Abstract
Constipation is one of the most common gastrointestinal complaints worldwide. This study was performed to determine whether Bifidobacterium adolescentis exerts inter-strain differences in alleviating constipation induced by loperamide in BALB/c mice and to analyze the main reasons for these differences. BALB/c mice underwent gavage with B. adolescentis (CCFM 626, 667, and 669) once per day for 17 days. The primary outcome measures included related constipation indicators, and the secondary outcome measures were the basic biological characteristics of the strains, the concentration changes of short-chain fatty acids in feces, and the changes in the fecal flora. B. adolescentis CCFM 669 and 667 relieved constipation symptoms by adhering to intestinal epithelial cells, growing quickly in vitro and increasing the concentrations of propionic and butyric acids. The effect of B. adolescentis on the gut microbiota in mice with constipation was investigated via 16S rRNA metagenomic analysis. The results revealed that the relative abundance of Lactobacillus increased and the amount of Clostridium decreased in the B. adolescentis CCFM 669 and 667 treatment groups. In conclusion, B. adolescentis exhibits strain-specific effects in the alleviation of constipation, mostly due to the strains’ growth rates, adhesive capacity and effects on the gut microbiome and microenvironment.
1. Introduction
Constipation is a common health problem and a predisposing factor for many conditions with high death rates. With changes in diet structure and the influence of psychological and social factors in recent years, the incidence of constipation has shown a clear upward trend, which has seriously affected human health and quality of life [1]. Irritant drugs are generally used to promote defecation, but this treatment has side effects [2,3]. Reports have shown that constipation and diarrhea are associated with gut microbes. The relative abundance of pathogens (methanogenic archaea [4] and clostridia [5]) increases in patient with constipation, and the relative abundance of enteropathogenic bacteria (Salmonella, Shigella, enterotoxigenic Escherichia coli, or Vibrio cholera) increases in patients with diarrhea [6,7], which disturbs the ecological balance in the intestines [8]. As research into the intestinal micro-ecology continues to develop, it is understandable to attempt treatment of constipation with intestinal micro-ecological therapy.
Increasing fiber intake or using laxatives is commonly recommended to alleviate or treat constipation. The intestinal microbiome is related to normal gastrointestinal (GI) functions such as GI motility, immune modulation, and drug metabolism [9,10,11]. Clinical studies have shown that gut microbiota in constipation differs from that in healthy subjects [12,13] and is mainly manifested in the levels of Bifidobacterium, Lactobacillus and pathogenic bacteria. Most studies have shown that changes in the intestinal flora in the constipation group mainly involve a decrease in Bifidobacteria and Lactobacilli and an increase in pathogenic bacteria (methanogenic archaea [4] and clostridia [5]). Therefore, supplementation with probiotics has become a new method to treat constipation. Probiotics have been defined as living microbes that, when administered in adequate amounts, such as 106 to 109 colony-forming units (CFU), confer health benefits to the host [14]. Some studies have supported the use of probiotics to prevent or treat constipation [15,16]. Some probiotic strains, either alone—Bifidobacterium infantis 36524 or Lactobacillus plantarum 299v—or combined—VSL#3 (Bifidobacterium (B. longum, B. infantis, and B. breve), Lactobacillus (L. acidophilus, L. casei, L. bulgaricus and L. plantarum)), and Streptococcus thermophiles—have been associated with significant alleviation of constipation [17,18], whereas others have proved ineffective [19,20]. The fundamental reason for the use of probiotics to treat constipation may be that the colonic microflora influences peristalsis of the colon [21]. Therefore, it has been suggested that an imbalance in the colonic microflora plays a role in constipation. Furthermore, Bifidobacteria produce lactic acid and acetic acid, which decrease the pH in the colon. This lower pH enhances peristalsis and decreases the colonic transit time, which is beneficial in the treatment of constipation [16,22]. This latter hypothesis was confirmed by showing a decrease in the colonic transit time in healthy adults who consumed a supplement with B. animalis [23].
Numerous probiotic supplementation trials have been carried out in animals and humans to test the efficacy of probiotics against constipation [15,24,25]. It has been shown that Bifidobacteria display inter-species differences in the alleviation of constipation [24]. Bifidobacteria may comprise as much as 25% of the cultivable gut microflora. B. adolescentis is recognized as one of the dominant anaerobes in adults and is considered to be beneficial to human health [26]. Therefore, we hypothesized that B. adolescentis could alleviate constipation and that inter-strain differences exist in the alleviation of constipation induced by loperamide in BALB/c mice.
Based on this background, the aims of this study were: (1) to determine whether B. adolescentis shows inter-strain differences in the alleviation of constipation induced by loperamide in mice; (2) to analyze the main reasons for the inter-strain differences, such as the basic biological characteristics of the strains, the concentration changes of short-chain fatty acids (SCFAs) in feces, and the changes in the fecal flora; and (3) to determine the changes in other indicators of constipation, including some parameters of the enteric nervous system, including motilin (MTL), gastrin (Gas), substance P (SP), endothelin (ET), somatostatin (SS) and vasoactive intestinal peptide (VIP).
2. Results
2.1. Growth Characteristics of B. adolescentis In Vitro
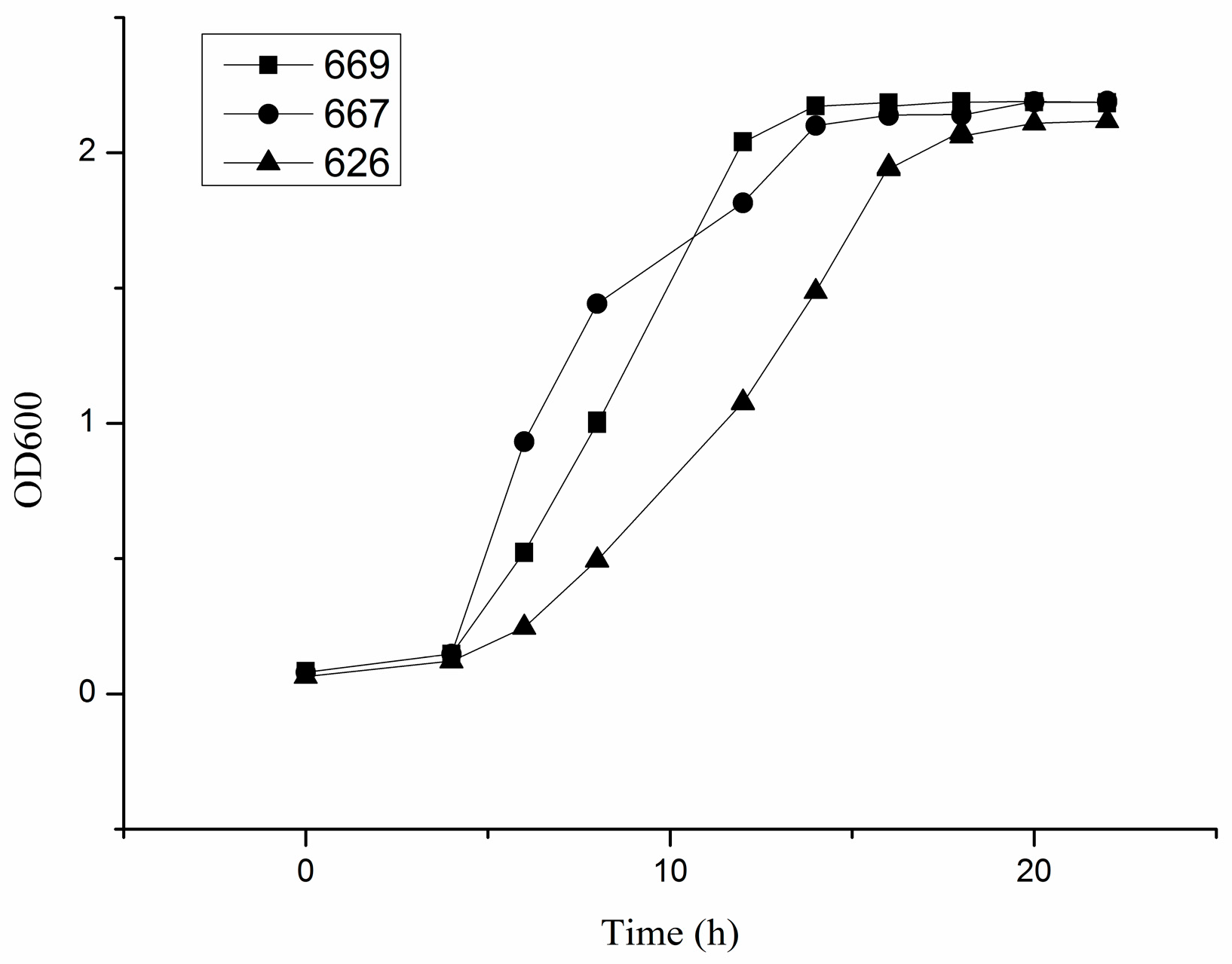
The growth curve of each strain of B. adolescentis cultured under anaerobic conditions at 37 °C in cMRS broth was drawn to determine the growth characteristics of B. adolescentis in vitro. The results showed that B. adolescentis CCFM 669 and 667 entered the exponential growth phase more quickly than B. adolescentis CCFM 626 (Figure 1).
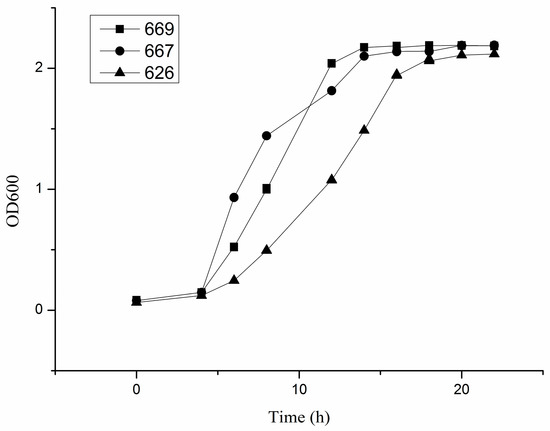
Figure 1.
Growth curves of three strains of Bifidobacterium adolescentis. The horizontal axis is the time of culturing B. adolescentis, and the vertical axis is the absorbance value of the culture solution at 600 nm: 669, the growth curve of B. adolescentis 669; 667, the growth curve of B. adolescentis 667; and 626, the growth curve of B. adolescentis 626. The curves shown are from a single experiment (mean ± SD, n = 3).
2.2. Tolerance Capacity of B. adolescentis to Simulated Gastric and Small Intestine Juices
To measure the tolerance capacity of B. adolescentis (667, 669 and 626) to gastric acid and bile salts, these three strains were cultured under anaerobic conditions at 37 °C in simulated gastric and intestinal juices. The tolerance capacity of B. adolescentis to simulated gastric and intestinal juices is presented in Table 1. Whether in gastric juice or in intestinal juice, the survival rates of the three examined strains showed a decline with the passage of incubation time, but they showed a preferable level of survival and were considered tolerant to gastric and intestinal juices.

Table 1.
Tolerance of Bifidobacterium adolescentis strains to simulated gastric and small intestinal juices.
2.3. Adhesion of Different B. adolescentis to HT-29 Cells
HT-29 cells that had been incubated and Bifidobacteria suspension (107 CFU/mL in DMEM) were cultured in six-well tissue culture plates to determine the adherence of B. adolescentis to intestinal epithelial cells. The results of three independent experiments performed in triplicate are shown in Table 2. Compared with the positive control L. plantarum ST-III, different strains of B. adolescentis exhibited different levels of adhesion to HT-29 cells (Table 2): B. adolescentis CCFM 669 showed remarkable adhesion, whereas B. adolescentis CCFM 626 and 667 showed no adhesion to HT-29 cells.

Table 2.
Adhesion of different bacteria to HT-29 cells.
