Macrophage diversity enhances tumor progression and metastasis. Cell 2010, 141, 39–51. [Google Scholar] [CrossRef] [PubMed]
- Ye, S.; Yuan, D.; Xie, Y.; Pan, Y.; Shao, C. Role of DNA methylation in long-term low-dose γ-rays induced adaptive response in human B lymphoblast cells. Int. J. Radiat. Biol. 2013, 89, 898–906. [Google Scholar] [CrossRef] [PubMed]
- Klug, F.; Prakash, H.; Huber, P.E.; Seibel, T.; Bender, N.; Halama, N.; Pfirschke, C.; Voss, R.H.; Timke, C.; Umansky, L.; et al. Low-dose irradiation programs macrophage differentiation to an iNOS(+)/M1 phenotype that orchestrates effective T cell immunotherapy. Cancer Cell 2013, 24, 589–602. [Google Scholar] [CrossRef] [PubMed]
- Kojima, S. Induction of glutathione and activation of immune functions by low-dose, whole-body irradiation with gamma-rays. Yakugaku Zasshi 2006, 126, 849–857. [Google Scholar] [CrossRef] [PubMed]
- Coussens, L.M.; Werb, Z. Inflammation and cancer. Nature 2002, 420, 860–867. [Google Scholar] [CrossRef] [PubMed]
- Li, X.Y.; Chen, Y.B.; Xia, F.Q. Effect of low dose radiation on growth of implanted tumor and cancer induction in mice. Chin. J. Radiol. Health 1996, 5, 21–23. [Google Scholar]
- Zhang, Y.; Liu, S.Z. Effect of low dose radiation on immune functions of tumor-bearing mice. Chin. J. Radiol. Health 1996, 5, 235–237. [Google Scholar]
- Schaue, D.; Marples, B.; Trott, K.R. The effects of low-dose X-irradiation on the oxidative burst in stimulated macrophages. Int. J. Radiat. Biol. 2002, 78, 567–576. [Google Scholar] [CrossRef] [PubMed]
- Banchereau, J.; Briere, F.; Caux, C.; Davoust, J.; Lebecque, S.; Liu, Y.J.; Pulendran, B.; Palucka, K. Immunobiology of dendritic cells. Ann. Rev. Immunol. 2000, 18, 767–811. [Google Scholar] [CrossRef] [PubMed]
- Jahns, J.; Anderegg, U.; Saalbach, A.; Rosin, B.; Patties, I.; Glasow, A.; Kamprad, M.; Scholz, M.; Hildebrandt, G. Influence of low dose irradiation on differentiation, maturation and T-cell activation of human dendritic cells. Mutat. Res. 2011, 710, 32–39. [Google Scholar] [CrossRef] [PubMed]
- Shigematsu, A.; Adachi, Y.; Koike-Kiriyama, N.; Suzuki, Y.; Iwasaki, M.; Koike, Y.; Nakano, K.; Mukaide, H.; Imamura, M.; Ikehara, S. Effects of low-dose irradiation on enhancement of immunity by dendritic cells. J. Radiat. Res. 2007, 48, 51–55. [Google Scholar] [CrossRef] [PubMed]
- Ishii, K.; Yamaoka, K.; Hosoi, Y.; Ono, T.; Sakamoto, K. Enhanced mitogen-induced proliferation of rat splenocytes by low-dose whole-body X-irradiation. Physiol. Chem. Phys. Med. NMR 1995, 27, 17–23. [Google Scholar] [PubMed]
- Liu, S.Z.; Han, Z.B.; Liu, W.H. Changes in lymphocyte reactivity to modulatory factors following low dose ionizing radiation. Biomed. Environ. Sci. 1994, 7, 130–135. [Google Scholar] [PubMed]
- Liu, S.Z.; Su, X.; Zhang, Y.C.; Zhao, Y. Signal transduction in lymphocytes after low dose radiation. Chin. Med. J. 1994, 107, 431–436. [Google Scholar] [PubMed]
- Song, K.H.; Kim, M.H.; Kang, S.M.; Jung, S.Y.; Ahn, J.; Woo, H.J.; Nam, S.Y.; Hwang, S.G.; Ryu, S.Y.; Song, J.Y. Analysis of immune cell populations and cytokine profiles in murine splenocytes exposed to whole-body low-dose irradiation. Int. J. Radiat. Biol. 2015, 91, 795–803. [Google Scholar] [CrossRef] [PubMed]
