Quantitative Determination of Stilbenoids and Dihydroisocoumarins in Shorea roxburghii and Evaluation of Their Hepatoprotective Activity
Abstract
:1. Introduction
2. Results and Discussion
2.1. Isolation
2.2. Simultaneous Quantitative Analysis
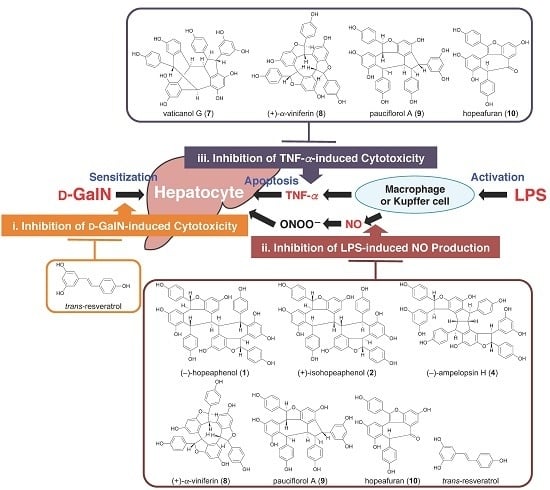
2.3. Protective Effects of Principal Polyphenols (1, 2, 8, and 13–15) on Liver Injury Induced by d-GalN/LPS in Mice
2.4. Effects on d-GalN-Induced Cytotoxicity in Primary Cultured Mouse Hepatocytes
2.5. Effects on LPS-Induced NO Production in Mouse Peritoneal Macrophages
2.6. Effects on TNF-α-Induced Cytotoxicity in L929 Cells
3. Materials and Methods
3.1. Chemicals and Reagents
3.2. Plant Materials
3.3. Standard Solution Preparation
3.4. Sample Solution Preparation
3.5. HPLC Instruments and Conditions
3.6. Calibration and Validation
3.7. Reagents for Bioassays
3.8. Animals
3.9. Effects on d-GalN/LPS-Induced Liver Injury in Mice
3.10. Effects on Cytotoxicity Induced by d-GalN in Primary Cultured Mouse Hepatocytes
3.11. Effects on Production of NO in LPS-Induced Mouse Peritoneal Macrophages
3.12. Effects on Cytotoxicity Induced by TNF-α in L929 Cells
3.13. Statistics
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Riviére, C.; Pawlus, A.D.; Mérillon, J.-M. Natural stilbenoids: Distribution in the plant kingdom and chemotaxonomic interest in Vitaceae. Nat. Prod. Rep. 2012, 29, 1317–1333. [Google Scholar] [CrossRef] [PubMed]
- Kovacic, P.; Somanathan, R. Multifaceted approach to resveratrol bioactivity—Focus on antioxidant action, cell signaling and safety. Oxid. Med. Cell. Longev. 2010, 3, 86–100. [Google Scholar] [CrossRef] [PubMed]
- Vang, O.; Ahmad, N.; Baile, C.A.; Baur, J.A.; Brown, K.; Csiszar, A.; Das, D.K.; Delmas, D.; Gottfried, C.; Lin, H.-Y.; et al. What is new for an old molecule? Systematic review and recommendations on the use of resveratrol. PLoS ONE 2011, 6, e19881. [Google Scholar] [CrossRef] [PubMed]
- Tomé-Carneiro, J.; Gonzálvez, M.; Larrosa, M.; Yáñez-Gascón, M.J.; García-Almagro, F.J.; Ruiz-Ros, J.A.; Tomás-Barberán, F.A.; García-Conesa, M.T.; Espín, J.C. Resveratrol in primary and secondary prevention of cardiovascular disease: A dietary and clinical perspective. Ann. N. Y. Acad. Sci. 2013, 1290, 37–51. [Google Scholar] [CrossRef] [PubMed]
- Tomé-Carneiro, J.; Larrosa, M.; González-Sarrías, A.; Tomás-Barberán, F.A.; García-Conesa, M.T.; Espín, J.C. Resveratrol and clinical trials: The crossroad from in vitro studies to human evidence. Curr. Pharm. Design 2013, 19, 6064–6093. [Google Scholar] [CrossRef]