2.4. Effects of B. adolescentis on Defecation Status of Mice
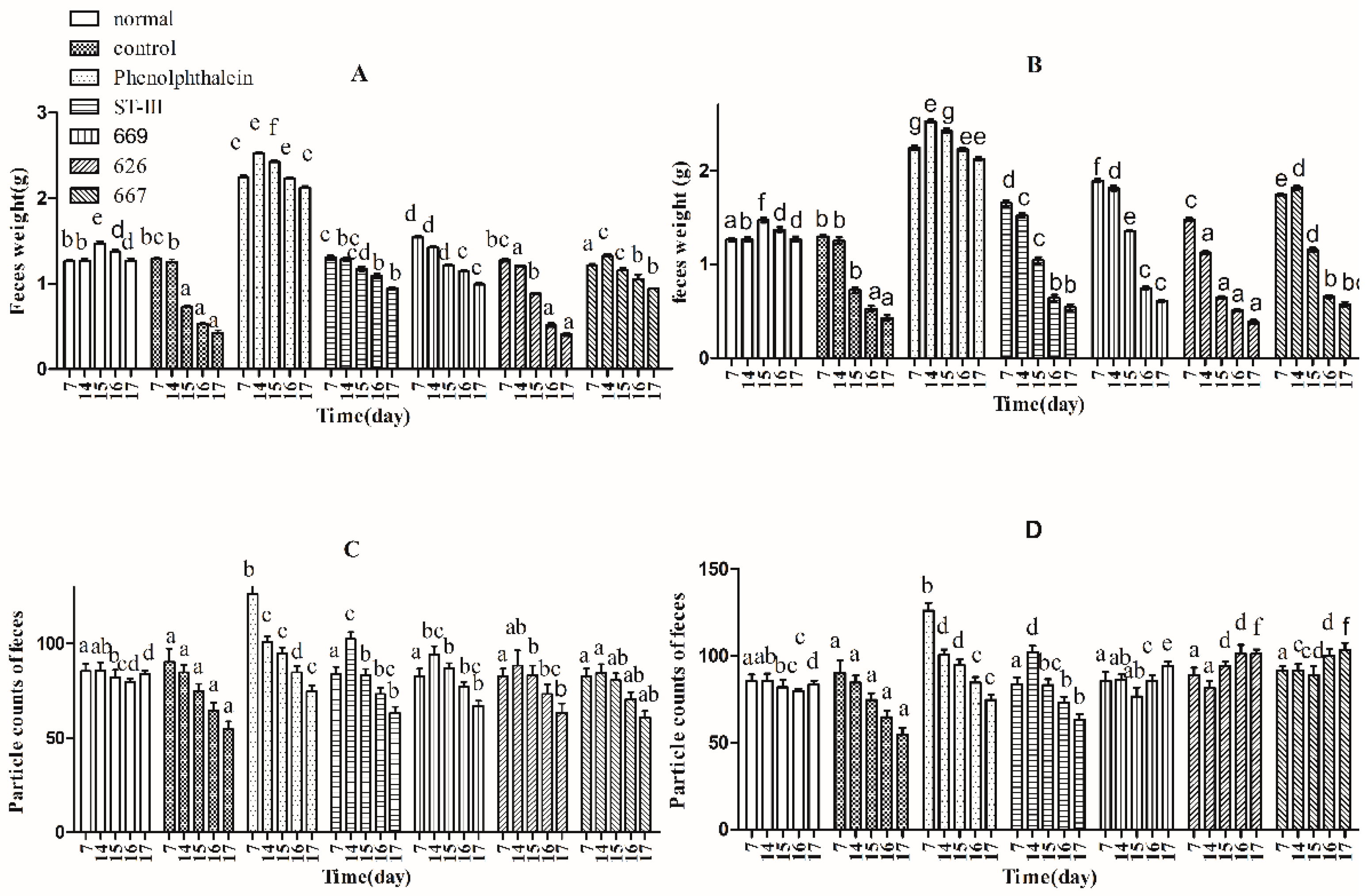
Three indicators, defecation wet weight, fecal pellet numbers and fecal water content, were used to evaluate defecation status. The results suggest that the defecation weight and fecal pellet numbers in the normal and control groups remained relatively stable before constipation from Day 1 to Day 14, whereas those of the other groups were significantly different by one-way analysis of variance and Duncan’s multiple range test (Figure 2A–D; p < 0.05). Furthermore, the water content of defecation in the B. adolescentis dose groups, the ST-III group and the phenolphthalein group increased significantly over time (Figure 2E,F; p < 0.05), whereas that in the normal and control groups remained stable. Meanwhile, the defecation weight and fecal pellet numbers in the phenolphthalein group were greater than those in the other groups. After constipation was induced by loperamide, the defecation weight, fecal pellet numbers and water content of defecation decreased in all of the treatment groups from Day 15 to Day 17, whereas those in the normal group remained stable (Figure 2A–F; p < 0.05). The defecation weight, fecal pellet numbers and water content of defecation increased significantly in the B. adolescentis CCFM 667 and 669 dose groups compared to those in the control group (loperamide group). The water content of defecation in the B. adolescentis CCFM 626 group was similar to that in the control group, which indicates that B. adolescentis CCFM 626 has no effect on constipation. Interestingly, although the particle counts of feces were higher in the low-dose B. adolescentis 626 and 667 groups than those in the high-dose groups (Figure 2C,D), the wet weight of total feces was lower in the low-dose B. adolescentis 626 and 667 groups than that in the high-dose groups (Figure 2A,B). The feces were dry, hard, and small in the low-dose 626 and 667 groups, indicating that the water content of defecation in the low-dose 626 and 667 groups was lower than that in the high-dose 626 and 667 groups.
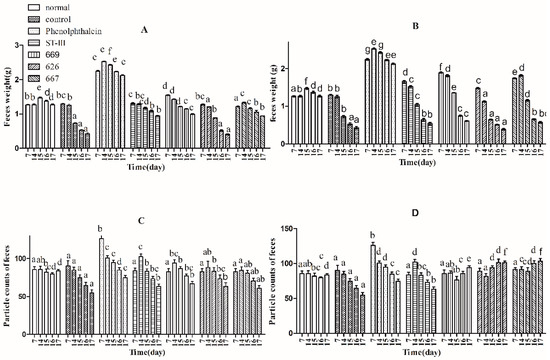
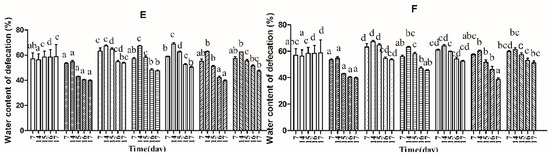
Figure 2.
Defecation status of mice during experiments in eight BALB/c mice in each group. Phenolphthalein: 10 mg/kg body weight; 626: B. adolescentis CCFM 626; 667: B. adolescentis CCFM 667; 669: B. adolescentis CCFM 669; ST-III: Lactobacillus plantarum ST-III; High dose: 1 × 1010 colony-forming units (CFU); Low dose: 1 × 108 CFU. (A,C,E) High-dose groups; and (B,D,F) low-dose groups. Mean values with different letters (a–g) over bars are significantly different (p < 0.05) according to one-way analysis of variance and Duncan’s multiple range test.
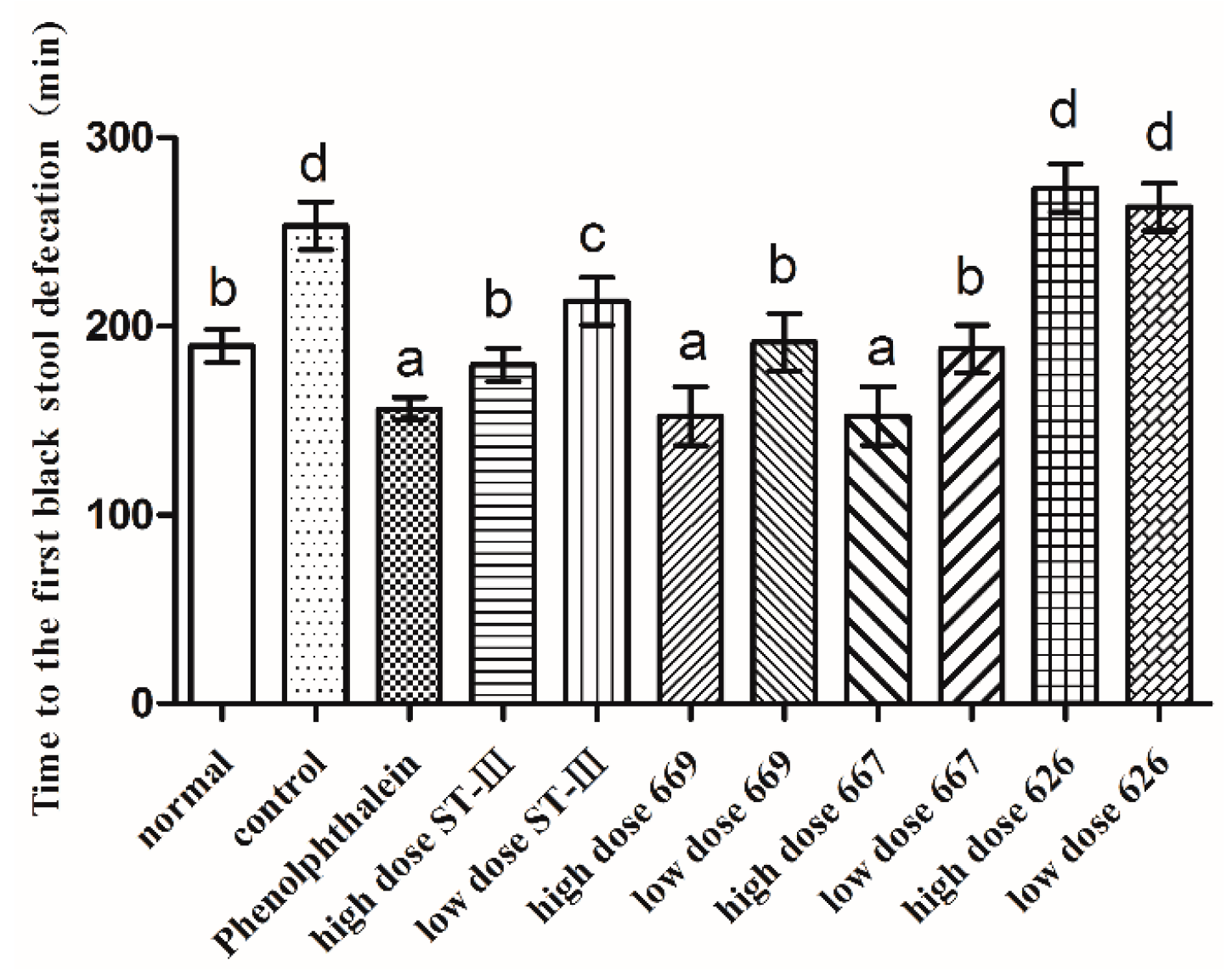
2.5. Time to the First Black Stool Defecation
The time to the first black stool defecation was measured to evaluate the effect of B. adolescentis on constipation. The results are shown in Figure 3. The defecation time was significantly different in each group. The time to the first black stool defecation in the normal group was similar to that in the low-dose B. adolescentis CCFM 669 and 667 groups and the high-dose ST-III group. Meanwhile, the defecation times in the phenolphthalein group and the high-dose B. adolescentis CCFM 669 and 667 groups were the shortest, and those in the control and B. adolescentis CCFM 626 groups were the longest, which indicates that B. adolescentis CCFM 626 has no effect on constipation.
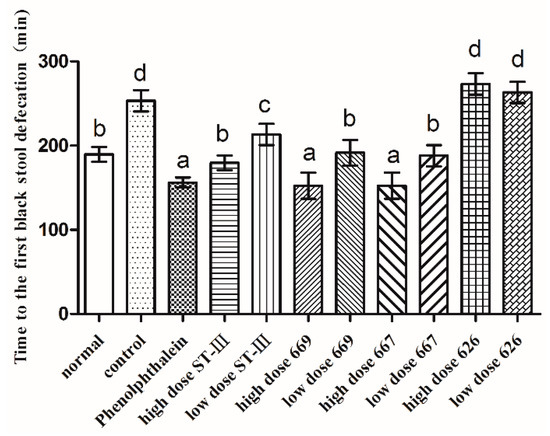
Figure 3.
First black stool defecation time of mice after constipation was induced by loperamide in eight BALB/c mice in each group. Phenolphthalein: 10 mg/kg body weight; 626: B. adolescentis CCFM 626; 667: B. adolescentis CCFM 667; 669: B. adolescentis CCFM 669; ST-III: L. plantarum ST-III; High dose: 1 × 1010 CFU; Low dose: 1 × 108 CFU. Mean values with different letters (a–d) over the bars are significantly different (p < 0.05) according to Duncan’s multiple range test.