- Shankar, B.; Pandey, R.; Sainis, K. Radiation-induced bystander effects and adaptive response in murine lymphocytes. Int. J. Radiat. Biol. 2006, 82, 537–548. [Google Scholar] [CrossRef] [PubMed]
- Rizvi, A.; Pecaut, M.J.; Slater, J.M.; Subramaniam, S.; Gridley, D.S. Low-dose gamma-rays modify CD4(+) T cell signalling response to simulated solar particle event protons in a mouse model. Int. J. Radiat. Biol. 2011, 87, 24–35. [Google Scholar] [CrossRef] [PubMed]
- Gridley, D.S.; Pecaut, M.J.; Rizvi, A.; Coutrakon, G.B.; Luo-Owen, X.; Makinde, A.Y.; Slater, J.M. Low-dose, low-dose-rate proton radiation modulates CD4(+) T cell gene expression. Int. J. Radiat. Biol. 2009, 85, 250–261. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.Z.; Zhang, Y.C.; Su, X. Effect of low dose radiation on the expression of TCR/CD3 and CD25 on mouse thymocyte plasma membrane. Chin. J. Pathophysiol. 1995, 11, 2–5. [Google Scholar]
- Sambani, C. Stimulatory effect of low dose X-irradiation on the expression of the human T lymphocyte CD2 surface antigen. Int. J. Radiat. Biol. 1996, 70, 711–717. [Google Scholar] [CrossRef] [PubMed]
- Lankford, K.V.; Mosunjac, M.; Hillyer, C.D. Effects of UVB radiation on cytokine generation, cell adhesion molecules, and cell activation markers in T-lymphocytes and peripheral blood HPCs. Transfusion 2000, 40, 361–367. [Google Scholar] [CrossRef] [PubMed]
- Liu, R.; Xiong, S.; Zhang, L.; Chu, Y. Enhancement of antitumor immunity by low-dose total body irradiationis associated with selectively decreasing the proportion and number of T regulatory cells. Cell. Mol. Immunol. 2010, 7, 157–162. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Li, B.; Dai, Z.; Ren, S.; Bai, M.; Wang, Z.; Li, Z.; Lin, S.; Wang, Z.; Huang, N.; et al. Low-dose splenic radiation inhibits liver tumor development of rats through functional changes in CD4+CD25+Treg cells. Int. J. Biochem. Cell. Biol. 2014, 55, 98–108. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.Z. Nonlinear dose-response relationship in the immune system following exposure to ionizing radiation: Mechanisms and implications. Nonlinearity Biol. Toxicol. Med. 2003, 1, 71–92. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.D.; Liu, S.Z.; Ma, S.M.; Liu, Y. Expression of IL-10 in mouse spleen at mRNA and protein level after whole-body X-irradiation. Chin. J. Radiol. Med. Prot. 2001, 22, 10–12. [Google Scholar]
- Weng, L.; Williams, R.O.; Vieira, P.L.; Screaton, G.; Feldmann, M.; Dazzi, F. The therapeutic activity of low-dose irradiation on experimental arthritis depends on the induction of endogenous regulatory T cell activity. Ann. Rheum. Dis. 2010, 69, 1519–1526. [Google Scholar] [CrossRef] [PubMed]
- Cho, S.J.; Kang, H.; Kim, M.Y.; Lee, J.E.; Kim, S.J.; Nam, S.Y.; Kim, J.Y.; Kim, H.S.; Pyo, S.; Yang, K.H. Site-specific phosphorylation of Ikaros induced by low-dose ionizing radiation regulates cell cycle progression of B lymphoblast through CK2 and AKT activation. Int. J. Radiat. Oncol. Biol. Phys. 2016, 94, 1207–1218. [Google Scholar] [CrossRef] [PubMed]
- Rho, H.S.; Park, S.S.; Lee, C.E. Gamma irradiation up-regulates expression of B cell differentiation molecule CD23 by NF-kappaB activation. J. Biochem. Mol. Biol. 2004, 37, 507–514. [Google Scholar] [PubMed]