- Lançon, A.; Frazzi, R.; Latruffe, N. Anti-oxidant, anti-inflammatory and anti-angiogenic properties of resveratrol in ocular diseases. Molecules 2016, 21, 304. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Xu, X.; Tao, Z.; Sun, C.; Pan, Y. Resveratrol derivatives: An updated patent review (2012–2015). Expert Opin. Ther. Pat. 2016, 26, 1189–1200. [Google Scholar] [CrossRef] [PubMed]
- Ito, T. Structures of oligostilbenoids in Dipterocarpaceaeous plants and their biological activities. Yakugaku Zasshi 2011, 131, 93–100. [Google Scholar] [CrossRef] [PubMed]
- Ito, T.; Ito, H.; Nehira, T.; Sawa, R.; Iinuma, M. Structure elucidation of highly condensed stilbenoids: Chiroptical properties and absolute configuration. Tetrahedron 2014, 70, 5640–5649. [Google Scholar] [CrossRef]
- Ito, T.; Hoshino, R.; Iinuma, M. Absolute configuration of resveratrol oligomers isolated from Hopea utilis. Helv. Chim. Acta 2015, 98, 32–46. [Google Scholar] [CrossRef]
- Ito, T.; Iinuma, M. Occurrence of non-heterocyclic resveratrol tetramer in Vatica chinensis. Phytochem. Lett. 2016, 15, 37–41. [Google Scholar] [CrossRef]
- Ito, T.; Hara, Y.; Kubota, Y.; Sawa, R.; Iinuma, M. Absolute structure of resveratrol hexamers in Dipterocarpaceaeous plants. Tetrahedron 2016, 72, 891–899. [Google Scholar] [CrossRef]
- Morikawa, T.; Chaipech, S.; Matsuda, H.; Hamao, M.; Umeda, Y.; Sato, H.; Tamura, H.; Ninomiya, K.; Yoshikawa, M.; Pongpiriyadacha, Y.; et al. Anti-hyperlipidemic constituents from the bark of Shorea roxburghii. J. Nat. Med. 2012, 66, 516–524. [Google Scholar] [CrossRef] [PubMed]
- Morikawa, T.; Chaipech, S.; Matsuda, H.; Hamao, M.; Umeda, Y.; Sato, H.; Tamura, H.; Kon’i, H.; Ninomiya, K.; Yoshikawa, M.; et al. Antidiabetogenic oligostilbenoids and 3-ethyl-4-phenyl-3,4-dihydroisocoumarins from the bark of Shorea roxburghii. Bioorg. Med. Chem. 2012, 20, 823–840. [Google Scholar] [CrossRef] [PubMed]
- Moriyama, H.; Moriyama, M.; Ninomiya, K.; Morikawa, T.; Hayakawa, T. Inhibitory effects of oligostilbenoids from the bark of Shorea roxburghii on malignant melanoma cell growth: Implications for novel topical anticancer candidates. Biol. Pharm. Bull. 2016, 39, 1675–1682. [Google Scholar] [CrossRef] [PubMed]
- Matsuda, H.; Asao, Y.; Nakamura, S.; Hamao, M.; Sugimoto, S.; Hongo, M.; Pongpiriyadacha, Y.; Yoshikawa, M. Antidiabetogenic constituents from the Thai traditional medicine Cotylelobium melanoxylon. Chem. Pharm. Bull. 2009, 57, 487–494. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Singh, R.; Lefkowitch, J.H.; Rigoli, R.M.; Czaja, M.J. Tumor necrosis factor-induced toxic liver injury results from JNK2-dependent activation of caspase-8 and the mitochondrial death pathway. J. Biol. Chem. 2006, 281, 15258–15267. [Google Scholar] [CrossRef] [PubMed]