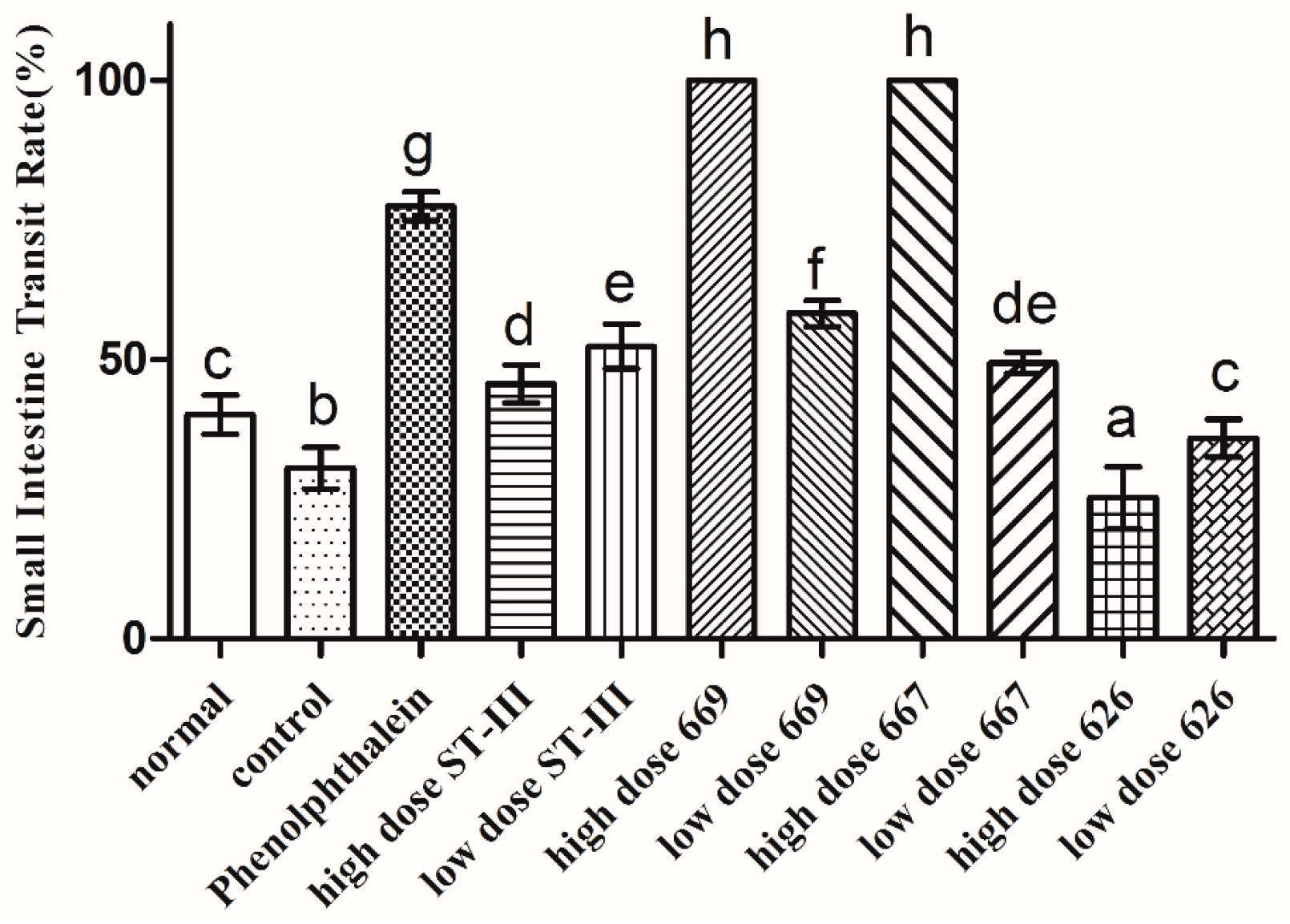
2.6. Gastrointestinal (GI) Transit Rate
The GI transit rate was measured to evaluate the effect of B. adolescentis on constipation. As shown in Figure 4, the small intestinal transit rate was significantly different in each group (p < 0.05). The transit rate in the control group was decreased compared to that in the normal group, indicating that the constipation model had been established. The two strains of B. adolescentis other than B. adolescentis CCFM 626 showed significant differences in the small intestinal transit rate. The small intestinal transit rates in the high-dose B. adolescentis CCFM 669 and CCFM 667 groups were the highest, that in the phenolphthalein group was the second, and that in the high-dose B. adolescentis CCFM 626 group was the lowest. The transit rates in the other groups had improved to different degrees. In conclusion, the three B. adolescentis strains showed a strain-specific effect on constipation in mice.
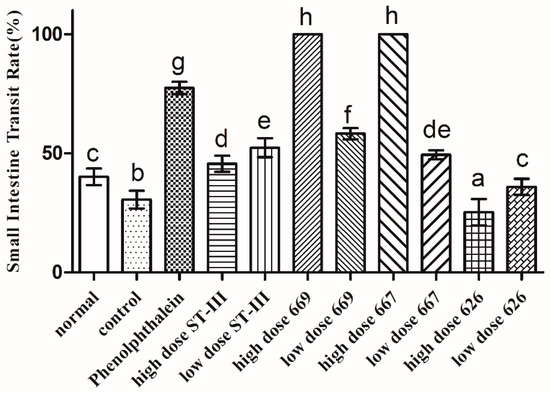
Figure 4.
Small intestinal transit rate of mice after constipation was induced by loperamide in eight BALB/c mice in each group. Phenolphthalein: 10 mg/kg body weight; 626: B. adolescentis CCFM 626; 667: B. adolescentis CCFM 667; 669: B. adolescentis CCFM 669; ST-III: L. plantarum ST-III; high dose: 1 × 1010 CFU; low dose: 1 × 108 CFU. Mean values with different letters (a–h) over the bars are significantly different (p < 0.05) according to Duncan’s multiple range test.
2.7. Short-Chain Fatty Acids (SCFAs) in Feces
The concentrations of SCFAs in the feces were determined to assess the effect of B. adolescentis on the gut microenvironment in mice with constipation. The results are shown in Table 3. From Day 1 to Day 14, the concentrations of acetic acid, propionic acid, butyric acid and total acid remained stable in the normal and control groups, whereas the other groups showed significant differences (Table 3; p < 0.05). The concentrations of propionic acid and butyric acid decreased in the ST-III and B. adolescentis CCFM 626 groups, whereas those of the other groups increased significantly. From Day 15 to Day 17, the concentrations of acetic acid, propionic acid, butyric acid and total acid decreased significantly from the first 14 days in the control group, whereas those in the normal group remained stable (Table 3; p < 0.05). The concentrations of propionic acid and butyric acid decreased in the B. adolescentis CCFM 626 group, whereas those in the other treated groups increased. From Day 1 to Day 17, the concentration of acetic acid increased in all treated groups except for the normal and control groups.

Table 3.
Short-chain fatty acids (SCFAs) in feces.
2.8. Effect of B. adolescentis on Composition of Fecal Microbiota
A data set consisting of 1,660,418 high-quality, classifiable 16S rRNA gene sequences was obtained from 88 fecal samples through MiSeq sequencing analysis. The average sequence read was 13,283 per sample. Representative sequences of all of the sequences were clustered, and a 97% sequence similarity cut-off was used. The number of operational taxonomic units (OTUs) per sample ranged from 791 to 6386.
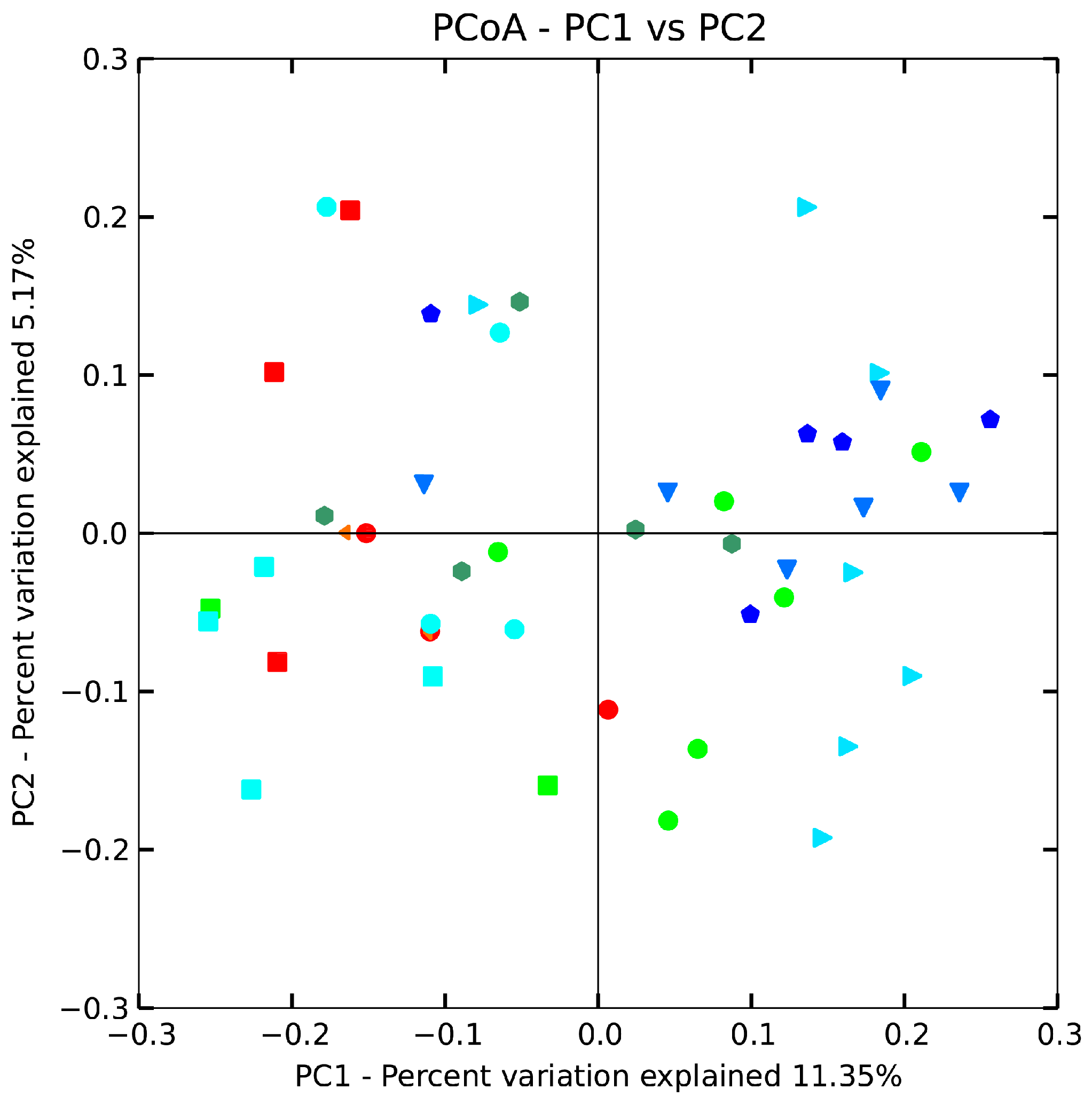
The beta diversity of gut microbiota in mice treated with B. adolescentis (CCFM 626,669 and 667) at the genus level was revealed by using an unweighted uniFrac matrix (Figure 5). However, the difference between the control, low-dose 626 and phenolphthalein groups was smaller, indicating that the taxa with the highest relative abundance were more similar between these three groups than the taxa that accounted for a smaller proportion of the community. The data points shifted from the right of the score plot to the left in two doses of 667 and 669 groups, indicating that B. adolescentis 669 and 667 changed the gut microbiota structure of mice. To test the hypothesis that the composition changed significantly, we performed one-way nonparametric multivariate analysis of variance with the total microbiota composition data. The results showed significant differences between the B. adolescentis CCFM 669, CCFM 667 and control groups, whereas the differences between the 626 group and control group were not statistically significant. In other words, B. adolescentis CCFM 669 and CCFM 667 significantly changed the gut microbiota structure of mice (Table 4). Interestingly, no significant differences were observed between the low-dose 669 group and the normal group. This result revealed that the gut microbiota of constipated mice treated with low-dose 669 recovered to normal status.
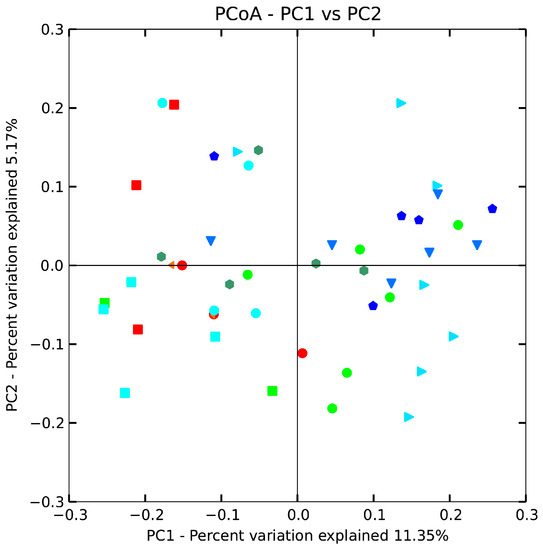
Figure 5.
Principal coordinates analysis (PCoA) plots based on unweighted UniFrac metric. Each colored symbol represents the composition of fecal microbiota of one mouse. Symbol:  , control;
, control;  , normal;
, normal;  , phenolphthalein;
, phenolphthalein;  , ST-III;
, ST-III;  , high-dose 667;
, high-dose 667;  , low-dose 667;
, low-dose 667;  , high-dose 669;
, high-dose 669;  , low-dose 669;
, low-dose 669;  , high-dose 626;
, high-dose 626;  , low-dose 626.
, low-dose 626.
 , control;
, control;  , normal;
, normal;  , phenolphthalein;
, phenolphthalein;  , ST-III;
, ST-III;  , high-dose 667;
, high-dose 667;  , low-dose 667;
, low-dose 667;  , high-dose 669;
, high-dose 669;  , low-dose 669;
, low-dose 669;  , high-dose 626;
, high-dose 626;  , low-dose 626.
, low-dose 626.

Table 4.
Effect size of the changes in community structure.