- Ohshima, Y.; Kitami, A.; Kawano, A.; Tsukimoto, M.; Kojima, S. Induction of extracellular ATP mediates increase in intracellular thioredoxin in RAW264.7 cells exposed to low-dose γ-rays. Free Radic. Biol. Med. 2011, 51, 1240–1248. [Google Scholar] [CrossRef] [PubMed]
- Lall, R.; Ganapathy, S.; Yang, M.; Xiao, S.; Xu, T.; Su, H.; Shadfan, M.; Asara, J.M.; Ha, C.S.; Ben-Sahra, I.; et al. Low-dose radiation exposure induces a HIF-1-mediated adaptive and protective metabolic response. Cell Death Differ. 2014, 21, 836–844. [Google Scholar] [CrossRef] [PubMed]
- Farooque, A.; Mathur, R.; Verma, A.; Kaul, V.; Bhatt, A.N.; Adhikari, J.S.; Afrin, F.; Singh, S.; Dwarakanath, B.S. Low-dose radiation therapy of cancer: Role of immune enhancement. Expert Rev. Anticancer Ther. 2011, 11, 791–802. [Google Scholar] [CrossRef] [PubMed]
- Gyuleva, I.M.; Penkova, K.I.; Rupova, I.T.; Panova, D.Y.; Djounova, J.N. Assessment of some immune parameters in occupationally exposed nuclear power plant workers: Flow cytometry measurements of T lymphocyte subpopulations and immunoglobulin determination. Dose Response 2015, 13. [Google Scholar] [CrossRef] [PubMed]
- Zablotska, L.B.; Bazyka, D.; Lubin, J.H.; Gudzenko, N.; Little, M.P.; Hatch, M.; Finch, S.; Dyagil, I.; Reiss, R.F.; Chumak, V.V.; et al. Radiation and the risk of chronic lymphocytic and other leukemias among chornobyl cleanup workers. Environ. Health Perspect. 2013, 121, 59–65. [Google Scholar] [PubMed]
- Abdel Meguid, M.H.; Hamad, Y.H.; Swilam, R.S.; Barakat, M.S. Relation of interleukin-6 in rheumatoid arthritis patients to systemic bone loss and structural bone damage. Rheumatol. Int. 2013, 33, 697–703. [Google Scholar] [CrossRef] [PubMed]
- Nakatsukasa, H.; Tsukimoto, M.; Ohshima, Y.; Tago, F.; Masada, A.; Kojima, S. Suppressing effect of low-dose gamma-ray irradiation on collagen-induced arthritis. J. Radiat. Res. 2008, 49, 381–389. [Google Scholar] [CrossRef] [PubMed]
- Hildebrandt, G.; Radlingmayr, A.; Rosenthal, S.; Rothe, R.; Jahns, J.; Hindemith, M.; Rödel, F.; Kamprad, F. Low-dose radiotherapy (LD-RT) and the modulation of iNOS expression in adjuvant-induced arthritis in rats. Int. J. Radiat. Biol. 2003, 79, 993–1001. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.S.; Son, Y.; Bae, M.J.; Lee, S.S.; Park, S.H.; Lee, H.J.; Lee, S.I.; Lee, C.G.; Kim, S.D.; Jo, W.S.; et al. Continuous exposure to low-dose-rate gamma irradiation reduces airway inflammation in ovalbumin-induced asthma. PLoS ONE 2015, 10, 0143403. [Google Scholar] [CrossRef] [PubMed]
- Seegenschmiedt, M.H.; Micke, O. Radiotherapy of non-malignant diseases: Past, present and future. Strahlenther Onkol. 2012, 188, 272–290. [Google Scholar] [CrossRef] [PubMed]
- Rödel, F.; Frey, B.; Manda, K.; Hildebrandt, G.; Hehlgans, S.; Keilholz, L.; Seegenschmiedt, M.H.; Gaipl, U.S.; Rödel, C. Immunomodulatory properties and molecular effects in inflammatory diseases of low-dose X-irradiation. Front. Oncol. 2012, 2, 120. [Google Scholar] [CrossRef] [PubMed]
- Leer, J.W.; van Houtte, P.; Seegenschmiedt, H. Radiotherapy of non-malignant disorders: Where do we stand? Radiother. Oncol. 2007, 83, 175–177. [Google Scholar] [CrossRef] [PubMed]