- Tilg, H.; Day, C.P. Management strategies in alcoholic liver disease. Nat. Rev. Gastroenterol. Hepatol. 2007, 4, 24–34. [Google Scholar] [CrossRef] [PubMed]
- Seronello, S.; Sheikh, M.Y.; Choi, J. Redox regulation of hepatitis C in nonalcoholic and alcoholic liver. Free Rad. Biol. Med. 2007, 43, 869–882. [Google Scholar] [CrossRef] [PubMed]
- Freudenberg, M.A.; Galanos, C. Tumor necrosis factor α mediates lethal activity of killed Gram-negative and Gram-positive bacteria in d-galactosamine-treated mice. Infect. Immun. 1991, 59, 2110–2115. [Google Scholar] [PubMed]
- Josephs, M.D.; Bahjat, F.R.; Fukuzuka, K.; Ksontini, R.; Solorzano, C.C.; Edwards, C.K., 3rd; Tannahill, C.L.; MacKay, S.L.; Copeland, E.M., 3rd; Moldawer, L.L. Lipopolysaccharide and d-galactosamine-induced hepatic injury is mediated by TNF-α and not by Fas ligand. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2000, 278, R1196–R1201. [Google Scholar] [PubMed]
- Matsuda, H.; Ninomiya, K.; Morikawa, T.; Yoshikawa, M. Inhibitory effect and action mechanism of sesquiterpenes from Zedoariae Rhizoma on d-galactosamine/lipopolysaccharide-induced liver injury. Bioorg. Med. Chem. Lett. 1998, 8, 339–344. [Google Scholar] [CrossRef]
- Matsuda, H.; Morikawa, T.; Ninomiya, K.; Yoshikawa, M. Hepatoprotective constituents from Zedoariae Rhizoma: Absolute stereostructures of three new carabrane-type sesquiterpenes, curcumenolactonea A, B, and C. Bioorg. Med. Chem. 2001, 9, 909–916. [Google Scholar] [CrossRef]
- Morikawa, T.; Matsuda, H.; Ninomiya, K.; Yoshikawa, M. Medicinal foodstuffs. XXIX. Potent protective effects of sesquiterpenes and curcumin from Zedoariae Rhizoma on liver injury induced by d-galactosamine/lipopolysaccharide or tumor necrosis factor-α. Biol. Pharm. Bull. 2002, 25, 627–631. [Google Scholar] [CrossRef] [PubMed]
- Morikawa, T. Search for bioactive constituents from several medicinal food: Hepatoprotective, antidiabetic, and antiallergic activities. J. Nat. Med. 2007, 61, 112–126. [Google Scholar] [CrossRef]
- Morikawa, T.; Ninomiya, K.; Imura, K.; Yamaguchi, T.; Aakgi, Y.; Yoshikawa, M.; Hayakawa, T.; Muraoka, O. Hepatoprotective triterpene from traditional Tibetan medicine Potentilla anserina. Phytochemistry 2014, 102, 169–181. [Google Scholar] [CrossRef] [PubMed]
- Ninomiya, K.; Miyazawa, S.; Ozeki, K.; Matsuo, N.; Muraoka, O.; Kikuchi, T.; Yamada, T.; Tanaka, R.; Morikawa, T. Hepatoprotective limonoids from Andiroba (Carapa guianensis). Int. J. Mol. Sci. 2016, 17, 591. [Google Scholar] [CrossRef] [PubMed]