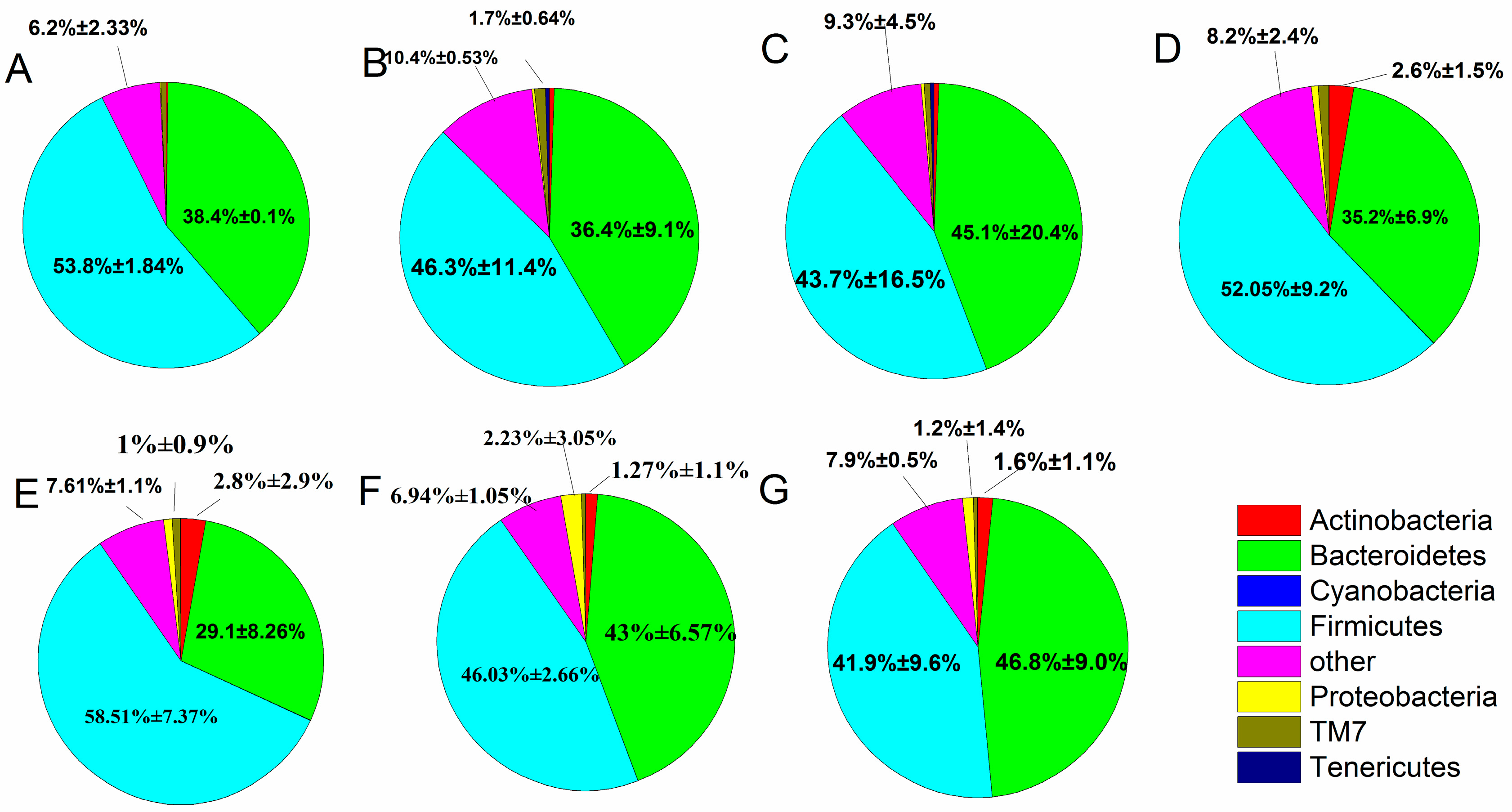
When individual OTUs were used to combine the phylum level (Figure 6), the intestinal flora was mostly dominated by Firmicutes and Bacteroidetes, which accounted for 88.04%, and Proteobacteria and Actinobacteria (3.89%). The fecal microbiota changed between the treatment, control and normal groups, as described in the following subsections. After constipation, the relative abundance of Firmicutes in the control group decreased compared with the normal group (from 53.8% to 46.3%), whereas the B. adolescentis CCFM 669 and ST-III groups showed an increase in the relative abundance of Firmicutes (from 46.3% to 58.51% and 52.05%) and a significant decrease in others (from 10.4% to 7.61% and 8.2%) (p < 0.05) compared with the control group by one-way analysis of variance. The relative abundance of Firmicutes and Bacteroidetes was similar in the B. adolescentis CCFM 667 group (46.03% vs. 43%) and the phenolphthalein group (43.7% vs. 45.1%). The B. adolescentis CCFM 626 group showed a decrease in Firmicutes and an increase in Bacteroidetes compared with the normal group.
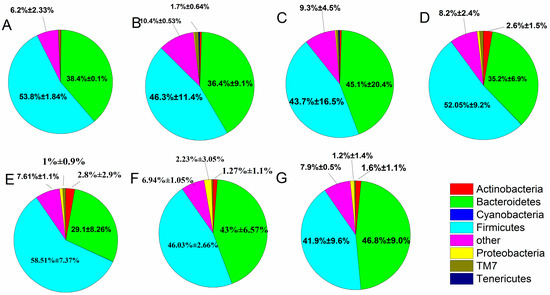
Figure 6.
Relative abundance of main phyla in the different groups after constipation: (A) normal; (B) control; (C) phenolphthalein; (D) ST-III; (E) B. adolescentis CCFM 669; (F) B. adolescentis CCFM 667; and (G) B. adolescentis CCFM 626.
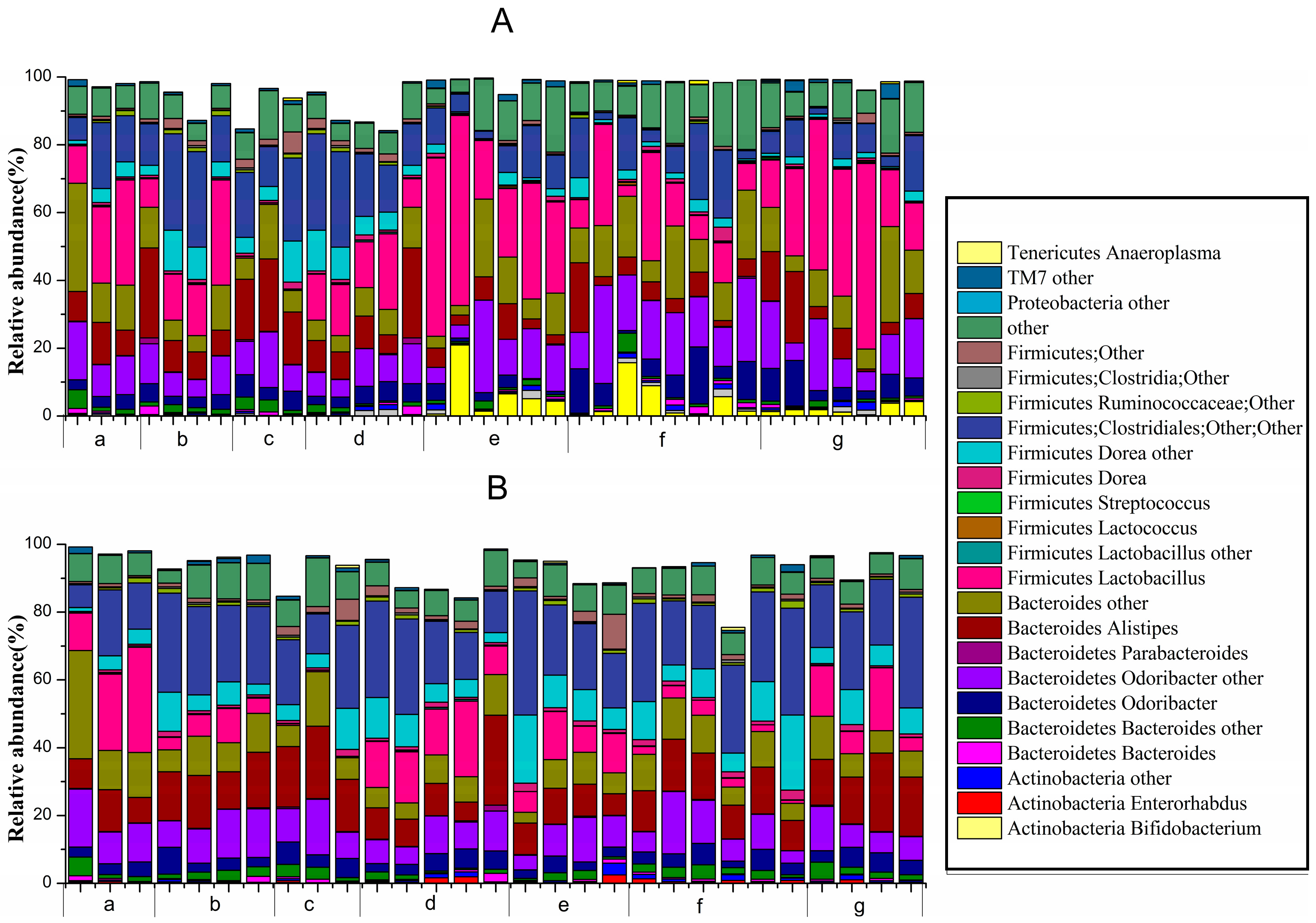
At the genus level (Figure 7), unknown genera belonging to Actinobacteria (Bifidobacterium, Enterorhabdus and others), Bacteroidetes (Bacteroides, Odoribacter, Parabacteroides, Alistipes and others), Firmicutes (Lactobacillus, Lactococcus, Dorea, Clostridiales, Ruminococcaceae and others) and Proteobacteria, TM7 and Tenericutes (Anaeroplasma) all comprised >0.1% of the microbiota.
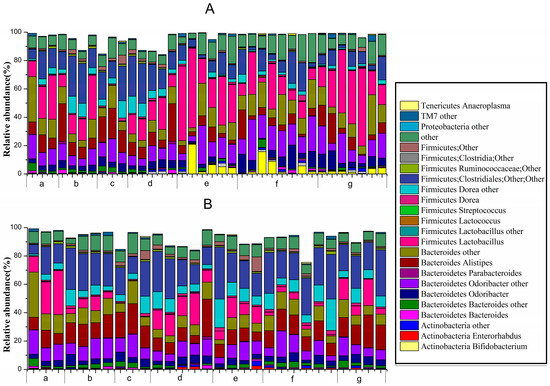
Figure 7.
Relative abundance of main genera >0.1% at d1422-0067/18/02/0317/s2ifferent periods in different groups: (A) before constipation (Days 1 to 14); and (B) during constipation (Days 15 to 17) ((a) normal group; (b) control group; (c) phenolphthalein group; (d) ST-III group; (e) B. adolescentis CCFM 669 group; (f) B. adolescentis CCFM 626 group; and (g) B. adolescentis CCFM 667 group).
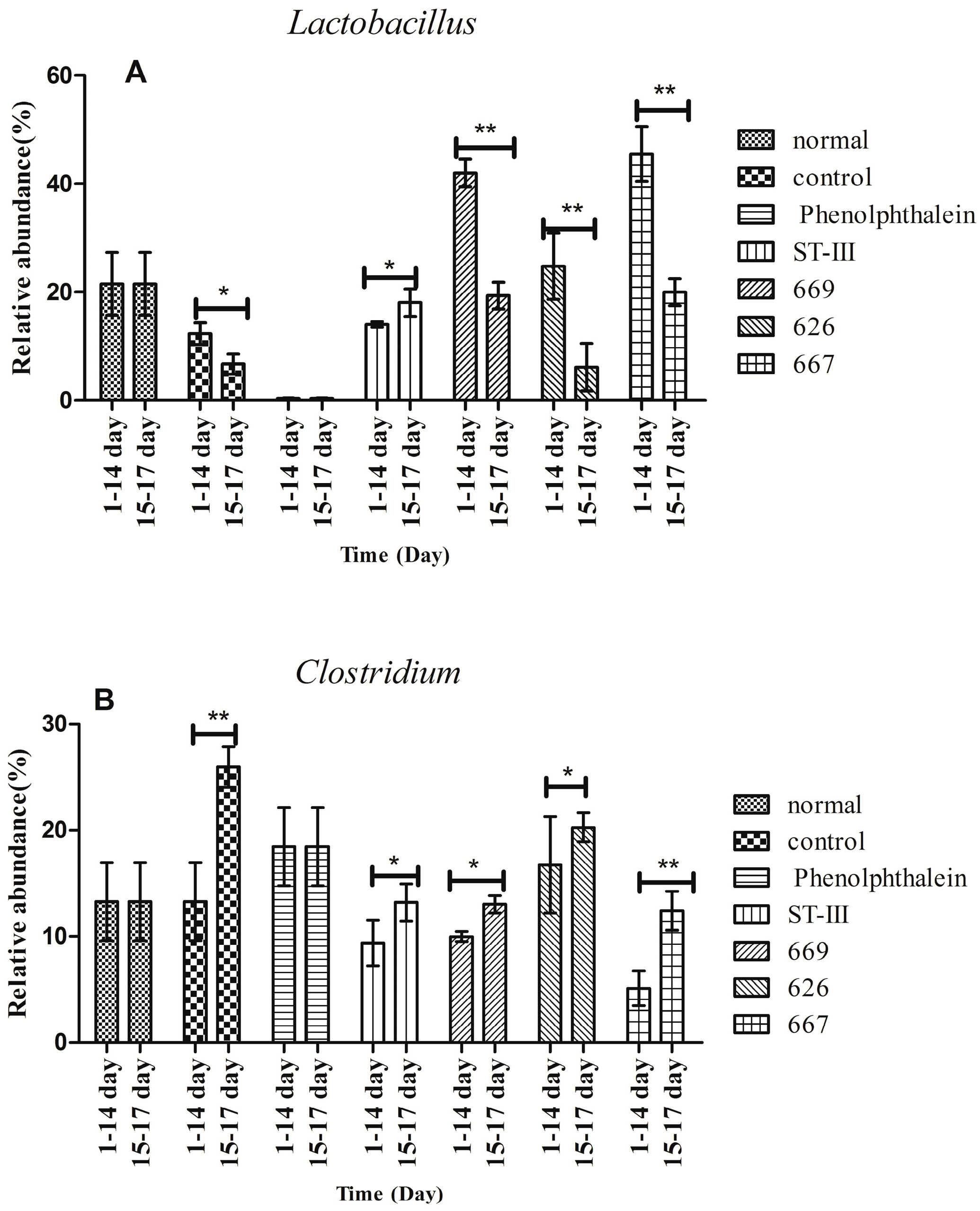
Before constipation (Day 1 to Day 14), the fecal flora structure changed tremendously during the two-week administration of B. adolescentis as suggested by the results of the one-way analysis of variance and Duncan’s multiple range test (Figure 7A and Figure 8). As expected, the relative abundance of Bifidobacterium increased remarkably from 0.24% to an average of 4.62% (shown in yellow in the histogram) (p < 0.01). Meanwhile, the level of Lactobacillus was significantly increased from 21.54% to an average ratio of 37.5% (shown in fuchsia in the histogram, Figure 8) (p < 0.05) during the B. adolescentis treatment periods. In addition, the levels of many other bacteria, such as Alistipes, Dorea and Clostridium, decreased after two weeks of B. adolescentis treatment.