- Artuković, M.; Ikić, M.; Kustelega, J.; Artuković, I.N.; Kaliterna, D.M. Influence of UV radiation on immunological system and occurrence of autoimmune diseases. Coll. Antropol. 2010, 34, 175–178. [Google Scholar] [PubMed]
- Tago, F.; Tsukimoto, M.; Nakatsukasa, H.; Kojima, S. Repeated 0.5-Gy gamma irradiation attenuates autoimmune disease in MRL-lpr/lpr mice with suppression of CD3+CD4−CD8−B220+ T-cell proliferation and with up-regulation of CD4+CD25+Foxp3+ regulatory T cells. Radiat. Res. 2008, 169, 59–66. [Google Scholar] [CrossRef] [PubMed]
- Valledor, A.F.; Comalada, M.; Santamaría-Babi, L.F.; Lloberas, J.; Celada, A. Macrophage proinflammatory activation and deactivation: A question of balance. Adv. Immunol. 2010, 108, 1–20. [Google Scholar] [PubMed]
- Liu, S.; Sun, X.; Luo, J.; Zhu, H.; Yang, X.; Guo, Q.; Song, Y.; Sun, X. Effects of radiation on T regulatory cells in normal states and cancer: Mechanisms and clinical implications. Am. J. Cancer Res. 2015, 5, 3276–3285. [Google Scholar] [PubMed]
- Fang, S.; Muto, Y.; Tago, F.; Shimura, N.; Kojima, S. Effect of repeated small-dose γ-ray irradiation on atopic dermatitis in NC/Nga mice. J. Health Sci. 2006, 52, 406–411. [Google Scholar] [CrossRef]
- Cardis, E.; Vijheid, M.; Blettner, M.; Gilbert, E.; Hakama, M.; Hill, C.; Howe, G.; Kaldor, J.; Muirhead, C.R.; Schubauer-Berigan, M.; et al. Risk of cancer after low doses of ionising radiation: Retrospective cohort study in 15 countries. BMJ 2005, 331, 77. [Google Scholar] [CrossRef] [PubMed]
- Cardis, E.; Vijheid, M.; Blettner, M.; Gilbert, E.; Hakama, M.; Hill, C.; Howe, G.; Kaldor, J.; Muirhead, C.R.; Schubauer-Berigan, M.; et al. The 15-country collaborative study of cancer risk among radiation workers in the nuclear industry: Estimates of radiation-related cancer risks. Radiat. Res. 2007, 167, 396–416. [Google Scholar] [CrossRef] [PubMed]
- Feinendegen, L.E. Evidence for beneficial low level radiation effects and radiation hormesis. Br. J. Radiol. 2005, 78, 3–7. [Google Scholar] [CrossRef] [PubMed]
- Li, X.J.; Yang, Y.; Li, X.Y.; Liu, S.Z. Immunologic mechanisms of reduction of radiation-induced thymic lymphoma by low dose radiation. J. Radat. Res. Radiat. Pros. 1999, 17, 125–128. [Google Scholar]
- Jin, A.X.; Wang, S.; Wei, D.Y. Mechanism of low level ionizing radiation in inhibiting B16 melanoma blood-born pulmonary metastasis. Chin. J. Radiol. Med. Prot. 1997, 17, 236–239. [Google Scholar]
- Kendall, G.M.; Muirhead, C.R.; MacGibbon, B.H.; O’Hagan, J.A.; Conquest, A.J.; Goodill, A.A.; Butland, B.K.; Fell, T.P.; Jackson, D.A.; Webb, M.A.; et al. Mortality and occupational exposure to radiation: First analysis of the National Registry for Radiation Workers. BMJ 1992, 304, 220–225. [Google Scholar] [CrossRef] [PubMed]
- Mifune, M.; Sobue, T.; Arimoto, H.; Komoto, Y.; Kondo, S.; Tanooka, H. Cancer mortality survey in a spa area (Misasa, Japan) with a high radon background. Jpn. J. Cancer Res. 1992, 83, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Miller, A.B.; Howe, G.R.; Sherman, G.J.; Lindsay, J.P.; Yaffe, M.J.; Dinner, P.J.; Risch, H.A.; Preston, D.L. Mortality from breast cancer after irradiation during fluoroscopic examinations in patients being treated for tuberculosis. N. Engl. J. Med. 1989, 321, 1285–1289. [Google Scholar] [CrossRef] [PubMed]