- Yoshikawa, M.; Nishida, N.; Ninomiya, K.; Ohgushi, T.; Kubo, M.; Morikawa, T.; Matsuda, H. Inhibitory effects of coumarin and acetylene constituents from the roots of Angellica furcijuga on D-galactosamine/lipopolysaccharide-induced liver injury in mice and on nitric oxide production in lipopolysaccharide-activated mouse peritoneal macrophages. Bioorg. Med. Chem. 2006, 14, 456–463. [Google Scholar] [CrossRef] [PubMed]
- Matsuda, H.; Ninomiya, K.; Morikawa, T.; Yasuda, D.; Yamaguchi, I.; Yoshikawa, M. Protective effects of amide constituents from the fruit of Piper chaba on d-galactosamine/TNF-α-induced cell death in mouse hepatocytes. Bioorg. Med. Chem. Lett. 2008, 18, 2038–2042. [Google Scholar] [CrossRef] [PubMed]
- Matsuda, H.; Ninomiya, K.; Morikawa, T.; Yasuda, D.; Yamaguchi, I.; Yoshikawa, M. Hepatoprotective amide constituents from the fruit of Piper chaba: Structural requirements, mode of action, and new amides. Bioorg. Med. Chem. 2009, 17, 7313–7323. [Google Scholar] [CrossRef] [PubMed]
- Morikawa, T. Search for TNF-α sensitivety degradation principles from medicinal foods—Hepatoprotective amide constituents from Thai natural medicine Piper chaba. Yakugaku Zasshi 2010, 130, 785–791. [Google Scholar] [CrossRef] [PubMed]
- Morikawa, T.; Pan, Y.; Ninomiya, K.; Imura, K.; Matsuda, H.; Yoshikawa, M.; Yuan, D.; Muraoka, O. Acylated phenylethanoid oligoglycosides with hepatoprotective activity from the desert plant Cistanche tubulosa. Bioorg. Med. Chem. 2010, 18, 1882–1890. [Google Scholar] [CrossRef] [PubMed]
- Yoshikawa, M.; Morikawa, T.; Kashima, Y.; Ninomiya, K.; Matsuda, H. Structures of new dammarane-type triterpene saponins from the flower buds of Panax notoginseng and hepatoprotective effects of principal ginseng saponins. J. Nat. Prod. 2003, 66, 922–927. [Google Scholar] [CrossRef] [PubMed]
- Osumi, W.; Jin, D.; Imai, Y.; Tashiro, K.; Li, Z.-L.; Otsuki, Y.; Maemura, K.; Komeda, K.; Hirokawa, F.; Hayashi, M.; et al. Recombinant human soluble thrombomodulin improved lipopolysaccharide/d-galactosamine-induced acute liver failure in mice. J. Pharmacol. Sci. 2015, 129, 233–239. [Google Scholar] [CrossRef] [PubMed]
- Cho, H.I.; Hong, J.M.; Choi, J.W.; Choi, H.S.; Kwak, J.H.; Lee, D.U.; Lee, S.K.; Lee, S.M. β-Caryophyllene alleviates d-galactosamine and lipopolysaccharide-induced hepatic injury through suppression of the TLR4 and RAGE signaling pathways. Eur. J. Pharmacol. 2015, 764, 613–621. [Google Scholar] [CrossRef] [PubMed]
- Sass, G.; Heinlein, S.; Agli, A.; Bang, R.; Schümann, J.; Tiegs, G. Cytokine expression in three mouse models of experimental hepatitis. Cytokine 2002, 19, 115–120. [Google Scholar] [CrossRef] [PubMed]