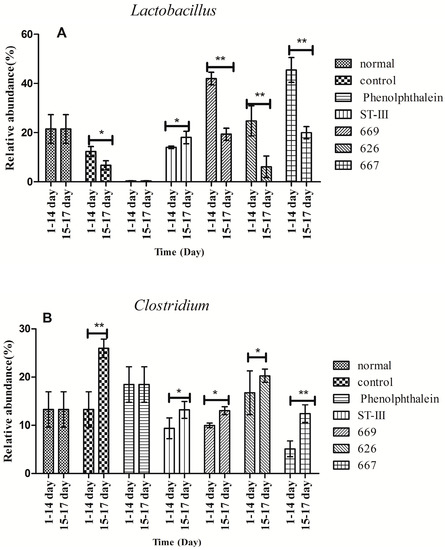
Figure 8.
Changes in abundance of selected genera at different periods. * p < 0.05; ** p < 0.01 according to one-way analysis of variance and Duncan’s multiple range test.
After loperamide-induced constipation (Days 15 to 17), the fecal flora structure changed slightly compared with that from the first 14 days (Figure 7B). The levels of Lactobacillus (Figure 7) and Bifidobacterium decreased in the B. adolescentis treatment groups but were still higher than that in the control group. In addition, Alistipes and Dorea tended to revert to their original levels before B. adolescentis treatment, as indicated by the cluster results between the data points of Figure 6A,E–G, and Figure 6B,E–G. The relative abundance of Clostridium showed a significantly decrease compared to the normal and phenolphthalein groups as suggested by the results of the one-way analysis of variance and Duncan’s multiple range test (Figure 8).
2.9. Characterization of Serum Parameters of Experimental Mice
The effects of B. adolescentis on constipation were further evaluated by measurement of serum parameters in the experimental mice, including MTL, Gas, SP, ET-1, SS and VIP. As shown in Table 5, no statistical differences were seen in these six indices between the B. adolescentis CCFM 626 group and the control group. In the treatment groups, the levels of MTL, Gas and SP were significantly increased, whereas the levels of ET-1, SS and VIP were significantly decreased (p < 0.05).

Table 5.
Effects of B. adolescentis on serum parameters in a mouse model of loperamide-induced constipation.
3. Discussion
Constipation is a common functional GI disorder whose main clinical symptoms include difficulty with defecation, reduced defecation frequency, dry and hard stools and a prolonged GI emptying time [27]. Patients with constipation have severe disturbances of intestinal flora and a large number of pathogen-produced nitrite amines, phenols, ammonia, azobenzene and carcinogenic substances. Direct contact with some of these harmful substances can cause inflammation of intestinal mucosal or even colon cancer. These toxins cannot be discharged in time and can be absorbed into the blood to induce breast cancer [28]. Disruption of the intestinal microflora balance may modify the intestinal barrier function and influence health. A micro-ecological view showed that a sufficient quantity of Bifidobacteria in the gut can ferment oligosaccharides and produce acetic acid and lactic acid to promote intestinal peristalsis, excretion of feces, and alleviation of constipation [29]. The fecal wet weight, the fecal water content, the time to the first black stool defecation and the rate of intestinal charcoal propulsion are important indices with which to evaluate the function of the GI tract.
The purpose of this study was to determine whether B. adolescentis exerts a strain-specific effect on constipation and the causes of these differences. Our study revealed that the three strains of B. adolescentis exerted a strain-specific effect on constipation caused by loperamide, and these differences were mainly caused by the fundamental properties of the strains and their effects on the intestinal flora and intestinal microenvironment.
A mouse constipation model was established by administration of loperamide, which is an agonist of μ-opioid receptors that prevents the release of acetylcholine and prostaglandin, resulting in inhibition of intestinal peristalsis and prolonged retention of the intestinal contents. Therefore, loperamide-induced constipation is considered a model of spastic constipation [30]. In this study, the loperamide (control) group showed evident symptoms of constipation, including significant decreases in fecal wet weight, fecal water content, small intestinal transit rate, and time to the first black stool defecation in comparison to the normal group. These symptoms were relieved in the B. adolescentis CCFM 669 and 667 treatment groups, but not in the B. adolescentis CCFM 626 treatment group. This finding indicates that B. adolescentis has a strain-specific effect on constipation.
There are three modes of small bowel movement: tension contraction, segmentation movement and peristalsis. The movement of intestinal contents is mainly promoted by peristalsis, which is detected by measuring the small intestine propulsion rate. A faster small intestine propulsion rate is conducive to the discharge of feces; otherwise, constipation is likely [31]. Our study revealed that B. adolescentis CCFM 667 and 669 relieve constipation by improving the small intestine propulsion rate. Although the high dose of B. adolescentis CCFM 626 did not alleviate constipation, the low dose could, which showed that a larger dose did not lead to a better effect; therefore, screening for the optimum dose was the next task. Previous studies have confirmed the efficacy of treatment with B. lactis DN-173010, B. longum 46 (DSM 14583), B. longum 2C (DSM 14579) and B. lactis HN019 (DR10TM) on the frequency of defecation and stool consistency [32,33]. All of these studies indicated that Bifidobacteria could relieve constipation by improving the fecal status and the rate of intestinal propulsion.
The whole intestinal transit time is reflected by the time to the first black stool defecation, which is the sum of the transit times of the small and large intestine. The main cause of constipation is the long retention time of feces in the intestinal tract and excessive absorption of water. The time to the first black stool defecation is an important indicator to judge both the effect of treatment and prognosis. A shorter time to the first black stool defecation indicates a better effect; otherwise, the effect will be worse. Our study showed that the time to the first black stool defecation was the shortest in the group that received a high dose of B. adolescentis CCFM 667 or 669 or phenolphthalein and the longest in the control and B. adolescentis CCFM 626 groups. Thus, CCFM 667 and 669 could relieve constipation, whereas CCFM 626 could not. Published data hold that supplementation of Bifidobacterium is associated with a slower whole intestinal transit time in the human gut [34], a finding that was confirmed by Favretto [35].
The water content of feces and the small intestinal transit rate were significantly greater in the low-dose B. adolescentis CCFM 669 group than those in the low-dose B. adolescentis CCFM 667 group, and the time to the first black stool defecation was similar to that in the B. adolescentis CCFM 667 group. These results revealed that B. adolescentis CCFM 667 relieves constipation by improving the colonic transit time and that B. adolescentis CCFM 669 relieves constipation by promoting small intestinal peristalsis. Furthermore, B. adolescentis CCFM 667 did not have adhesion ability, so it passed into the large intestine with chyme. It would quickly produce SCFAs from carbohydrates that could not be absorbed by the small intestine and would stimulate colon peristalsis, ultimately accelerating colonic transit time. The action of B. adolescentis CCFM 669 was completely contrary to that of B. adolescentis CCFM 667. It partly colonized in the small intestine, promoted intestinal peristalsis and improved the small intestinal transit rate. The remainder passed into the large intestine with chyme and played the same role as B. adolescentis CCFM 667.
The changes in the intestinal flora in mice were mainly detected by the changes in the bacteria in their feces. How do mouse intestinal flora change before and after constipation, and after supplementation with B. adolescentis? The results clearly showed that the composition of the gut microbiota community both before and after intervention was dominated by two phyla, Bacteroidetes (which includes Bacteroides and Odoribacter) and Firmicutes (which includes Clostridium, Streptococcus, Lactobacillus and Dorea), whereas Actinobacteria (mainly Bifidobacterium), Proteobacteria and Tenericutes played minor roles. In general, at the phylum level, the ratio of Firmicutes to Bacteroidetes increased and the abundance of other phyla decreased in the B. adolescentis treatment groups. Similarly, at the genus level, B. adolescentis did not increase the abundance of Bifidobacterium in Actinobacteria but did increase the relative abundance of Lactobacillus in Firmicutes and reduced the abundance of Clostridium. Some species of the Clostridium genera are associated with the emergence of intestinal complications such as colitis [36], necrotizing enterocolitis and gastroenteritis [37]. Therefore, the reduction of harmful Clostridium after B. adolescentis ingestion indicates a potential benefit for gut health. The intestinal flora comprises a complex and dynamic bacterial community that plays an important role in human health [38]. Previous studies described the changes in the gut microbiota of patients with constipation, which are characterized by a relative decrease in the Bifidobacterium and Lactobacillus species and an increase in potentially pathogenic microorganisms [39]. Previous studies reported no significant differences in the microbial profiles of patients with constipation and healthy subjects [4]. The conflicting results might also relate to differences in the definition of constipation in the various studies. We measured objective indices such as the whole intestinal transit time rather than more subjective indices. For example, Khalif et al. [39] observed little difference in the gut bacteria between healthy subjects and those with constipation, but only constipated subjects with a severely prolonged transit time were considered in the comparison. It is possible that symptom-based diagnosis such as that with a subjective index may not be sufficient to differentiate gut microbial differences between subjects with and without constipation. In brief, altering the composition of the gut microbiota community in patients with constipation to resemble that in normal subjects may decrease constipation-associated changes in GI function.
Microbes do not simply remain within the gut; they must be metabolically active to survive in that environment. Hence, B. adolescentis would have an influence not only on the composition and numbers of various microbes, but also on their fermentation products, such as SCFAs. SCFAs are the final product of microbial fermentation in the mammalian colon, in which they represent the major organic anions. The ability of the bacteria to produce SCFAs is influenced by the number of bacteria, the pH and the substrate. The total amount and proportion of SCFAs produced by different substrates differ [40]. SCFAs are involved in important physiological metabolic processes in vivo [41]. Thus, measuring the content of SCFAs in the intestine has become the main method to detect differences between strains in the relief of constipation. Our study showed that B. adolescentis increased the concentration of propionic acid and butyric acid to relieve constipation. Propionate is primarily used in the liver and has been suggested as a potential modulator of cholesterol synthesis and a precursor in lipo-neogenesis, which may influence body weight [42]. Butyrate is the preferred energy source for colonocytes and thus is extensively metabolized by the colon. Butyrate and other SCFAs have also been shown to provide protection against colon cancer [43]. Compared with other similar investigations, some have observed significantly higher levels of isobutyrate in samples from subjects with constipation than in those from healthy subjects [4], and others reported increases in butyrate levels [44]. These phenomena might be related to diet because SCFAs originate from the degradation of polysaccharides, which increases when the system operates with longer retention times [45]. Our study incorporated dietary and gut transit time, and the results are more reliable. Further studies are also needed to provide direct evidence for increased propionate and butyrate acid and to examine the possible role of propionate and butyrate in the pathogenesis of constipation. As to the molecular mechanism of the beneficial effects of SCFAs, butyrate was reported to be a physiological regulator of major pathways of colonic epithelial cell maturation including cell cycle arrest, lineage-specific differentiation, and apoptosis [46]. In addition, increased expression of genes that favor apoptosis (Bax, and Bak) and a reduced expression of counter-players that prevent apoptosis (Bcl-2, and Bcl-XL) were all reported to be the molecular mechanisms of SCFAs that mediate colorectal cancer [47]. Compared to the in-depth investigation of the molecular mechanism of SCFAs intervention in colorectal cancer, few studies have reported molecular evidence of the mediation of SCFAs in constipation. It is believed that SCFAs may stimulate water and electrolyte absorption, potentiate the proliferation of epithelial cells, influence GI motility, increase mesenteric blood flow and exert other physiological effects [41]. However, the molecular mechanism still needs to be investigated.