- Nambi, K.S.; Soman, S.D. Environmental radiation and cancer in India. Health Phys. 1987, 52, 653–657. [Google Scholar] [CrossRef] [PubMed]
- Jiang, H.; Xu, Y.; Li, W.; Ma, K.; Cai, L.; Wang, G. Low-dose radiation does not induce proliferation in tumor cells in vitro and in vivo. Radiat. Res. 2008, 170, 477–487. [Google Scholar] [CrossRef] [PubMed]
- Liang, X.; Gu, J.; Yu, D.; Wang, G.; Zhou, L.; Zhang, X.; Zhao, Y.; Chen, X.; Zheng, S.; Liu, Q.; et al. Low-dose radiation induces cell proliferation in human embryonic lung fibroblasts but not in lung cancer cells: Importance of ERK1/2 and AKT signaling pathways. Dose Response 2016, 14. [Google Scholar] [CrossRef] [PubMed]
- Yang, G.; Yu, D.; Li, W.; Zhao, Y.; Wen, X.; Liang, X.; Zhang, X.; Zhou, L.; Hu, J.; Niu, C.; et al. Distinct biological effects of low-dose radiation on normal and cancerous human lung cells are mediated by ATM signaling. Oncotarget 2016. [Google Scholar] [CrossRef] [PubMed]
- Park, S.H.; Lee, Y.; Jeong, K.; Yoo, S.Y.; Cho, C.K.; Lee, Y.S. Different induction of adaptive response to ionizing radiation in normal and neoplastic cells. Cell. Biol. Toxicol. 1999, 15, 111–119. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.J.; Choi, S.A.; Cho, C.K.; Kim, T.H.; Jeong, K.S.; Yoo, S.Y.; Lee, Y.S. Adaptive response is differently induced depending on the sensitivity to radiation-induced cell death in mouse epidermal cells. Cell. Biol. Toxicol. 2000, 16, 175–184. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Sakai, K. Enhancement of radiation-induced apoptosis by preirradiation with low-dose X-rays in human leukemia MOLT-4 cells. J. Radiat. Res. 2004, 45, 239–243. [Google Scholar] [CrossRef] [PubMed]
- Gerashchenko, B.I.; Ryabchenko, N.M.; Glavin, O.A.; Rodionova, N.K.; Makovetska, L.I.; Ganzha, O.B.; Druzhyna, M.O.; Mikhailenko, V.M. Fractionated low-dose radiation exposure potentiates proliferation of implanted tumor cells. Exp. Oncol. 2013, 35, 69–71. [Google Scholar] [PubMed]
- Raaphorst, G.P.; Boyden, S. Adaptive response and its variation in human normal and tumour cells. Int. J. Radiat. Biol. 1999, 75, 865–873. [Google Scholar] [PubMed]

© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cui, J.; Yang, G.; Pan, Z.; Zhao, Y.; Liang, X.; Li, W.; Cai, L. Hormetic Response to Low-Dose Radiation: Focus on the Immune System and Its Clinical Implications. Int. J. Mol. Sci. 2017, 18, 280. https://doi.org/10.3390/ijms18020280
Cui J, Yang G, Pan Z, Zhao Y, Liang X, Li W, Cai L. Hormetic Response to Low-Dose Radiation: Focus on the Immune System and Its Clinical Implications. International Journal of Molecular Sciences. 2017; 18(2):280. https://doi.org/10.3390/ijms18020280
Chicago/Turabian StyleCui, Jiuwei, Guozi Yang, Zhenyu Pan, Yuguang Zhao, Xinyue Liang, Wei Li, and Lu Cai. 2017. "Hormetic Response to Low-Dose Radiation: Focus on the Immune System and Its Clinical Implications" International Journal of Molecular Sciences 18, no. 2: 280. https://doi.org/10.3390/ijms18020280
APA StyleCui, J., Yang, G., Pan, Z., Zhao, Y., Liang, X., Li, W., & Cai, L. (2017). Hormetic Response to Low-Dose Radiation: Focus on the Immune System and Its Clinical Implications. International Journal of Molecular Sciences, 18(2), 280. https://doi.org/10.3390/ijms18020280