- Hamadi, N.; Mansour, A.; Hassan, M.H.; Khalifi-Touhami, F.; Badary, O. Ameliorative effects of resveratrol on liver injury in streptozotocin-induced diabetic rats. J. Biochem. Mol. Toxicol. 2012, 26, 384–392. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Jiang, Y.; Fan, X.; Tan, H.; Zeng, H.; Wang, Y.; Chen, P.; Huang, M.; Bi, H. Hepato-protective effect of resveratrol against acetaminophen-induced liver injury is associated with inhibition of CYP-mediated bioactivation and regulation of SIRT1-p53 signaling pathways. Toxicol. Lett. 2015, 236, 82–89. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.-M.; Shaw, L.-H.; Chang, P.-J.; Tung, S.-Y.; Chang, T.-S.; Shen, C.-H.; Hsieh, Y.-Y.; Wei, K.-L. Hepatoprotective effect of resveratrol against ethanol-induced oxidative stress through induction of superoxide dismutase in vivo and in vitro. Exp. Ther. Med. 2016, 11, 1231–1238. [Google Scholar] [CrossRef] [PubMed]
- Nabavi, S.F.; Daglia, M.; Moghaddam, A.H.; Habtemariam, S.; Nabavi, S.M. Curcumin and liver disease: From chemistry to medicine. Compr. Rev. Food Sci. Food Saf. 2013, 13, 62–77. [Google Scholar] [CrossRef]
- Fraschini, F.; Demartini, G.; Esposti, D. Pharmacology of silymarin. Clin. Drug Investig. 2002, 22, 51–65. [Google Scholar] [CrossRef]
- Kren, V.; Walteravá, D. Silybin and silymarin—New effects and applications. Biomed. Pap. 2005, 149, 29–41. [Google Scholar] [CrossRef]



| Extraction Method | Extraction Yield (%) | Contents (mg/g in Dry Material) | Total a | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13 | 14 | 15 | |||
| Methanol, reflux | 17.74 | 13.31 | 10.21 | 7.89 | 2.41 | 4.84 | 11.57 | 1.25 | 2.35 | 0.91 | 0.52 | 1.71 | 0.34 | 13.81 | 1.03 | 0.45 | 72.60 (100) |
| 50% Methanol, reflux | 20.21 | 13.31 | 10.17 | 7.90 | 2.39 | 4.85 | 11.56 | 1.26 | 2.23 | 0.92 | 0.52 | 1.71 | 0.31 | 14.01 | 1.01 | 0.39 | 72.54 (100) |
| H2O, reflux | 12.28 | 4.72 | 4.64 | 1.62 | 0.57 | 1.65 | 3.51 | 0.65 | 0.15 | 0.37 | 0.17 | 0.69 | 0.16 | 9.66 | 0.67 | 0.36 | 29.59 (41) |
| Methanol, sonication | 13.47 | 11.46 | 8.49 | 6.81 | 2.00 | 4.08 | 9.93 | 1.04 | 1.68 | 0.80 | 0.43 | 1.35 | 0.25 | 11.00 | 0.71 | 0.36 | 60.39 (83) |
| 50% Methanol, sonication | 11.67 | 8.42 | 6.47 | 4.83 | 1.49 | 3.06 | 6.18 | 0.85 | 1.12 | 0.61 | 0.36 | 1.00 | 0.20 | 10.95 | 0.65 | 0.31 | 46.50 (64) |
| H2O, sonication | 12.71 | 6.51 | 6.11 | 2.07 | 0.74 | 2.23 | 4.93 | 0.89 | n.d. b | 0.70 | 0.18 | 0.80 | 0.16 | 10.45 | 0.63 | 0.32 | 36.72 (51) |
| Analyte | Regression Equation a | Correlation Coefficient | Detection Limit b (ng) | Quantitation Limit b (ng) | Precision c (RSD, %) | |
|---|---|---|---|---|---|---|
| Intra-Day | Inter-Day | |||||