GI hormones such as MTL, Gas, SP, ET, SS, and VIP play important roles in the regulation of GI motility [48] and have been implicated to different extents in normal and pathophysiological situations. MTL, Gas and SP are excitatory peptide neurotransmitters, whereas ET, SS and VIP are inhibitory peptide neurotransmitters. In this study, the control group showed evident constipation symptoms in relation to neurotransmitters, including significantly decreased levels of MTL, Gas and SP and increased levels of ET, SS and VIP in comparison to the normal group and the B. adolescentis CCFM 669 and 667 groups, whereas no statistical differences were seen between the B. adolescentis CCFM 626 group and the control group. B. adolescentis not only improved the symptoms of constipation and changed the mice’s intestinal flora and microenvironment, but also affected the GI neurotransmitters related to constipation. MTL influences the transport of water and electrolytes, promotes gastric contractions and small intestine segmental movement, accelerates intestinal transfer time and increases colon movement. Gas stimulates the secretion of gastric acid and pepsinogen and promotes the growth of digestive tract mucosa, the contraction of the GI smooth muscle and the relaxation of the pyloric sphincter. SP adjust the contraction of the GI tract, intestinal motility and gastric acid secretion. Therefore, the promotion of the serum levels of MTL, Gas and SP accelerates intestinal peristalsis and the transport of contents. In our study, the levels of MTL, Gas and SP in the control group were significantly decreased compared with those in the normal and B. adolescentis treatment groups. This phenomenon confirmed that the decreases in MTL, Gas and SP might be amongst the causes of constipation. These results are consistent with those of several other reports [49,50,51].
ET, as a multifunctional peptide, can exert important effects on numerous aspects of cardiovascular, neuroendocrine and gastrointestinal function [52]. Meanwhile, it plays an important role in the stability of vascular tension and maintains the basic cardiovascular system. SS inhibits the release of GI hormones, such as MTL and Gas, and the secretion of gastric acid, trypsin and amylase. Vecht [53] revealed that SS could slow the small intestinal transit time significantly, whether during eating or fasting. VIP is a polypeptide composed of 28 amino acids whose function is to relax the GI tract and GI sphincter, and it significantly promotes the colon cancer induced by carcinogens in mice. Fasth et al. [54] held that VIP is an important factor in the production of descending inhibition, resulting in slow transmission. Studies have found that the levels of ET, SS and VIP in control groups were higher than those in normal and B. adolescentis treatment groups, which means that B. adolescentis influenced the level of GI hormones, an index that could reflect the status of constipation.
4. Materials and Methods
4.1. Chemicals and Reagents
Kits used to measure the levels of MTL, Gas, SP, ET, SS, and VIP were purchased from Wen LE Bioengineering Institute (Shanghai, China). MRS broth was purchased from Qingdao Hopebio Company (Qingdao, China). Dulbecco’s minimum essential medium–high glucose (DMEM), fetal bovine serum, penicillin, and streptomycin were obtained from HyClone (Logan, UT, USA).
Loperamide hydrochloride, (2 mg per capsule) was purchased from Wuxi big drugstore (Xi’an Janssen Pharmaceutical Ltd., Xi’an, China). Loperamide was dissolved in distilled water to reach its final concentration of 1 mg/mL. Phenolphthalein (0.1 g, 100 tablets) was dissolved in distilled water to a final concentration of 7 mg/mL.
For activated carbon meal solution, gum arabic 100 g and water 800 mL were boiled until the solution was transparent. Activated carbon 50 g was then added and boiled three times. After the solution was cool, it was diluted with water to 1000 mL, stored at 4 °C, and mixed before use. All other chemicals and reagents used in this study were of analytical grade. The Bifidobacterium species used in this study were obtained from different samples in different regions and kept in the culture collection of food microorganisms of Jiangnan University (Wuxi, China).
4.2. Bacterial Strain and Culture Conditions
The Bifidobacterium species used in this study, including B. adolescentis CCFM 626, 667 and 669, were stored at −80 °C in 30% (v/v) glycerol broth and then cultured under anaerobic conditions for 24 to 48 h at 37 °C in modified MRS (cMRS) broth supplemented with 0.05% w/v l-cysteine-HCl (Merck) [55]. To prepare active cultures for all experiments, all strains were consecutively reactivated in an anaerobic atmosphere at least three times using 3% (v/v) inoculum in cMRS broth at 37 °C for 24 to 48 h before use.
To use these strains in the animal experiments, the bacterial culture was centrifuged at 3000× g for 10 min, washed twice with sterile saline solution, and centrifuged again at 3000× g for 10 min to obtain the bacteria. The bacteria were lyophilized using skimmed milk as a protectant and stored at −20 °C. The viability of the freeze-dried cells was measured by colony counting before the animal experiments. The bacterial suspensions were prepared daily by diluting 4 × 1010 CFU of freeze-dried B. adolescentis in sterile saline solution before the animal experiments.
4.3. Growth Curve of B. adolescentis
An early stationary phase culture of each B. adolescentis strain (about 107 CFU/mL) was inoculated into fresh cMRS medium. The cultures were grown in an anaerobic atmosphere at 37 °C, and OD600 values were measured every 2 h. The growth curve of each B. adolescentis culture was made with the incubation time as the abscissa and the corresponding absorbance values as the ordinate.
4.4. Tolerance Capacity of B. adolescentis to Simulated Gastric and Small Intestine Juices
Simulated gastric juices were prepared with 3 g/L pepsin (1:10,000, Sigma, St. Louis, MO, USA) in phosphate-buffered saline solution (PBS; pH 3.0) and filtered with a 0.22-μm filter before use. Simulated small intestinal juices were prepared with 1 g/L trypsin (1:250, Sigma, St. Louis, MO, USA) in PBS (pH 8.0) and filtered with a 0.22-μm filter before use. Washed cell suspensions (1 mL) of B. adolescentis were mixed with 9 mL of the simulated gastric juice and cultured at 37 °C in anaerobic conditions. The samples were periodically withdrawn at 0, 1, 2 and 3 h, and a 1-mL sample was then transferred into 9 mL of sterile saline solution by serial dilutions. A 0.1-mL portion of the suspension was cultured anaerobically on a cMRS agar plate at 37 °C for 48 h. Strains sampled at 0 h were used as a control. CFUs were counted, and the survival rate in gastric juices was determined [56]. Washed cell suspensions (1 mL) sampled at 3 h in gastric juice were then inoculated into 9 mL of sterile small intestine juice and cultured in anaerobic conditions at 37 °C. A sample volume of 1 mL was taken at 2, 4 and 8 h, transferred into 9 mL of sterile saline solution and mixed. The survival rate in the intestinal juice was then detected with the same manipulation.
where N1 = total viable count of B. adolescentis strains after treatment with simulated GI juices and N0 = total viable count of B. adolescentis strains before treatment.
4.5. Determination of Adhesion Properties of Bifidobacterium spp. to HT-29 Cells In Vitro
The adherence of B. adolescentis to intestinal epithelial cells of the HT-29 cell line was examined as described by Coconnier et al. [57]. In brief, HT-29 cells were purchased from the cell bank of the type culture collection of the Chinese Academy of Sciences (Shanghai, China). The cells were grown in DMEM containing 10% heat-inactivated (30 min at 56 °C) fetal bovine serum, 100 U/mL penicillin and 100 U/mL streptomycin at 37 °C in a humidified atmosphere of 5% CO2 and 95% air. For the adherence assays, the HT-29 cells were cultured in six-well tissue culture plates without antibiotics. Approximately 10 h after incubation, the HT-29 cells were carefully collected and washed three times with sterile PBS (pH 7.8), and 1 mL fresh DMEM and 1 mL of Bifidobacteria suspension (107 CFU/mL in DMEM) were then added. The inoculated cultures were incubated for 3 h at 37 °C in 5% CO2 and 95% air. The treated cells were washed three times with sterile PBS (pH 7.8), fixed in methanol, gram-stained and observed microscopically. The strain L. plantarum ST-III was used as a control.
4.6. Animal Experiments
4.6.1. Animals
Seven-week-old male BALB/c mice (initial body weight, 18 to 21 g) were purchased from Jiangsu Laboratory Animal Centre (Suzhou China) and used in all of the experiments; they were fed under standard conditions at a room temperature of 25 ± 2 °C and humidity of 50% ± 5% with a 12-h light–dark cycle. All protocols for this study were approved by the Ethics Committee of Jiangnan University, China (JN. No20150326-0110-21). The procedures were carried out in accordance with the European Community guidelines (Directive 2010/63/EU) for the care and use of experimental animals.
4.6.2. Induction of Constipation and Experimental Design
After adaptation to the environment for 7 days, mice were fed with standard diet (Nanjing Qinglong animal farm, Nanjing, China), and 88 mice were used to investigate the strain-specific preventive effects of B. adolescentis in constipation. The mice were randomly divided with eight mice in each of 11 groups: the normal group (saline solution or distilled water), the constipation control group (loperamide 10 mg/kg body weight), two groups with different doses of B. adolescentis CCFM 626, 667 or 669 (i.e., 1 × 1010 CFU or 1 × 108 CFU B. adolescentis 626, 667 or 669; for a total of six groups), a positive drug control group (phenolphthalein 70 mg/kg body weight [58]) and two positive control groups (L. plantarum ST-III at either a high dose of 1 × 1010 CFU or a low dose of 1 × 108 CFU).
The experiment was designed as follows. All animals were fasted overnight (approximately 18 h) before the first experiment (water was not restricted). The control groups were given normal saline solution once per day via gavage for 17 days. The high-dose B. adolescentis CCFM 626, CCFM 667 and CCFM 669 and L. plantarum ST-III groups and the low-dose B. adolescentis CCFM 626, CCFM 667 and CCFM 669 and L. plantarum ST-III groups received 0.25 mL of 4 × 1010 CFU/mL or 4 × 108 CFU/mL bacterial suspension, respectively, in the same manner as the treatment groups for 14 days, and the positive drug control group was treated with a 70 mg/kg body weight dose of phenolphthalein once per day for 14 days. Phenolphthalein is a laxative drug that acts to stimulate intestinal peristalsis. It is typically prescribed for the relief of constipation and for the management of neurogenic bowel dysfunction [59]. The control and treatment groups were treated with loperamide (10 mg/kg body weight; 0.25 mL) via gavage from Day 15 to 17 to induce constipation [60]. Fecal water content, the time to the first black stool defecation, GI transit (the rate at which carbon powder is propelled in the small intestine), SCFAs and gut microbiota in feces were measured. The excreted feces of the individual mice were collected in a tube and their wet weight was measured immediately after excretion every day during the experimental period. The feces were then thoroughly dried and weighed, and the water content of the fecal pellet was calculated as the difference between its wet and dry weights. The specific arrangements of the experiment are shown in Table 6.

Table 6.
Specifics of the animal experiment.
4.6.3. Measurement of Defecation Status of Mice
During the 17-day experimental period, each mouse was moved into a clean, empty cage every day for 3 h, during which stool samples were collected, counted, and weighed. The water content was calculated as the difference between the wet and dry weights of the stool as described previously [61]. This measurement was performed to determine the effect of B. adolescentis in improving intestinal motility and feces status.
4.6.4. Determination of the Time to the First Black Stool Defecation
The mice were fasted overnight with water provided; after 18 h, the mice were treated with loperamide (10 mg/kg body weight, 0.25 mL) or normal saline solution (normal group), and 1 h later, all mice were treated with activated carbon meal by the same method. The animals were then immediately moved into clean, empty individual cages and allowed food and water ad libitum. The length of time from the administration of activated carbon meal to the appearance of darkened feces was recorded.
4.6.5. Determination of GI Transit
The small intestinal transit time was measured with the method of Nagakura et al. [62], with minor modification. Mice were fasted overnight (approximately 18 h, with water provided) from Day 17 at 6:00 p.m. After 16 h, 0.25 mL of activated carbon meal was administered to each mouse via gavage. One hour later, the mice were killed with light ether anesthesia, and their blood was collected in tubes for almost 2 h and centrifuged at 3000× g for 15 min to obtain serum. The abdomen was opened, and the entire small intestine starting from the pylorus to the cecum was carefully removed and placed on blotting paper. The distance over which the activated carbon had travelled and the total length of the small intestine were measured. The GI transit of each mouse was calculated as the percentage of the distance travelled by the activated carbon meal relative to the total length of the small intestine.
4.6.6. Determination of SCFAs in Feces
The concentrations of SCFAs were determined according to Mao et al. by gas chromatography mass spectrometry (GCMS-QP2010 Ultra system, Shimadzu Corporation, Kyoto, Japan) [63], which was equipped with a Rtx-Wax column (30 m × 0.25 μm × 0.25 μm). The carrier gas was helium, the flow rate was 2 mL/min, the split ratio was 10:1 and the volume of sampling was 1 μL. The injection temperature was 240 °C, and the GC temperature program was as follows: the initial temperature of 100 °C was increased to 140 °C at the rate of 7.5 °C/min, and then was increased by 60 °C/min to 200 °C, which was maintained for 3 min. The ionization temperature was 220 °C. Mass scanning was performed in full scan mode. A standard curve was generated by the external standard method, and the concentrations of SCFAs were calculated according to the standard curve in units of μmol/g sample. Fecal samples separately collected from each mouse, were subjected to soaking, acidification, and extraction using saturated NaCl solution, sulfuric acid (10%), and diethyl ether, respectively, and the concentration of total SCFAs consisting of acetic acid, propionic acid and butyric acid was calculated.
4.6.7. MiSeq Genome Sequencing Analysis of Community Structures
Metagenomic DNA from the fecal samples was obtained using a FastDNA Spin Kit for Soil (MP Biomedical, catalog No. 6560-200) following the manufacturer’s instructions. The V4 region of the 16S rRNA was amplified from microbial genome DNA (forward primer, 5′-AYTGGGYDTAAAGNG-3′; reverse primer, 5′-TACNVGGGTATCTAATCC-3′) as described previously by polymerase chain reaction [64]. The products were excised from a 1.5% agarose gel, purified by Gene Clean Turbo (MP Biomedical, Beijing, China) and quantified with a Quant-iT PicoGreen dsDNA Assay Kit (Life Technologies, Carlsbad, CA, USA) according to the manufacturer’s instructions. Libraries were prepared using TruSeq DNA LT Sample Preparation Kit (Illumina, San Diego, CA, USA) and sequenced for 500 + 7 cycles on an Illumina MiSeq using the MiSeq Reagent Kit. The read length is 2 × 250 bp.
After sequencing, 16S rRNA reads were analyzed using the QIIME pipeline. [65] The raw sequences were screened. The short lengths (<200 bp) were then removed, and the pair-end reads that overlapped longer than 10 bp and without any mismatch were assembled according to their overlap sequence. For the high quality sequences obtained by assembling, the sequences with similarity greater than 97% were defined as an OTU by QIIME software (http://qiime.sourceforge.net/), and finally, representative sequences of OTUs were used to identify a species. The beta diversity of microbial communities was investigated by visual assessment using principle coordinate analysis plots and by an analysis of similarity calculated based on weighted UniFrac distances (QIIME) according to one-way nonparametric multivariate analysis of variance.
4.6.8. Determination of MTL, Gas, ET, SS, SP and VIP Levels in Serum
MTL, Gas, ET, SS, SP and VIP levels in the serum were determined by an enzyme-linked immunosorbent assay (ELISA) instrument according to the manufacturer’s instructions (Microplate Spectrophotometer Multiskan Go, Thermo Scientific, Waltham, MA, USA). A standard curve was generated from the concentration of the standard sample and the corresponding OD450 values. The concentration of MTL, Gas, ET, SS, SP and VIP was calculated according to the standard curve in units of ng/L. The main experimental steps in this study were as follows: first, samples (serum, standard sample, biotin-labeled secondary antibody and ELISA reagent) were added according to the manufacturer’s instructions, maintained for 1 h at 37 °C, and then washed 5 times. Then color reagents A and B were added and incubated for 10 min at 37 °C. Finally, the termination reagent was added, OD450 values were read, and the concentrations of MTL, Gas, ET, SS, SP and VIP were calculated.
4.7. Statistical Analysis
The data are presented as mean ± SD for each group. The differences between the mean values for the groups were analyzed by one-way analysis of variance with Duncan’s multiple range test. A p value of less than 0.05 was considered to indicate statistical significance. All statistical analyses were performed with GraphPad Prism 5 (GraphPad Software Inc., San Diego, CA, USA) and OriginPro 8.5 (OriginLab Corporation, Northampton, MA, USA).
5. Conclusions
In conclusion, by measuring related constipation indicators (stool status, water content of defecation, GI transit rate and time to the first black stool defecation), it was found that B. adolescentis CCFM 669 and 667 could alleviate constipation. In addition, B. adolescentis CCFM 667 exerted an effect on slow transit constipation, whereas B. adolescentis CCFM 626 could not. Furthermore, B. adolescentis CCFM 669 and 667 increased the ratio of Firmicutes to Bacteroidetes at the phylum level, increased the abundance of Lactobacillus and reduced the level of Clostridium at the genus level, and increased the amounts of propionic acid and butyric acid in feces of constipated mice. These results revealed that B. adolescentis (CCFM 669, CCFM 667 and CCFM 626) exerted strain-specific effects on relieving constipation. The main causes of the strain-specific effects on constipation might be the differences in the growth rate and the adhesion properties of the different strains, which exerted different effects on gut flora, the intestinal microenvironment and the related GI peptide neurotransmitter.
Acknowledgments
This work was supported by the Natural Science Foundation of China (No. 31671839), the Program of Introducing Talents of Discipline to Universities (B07029), and the Fundamental Research Funds for the Central Universities (JUSRP51501), a project funded by the Priority Academic Program Development of Jiangsu Higher Education Institutions and the Program of Collaborative Innovation Centre of Food Safety and Quality Control in Jiangsu Province.
Author Contributions
Linlin Wang, Gang Wang and Hao Zhang conceived and designed the experiments; Linlin Wang and Lujun Hu performed the experiments; Linlin Wang, Gang Wang and Hao Zhang analyzed the data; Qi Xu, Boxing Yin, Dongsheng Fang, Jianxin Zhao and Wei Chen contributed reagents/materials/analysis tools; and Linlin Wang and Gang Wang wrote the paper.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Stern, T.; Davis, A.M. Evaluation and treatment of constipation. JAMA Clin. Guidel. Synop. 2016, 315, 192–193. [Google Scholar]
- McQuaid, K.R.; Katzung, B.G.; Masters, S.B. Drugs Used in the Treatment of Gastrointestinal Diseases. In Basic & Clinical Pharmacology, 12th ed.; Trevor, A.J., Ed.; McGraw-Hill: New York, NY, USA, 2012. [Google Scholar]
- Xu, J.; Zhou, X.; Chen, C.; Deng, Q.; Huang, Q.; Yang, J.; Yang, N.; Huang, F. Laxative effects of partially defatted flaxseed meal on normal and experimental constipated mice. BMC Complement. 2012, 12, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Kang, D.; di Baise, J.K.; Ilhan, Z.E.; Crowell, M.D.; Rideout, J.R.; Caporaso, J.G.; Rittmann, B.E.; Krajmalnik-Brown, R. Gut microbial and short-chain fatty acid profiles in adults with chronic constipation before and after treatment with lubiprostone. Anaerobe 2015, 33, 33–41. [Google Scholar] [CrossRef] [PubMed]
- Zoppi, G.; Cinquetti, M.; Luciano, A.; Benini, A.; Muner, A.; Bertazzoni Minelli, E. The intestinal ecosystem in chronic functional constipation. Acta Paediatr. 1998, 87, 836–841. [Google Scholar] [CrossRef] [PubMed]
- De Vrese, M.; Marteau, P.R. Probiotics and Prebiotics: Effects on Diarrhea. J. Nutr. 2007, 137, 803S–811S. [Google Scholar] [PubMed]
- Gopal, P.K.; Prasad, J.; Smart, J.; Gill, H.S. In vitro adherence properties of Lactobacillus rhamnosus DR20 and Bifidobacterium lactis DR10 strains and their antagonistic activity against an enterotoxigenic Escherichia coli. Int. J. Food Microbiol. 2001, 67, 207–216. [Google Scholar] [CrossRef]
- Quigley, E.M. The enteric microbiota in the pathogenesis and management of constipation. Best Pract. Res. Clin. Gastroenterol. 2011, 25, 119–126. [Google Scholar] [CrossRef] [PubMed]
- Maurice, C.F.; Haiser, H.J.; Turnbaugh, P.J. Xenobiotics shape the physiology and gene expression of the active human gut microbiome. Cell 2013, 152, 39–50. [Google Scholar] [CrossRef] [PubMed]
- Malaguarnera, G.; Leggio, F.; Vacante, M.; Motta, M.; Giordano, M.; Biondi, A.; Basile, F.; Mastrojeni, S.; Mistretta, A.; Malaguarnera, M.; et al. Probiotics in the gastrointestinal diseases of the elderly. J. Nutr. Health Aging 2012, 16, 402–410. [Google Scholar] [CrossRef] [PubMed]
- Singh, N.; Arioli, S.; Wang, A.; Villa, C.R.; Jahani, R.; Song, Y.S.; Mora, D.; Guglielmetti, S.; Comelli, E.M. Impact of Bifidobacterium bifidum MIMBb75 on mouse intestinal microorganisms. FEMS Microbiol. Ecol. 2013, 85, 369–375. [Google Scholar] [CrossRef] [PubMed]
- O’mahony, L.; Mccarthy, J.; Kelly, P.; Hurley, G.; Luo, F.; Chen, K.; O’sullivan, G.C.; Kiely, B.; Collins, J.K.; Shanahan, F.; et al. Lactobacillus and bifidobacterium in irritable bowel syndrome: Symptom responses and relationship to cytokine profiles. Gastroenterology 2005, 38, 1–8. [Google Scholar] [CrossRef]
- Kassinen, A.; Krogius-Kurikka, L.; Mäkivuokko, H.; Rinttilä, T.; Paulin, L.; Corander, J.; Malinen, E.; Apajalahti, J.; Palva, A. The Fecal Microbiota of Irritable Bowel Syndrome Patients Differs Significantly From That of Healthy Subjects. Gastroenterology 2007, 131, 24–33. [Google Scholar] [CrossRef] [PubMed]
- Quigley, E.M. Probiotics in the Management of Functional Bowel Disorders Promise Fulfilled? Gastroenterol. Clin. N. Am. 2012, 41, 805–810. [Google Scholar] [CrossRef] [PubMed]
- Urita, Y.; Goto, M.; Watanabe, T.; Matsuzaki, M.; Gomi, A.; Kano, M.; Miyazaki, K.; Kaneko, H. Continuous consumption of fermented milk containing Bifidobacterium bifidum YIT 10347 improves gastrointestinal and psychological symptoms in patients with functional gastrointestinal disorders. Biosci. Microbiota Food Health 2015, 34, 37–44. [Google Scholar] [CrossRef] [PubMed]
- Ojetti, V.; Ianiro, G.; Tortora, A.; D’Angelo, G.; di Rienzo, T.A.; Bibbo, S.; Migneco, A.; Gasbarrini, A. The Effect of Lactobacillus reuteri Supplementation in Adults with Chronic Functional Constipation: A Randomized, Double-Blind, Placebo-Controlled Trial. J. Gastrointest. Liver Dis. 2014, 23, 387–391. [Google Scholar]
- Kim, S.; Choi, S.C.; Park, K.S.; Park, M.I.; Shin, J.E.; Lee, T.H.; Jung, K.W.; Koo, H.S.; Myung, S.; Constipation Research Group of Korean Society of Neurogastroenterology and Motili. Change of Fecal Flora and Effectiveness of the Short-term VSL#3 Probiotic Treatment in Patients with Functional Constipation. J. Neurogastroenterol. Motil. 2015, 21, 111–120. [Google Scholar] [PubMed]
- Waitzberg, D.L.; Quilici, F.A.; Michzputen, S.; Passos, M.D.C.F. The effect of probiotic fermented milk that includes Bifidobacterium lactis CNCM I-2494 on the reduction of gastrointestinal discomfort and symptoms in adults: A narrative review. Nutr. Hosp. 2015, 32, 501–509. [Google Scholar] [PubMed]
- Riezzo, G.; Orlando, A.; D’Attoma, B.; Guerra, V.; Valerio, F.; Lavermicocca, P.; de Candia, S.; Russo, F. Randomised clinical trial: Efficacy of Lactobacillus paracasei-enriched artichokes in the treatment of patients with functional constipation—A double-blind, controlled, crossover study. Aliment. Pharmacol. Ther. 2012, 35, 441–450. [Google Scholar] [CrossRef] [PubMed]
- Mazlyn, M.M.; Nagarajah, L.H.L.; Fatimah, A.; Norimah, A.K.; Goh, K.L. Effects of a probiotic fermented milk on functional constipation: A randomized, double-blind, placebo-controlled study. J. Gastroenterol. Hepatol. 2013, 28, 1584. [Google Scholar] [CrossRef] [PubMed]
- MacDonald, R.S.; Wagner, K. Influence of dietary phytochemicals and microbiota on colon cancer risk. J. Agric. Food Chem. 2012, 60, 6728–6735. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Huang, X.E. Efficacy of Bifidobacterium tetragenous Viable Bacteria Tablets for Cancer Patients with Functional Constipation. Asian Pac. J. Cancer Prev. 2014, 15, 10241–10244. [Google Scholar] [CrossRef] [PubMed]
- Marteau, P.; Cuillerier, E.; Meance, S.; Gerhardt, M.F.; Myara, A.; Bouvier, M.; Bouley, C.; Tondu, F.; Bommelaer, G.; Grimaud, J.C. Bifidobacterium animalis strain DN-173 010 shortens the colonic transit time in healthy women: A double-blind, randomized, controlled study. Aliment. Pharmacol. Ther. 2002, 16, 587–593. [Google Scholar] [CrossRef] [PubMed]
- Shimoyama, T.; Takahashi, R.; Kimura, M.; Fukuda, Y. Study of the mechanisms of a Japanese traditional fermented medicine in the improvement of constipation. J. Gastroenterol. Hepatol. 2015, 30, 53–59. [Google Scholar] [CrossRef] [PubMed]
- Dimidi, E.; Christodoulides, S.; Fragkos, K.C.; Scott, S.M.; Whelan, K. The effect of probiotics on functional constipation in adults: A systematic review and meta-analysis of randomized. Am. J. Clin. Nutr. 2014, 100, 1075–1084. [Google Scholar] [CrossRef] [PubMed]
- Hopkins, M.J.; Cummings, J.H.; Macfarlane, G.T. Inter-species differences in maximum specific growth rates and cell yields of Bifidobacteria cultured on oligosaccharides and other simple carbohydrate sources. J. Appl. Microbiol. 1998, 85, 381–386. [Google Scholar] [CrossRef]
- Bharucha, A.E.; Pemberton, J.H.; Locke, G.R., III. American Gastroenterological Association technical review on constipation. Gastroenterology 2013, 144, 218–238. [Google Scholar] [CrossRef] [PubMed]
- Aichbichler, B.W.; Wenzl, H.H.; Santa Ana, C.A.; Porter, J.L.; Schiller, L.R.; Fordtran, J.S. A comparison of stool characteristics from normal and constipated people. Dig. Dis. Sci. 1998, 43, 2353–2362. [Google Scholar] [CrossRef] [PubMed]
- Sohji, Y.; Kawashima, K.; Shimizu, M. Pharmacological studies of loperamide, an anti-diarrheal agent II. Effects on peristalsis of the small intestine and colon in guinea pigs. Folia Pharmacol. Jpn. 1978, 74, 155–163. [Google Scholar] [CrossRef]
- Salminen, S.; Bouley, C.; Boutron-Ruault, M.C.; Cummings, J.H.; Franck, A.; Gibson, G.R.; Isolauri, E.; Moreau, M.C.; Roberfroid, M.; Rowland, I. Functional food science and gastrointestinal physiology and function. Br. J. Nutr. 1998, 80, S147–S171. [Google Scholar] [CrossRef] [PubMed]
- Van der Werf, M.J.; Venema, K. Bifidobacteria: Genetic modification and the study of their role in the colon. J. Agric. Food Chem. 2001, 49, 378–383. [Google Scholar] [CrossRef] [PubMed]
- De Milliano, I.; Tabbers, M.M.; van der Post, J.A.; Benninga, M.A. Is a multispecies probiotic mixture effective in constipation during pregnancy? ‘A pilot study’. Nutr. J. 2012, 11, 2–6. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, M.; Prasad, J.; Gill, H.; Stevenson, L.; Gopal, P. Impact of consumption of different levels of Bifidobacterium lactis HN019 on the intestinal microflora of elderly human subjects. J. Nutr. Health Aging 2007, 11, 26–31. [Google Scholar] [PubMed]
- Waller, P.A.; Gopal, P.K.; Leyer, G.J.; Ouwehand, A.C.; Reifer, C.; Stewart, M.E.; Miller, L.E. Dose-response effect of Bifidobacterium lactis HN019 on whole gut transit time and functional gastrointestinal symptoms in adults. Scand. J. Gastroenterol. 2011, 46, 1057–1064. [Google Scholar] [CrossRef] [PubMed]
- Favretto, D.C.; Pontin, B.; Moreira, T.R. Effect of the consumption of a cheese enriched with probiotic organisms (Bifidobacterium lactis bi-07) in improving symptoms of constipation. Arq. Gastroenterol. 2013, 50, 196–201. [Google Scholar] [CrossRef] [PubMed]
- Vaishnavi, C. Clinical spectrum & pathogenesis of Clostridium difficile associated diseases. Indian J. Med. Res. 2010, 131, 487–499. [Google Scholar] [PubMed]
- Kleessen, B.; Sykura, B.; Zunft, H.-J.; Blaut, M. Effects of inulin and lactose on fecal microflora, microbial activity, and bowel habit in elderly constipated persons. Am. J. Clin. Nutr. 1997, 65, 1397–1402. [Google Scholar] [PubMed]
- Lloyd-Price, J.; Abu-Ali, G.; Huttenhower, C. The healthy human microbiome. Genome Med. 2016, 8, 2–11. [Google Scholar] [CrossRef] [PubMed]
- Khalif, I.L.; Quigley, E.M.M.; Konovitch, E.A.; Maximova, I.D. Alterations in the colonic flora and intestinal permeability and evidence of immune activation in chronic constipation. Dig. Liver Dis. 2005, 37, 838–849. [Google Scholar] [CrossRef] [PubMed]
- Ritzhaupt, A.; Wood, I.S.; Ellis, A.; Hosie, K.B.; Shirazi-Beechey, S.P. Identification and characterization of a monocarboxylate transporter (MCT1) in pig and human colon: Its potential to transport l-lactate as well as butyrate. J. Physiol. 1998, 513, 719–732. [Google Scholar] [CrossRef] [PubMed]
- Ríos-Covián, D.; Ruas-Madiedo, P.; Margolles, A.; Gueimonde, M.; de los Reyes-Gavilán, C.G.; Salazar, N. Short chain fatty acids and intestinal microflora. Front. Microbiol. 2016, 7, 185. [Google Scholar] [CrossRef] [PubMed]
- Satapati, S.; Kucejova, B.; Duarte, J.A.; Fletcher, J.A.; Reynolds, L.; Sunny, N.E.; He, T.; Nair, L.A.; Livingston, K.; Fu, X.; et al. Mitochondrial metabolism mediates oxidative stress and inflammation in fatty liver. J. Clin. Investig. 2015, 125, 4447–4462. [Google Scholar] [CrossRef] [PubMed]
- Roy, C.C.; Kien, C.L.; Bouthillier, L.; Levy, E. Short-chain fatty acids: Ready for prime time? Nutr. Clin. Pract. 2006, 21, 351–366. [Google Scholar] [CrossRef] [PubMed]
- Chassard, C.; Dapoigny, M.; Scott, K.P.; Crouzet, L.; Del’homme, C.; Marquet, P.; Martin, J.C.; Pickering, G.; Ardid, D.; Eschalier, A.; et al. Functional dysbiosis within the gut microbiota of patients with constipated-irritable bowel syndrome. Aliment. Pharmacol. Ther. 2012, 35, 828–838. [Google Scholar] [CrossRef] [PubMed]
- Macfarlane, G.T.; Macfarlane, S.; Gibson, G.R. Validation of a three-stage compound continuous culture system for investigating the effect of retention time on the ecology and metabolism of bacteria in the human colon. Microb. Ecol. 1998, 35, 180–187. [Google Scholar] [CrossRef] [PubMed]
- Mariadason, J.M.; Corner, G.A.; Augenlicht, L.H. Genetic reprogramming of colonic cell maturation induced by short chain fatty acids: Comparison with trichostatin A, sulindac, and curcumin and implications for chemoprevention of colon cancer. Cancer Res. 2000, 60, 4561–4572. [Google Scholar] [PubMed]
- Menzel, T.; Schauber, J.; Kreth, F.; Kudlich, T.; Melcher, R.; Gostner, A.; Scheppach, W.; Lührs, H. Butyrate and aspirin in combination have an enhanced effect on proliferation and apoptosis in human colorectal cancer cells. Eur. J. Cancer Prev. 2002, 11, 271–281. [Google Scholar] [CrossRef] [PubMed]
- Schulz, S.; Rocken, C.; Mawrin, C.; Weise, W.; Hollt, V.; Schulz, S. Immunocyto chemical identification of VPAC1, VPAC2, and PAC1 receptors in normal and neoplastic human tissues with subtype-specific antibodies. Clin. Cancer Res. 2004, 10, 8235–8242. [Google Scholar] [CrossRef] [PubMed]
- Peracchi, M.; Basilisco, G.; Tagliabue, R.; Terrani, C.; Locati, A.; Bianchi, P.A.; Velio, P. Postprandial gut peptide plasma levels in women with idiopathic slow transit constipation. Scand. J. Gastroenterol. 1999, 34, 25–28. [Google Scholar]
- Stern, H.P.; Stroh, S.E.; Fiedorek, S.C.; Kelleher, K.; Mellon, M.W.; Pope, S.K.; Rayford, P.L. Increased plasma levels of pancreatic polypeptide and decreased plasms levels of motilin in encopretic children. Rayford PL Pediatr. 1995, 96, 111–117. [Google Scholar]
- EL-Salhy, M.; Norrgard, O. Colonic neuroendocrine peptide levels in patients with chronic idiopathic slow transit constipation. Ups. J. Med. Sci. 1998, 103, 223–230. [Google Scholar] [CrossRef] [PubMed]
- Colucci, W.S. Myocardial Endothelin Does It Play a Role in Myocardial Failure? Circulation 1996, 93, 1069–1072. [Google Scholar] [CrossRef] [PubMed]
- Shi, Y.; Chen, Q.; Huang, Y.; Ni, L.; Liu, J.; Jiang, J.; Li, N. Function and clinical implications of short-chain fatty acids in patients with mixed refractory constipation. Colorectal Dis. 2016, 18, 803–810. [Google Scholar] [CrossRef] [PubMed]
- Fasth, S. Neuropeptides in idiopathic chronic constipation (slow transit constipation). Neurogastroenterol. Motil. 1995, 9, 143–150. [Google Scholar]
- Beerens, H. An elective and selective insulation medium for Bifidobacterium spp. Lett. Appl. Microbiol. 1990, l11, 155–157. [Google Scholar] [CrossRef]
- Bibiloni, R.; Perez, P.F.; de Antoni, G.L. An enzymatic–colourimetric assay for the quantification of Bifidobacterium. J. Food Prot. 2000, 63, 322–326. [Google Scholar] [CrossRef] [PubMed]
- Coconnier, M.; Klaenhammer, T.R.; Kerneis, S.; Bernet, M.; Servin, A.L. Protein-Mediated Adhesion of Lactobacillus acidophilus BG2FO4 on Human Enterocyte and Mucus-Secreting Cell Lines in Culture. Appl. Environ. Microbiol. 1992, 58, 2034–2039. [Google Scholar] [PubMed]
- Lin, Z.; Wu, J.; Xiao, Z. The curative effect of aloe on constipation and its primary mechanism. Guangdong Med. J. 2005, 26, 1336–1338. [Google Scholar]
- Battish, R.; Cao, G.Y.; Lynn, R.B.; Chakder, S.; Rattan, S. Heme oxygenase-2 distribution in anorectum: Colocalization with neuronal nitric oxide synthase. Am. J. Physiol. Gastrointest. Liver Physiol. 2000, 278, 148–155. [Google Scholar]
- Qian, Y.; Zhao, X.; Kan, J. Preventive effect of resistant starch on activated carbon-induced constipation in mice. Exp. Ther. Med. 2013, 6, 228–232. [Google Scholar] [PubMed]
- Lee, H.Y.; Kim, J.H.; Jeung, H.W.; Lee, C.U.; Kim, D.S.; Li, B.; Lee, G.H.; Sung, M.S.; Ha, K.C.; Back, H.I.; et al. Effects of Ficuscarica paste on loperamide-induced constipation in rats. Food Chem. Toxicol. 2012, 50, 895–902. [Google Scholar] [CrossRef] [PubMed]
- Nagakura, Y.; Naitoh, Y.; Kamato, T.; Amano, M.; Miyata, K. Compounds possessing 5-HT3 receptor antagonistic activity inhibit intestinal propulsion in mice. Eur. J. Pharmacol. 1996, 311, 67–72. [Google Scholar] [CrossRef]
- Mao, B.; Li, D.; Ai, C.; Zhao, J.; Zhang, H.; Chen, W. Lactulose Differently Modulates the Composition of Luminal and Mucosal Microbiota in C57BL/6J Mice. J. Agric. Food Chem. 2016, 64, 6240–6247. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.; Wang, G.; Siegel, P.; He, C.; Wang, H.; Zhao, W.; Zhai, Z.; Tian, F.; Zhao, J.; Zhang, H.; et al. Quantitative genetic background of the host influences gut microbiomes in chickens. Sci. Rep. 2013, 3, 1163. [Google Scholar] [CrossRef] [PubMed]
- Caporaso, J.G.; Kuczynski, J.; Stombaugh, J.; Knight, R. QIIME allows analysis of high-throughput community sequencing data. Nat. Methods 2010, 7, 335–336. [Google Scholar] [CrossRef] [PubMed]
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license ( http://creativecommons.org/licenses/by/4.0/).