| (−)-Hopeaphenol (1) | y = 4404 x − 822 | 0.9997 | 0.11 | 0.34 | 0.30 | 0.28 |
| (+)-Isohopeaphenol (2) | y = 4465 x − 850 | 0.9998 | 0.11 | 0.32 | 0.90 | 1.08 |
| Hemsleyanol D (3) | y = 3506 x − 923 | 0.9999 | 0.11 | 0.34 | 1.09 | 0.81 |
| (−)-Ampelopsin H (4) | y = 3743 x − 885 | 0.9998 | 0.11 | 0.34 | 0.80 | 0.78 |
| Vaticanol A (5) | y = 3747 x − 507 | 1.000 | 0.14 | 0.42 | 1.32 | 0.13 |
| Vaticanol E (6) | y = 3009 x − 765 | 0.9994 | 0.14 | 0.44 | 0.73 | 0.91 |
| Vaticanol G (7) | y = 3195 x − 487 | 1.000 | 0.14 | 0.44 | 0.49 | 1.25 |
| (+)-α-Viniferin (8) | y = 4585 x − 166 | 0.9999 | 0.08 | 0.26 | 1.54 | 1.23 |
| Pauciflorol A (9) | y = 2592 x − 441 | 1.000 | 0.22 | 0.66 | 0.46 | 0.78 |
| Hopeafuran (10) | y = 4518 x − 150 | 1.000 | 0.10 | 0.30 | 0.84 | 1.29 |
| (−)-Balanocarpol (11) | y = 5666 x + 325 | 0.9999 | 0.12 | 0.36 | 0.46 | 1.58 |
| (−)-Ampelopsin (12) | y = 4516 x − 427 | 1.000 | 0.10 | 0.32 | 0.35 | 0.32 |
| Trans-resveratrol 10-C-Glc (13) | y = 11461 x − 872 | 1.000 | 0.05 | 0.14 | 0.13 | 0.96 |
| Phayomphenol A1 (14) | y = 2700 x − 564 | 0.9999 | 0.24 | 0.72 | 0.93 | 0.60 |
| Phayomphenol A2 (15) | y = 2059 x − 189 | 0.9999 | 0.26 | 0.80 | 0.36 | 0.93 |
| Add (µg/mL) | Recovery a (%) | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13 | 14 | 15 | |
| 50 | 96.1 ± 1.0 | 97.1 ± 1.1 | 95.1 ± 1.1 | 101.1 ± 1.0 | 98.0 ± 0.9 | 95.8 ± 0.7 | 99.8 ± 0.9 | 100.6 ± 1.1 | 104.9 ± 1.2 | 102.7 ± 1.0 | 96.2 ± 1.3 | 98.8 ± 0.8 | 95.7 ± 0.9 | 98.1 ± 0.5 | 98.3 ± 1.0 |
| 125 | 104.9 ± 0.9 | 103.1 ± 1.2 | 101.6 ± 0.6 | 100.7 ± 1.0 | 99.0 ± 1.1 | 100.7 ± 1.0 | 96.3 ± 1.1 | 95.9 ± 1.0 | 104.3 ± 1.0 | 103.7 ± 1.2 | 101.5 ± 0.9 | 96.5 ± 0.5 | 101.2 ± 0.5 | 102.4 ± 0.6 | 99.4 ± 0.3 |
| Extraction Method | Loss of Drying a (%) | Extraction Yield b (%) | Contents (mg/g in Dry Material) | Total | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13 | 14 | 15 | ||||
| S. roxburghii, bark | 7.44 | 17.74 | 13.31 | 10.21 | 7.89 | 2.41 | 4.84 | 11.57 | 1.25 | 2.35 | 0.91 | 0.52 | 1.71 | 0.34 | 13.81 | 1.03 | 0.45 | 72.60 |
| S. roxburghii, wood | 6.76 | 8.32 | 3.39 | 3.03 | 1.83 | 0.51 | 2.04 | 3.75 | 0.40 | n.d. c | n.d. c | n.d. c | 2.03 | 0.28 | 3.94 | n.d. c | n.d. c | 21.20 |
| C. melanoxylon, bark | 8.96 | 31.40 | n.d. c | n.d. c | 24.59 | n.d. c | 76.45 | 120.75 | 63.81 | n.d. c | 1.13 | n.d. c | n.d. c | n.d. c | n.d. c | n.d. c | n.d. c | 286.73 |
| C. melanoxylon, wood | 7.77 | 27.89 | n.d. c | n.d. c | 4.91 | n.d. c | 7.45 | 3.45 | 181.69 | n.d. c | n.d. c | n.d. c | n.d. c | n.d. c | n.d. c | n.d. c | n.d. c | 197.50 |
| Treatment | Dose (mg/kg, p.o.) | n | Inhibition (%) | |
|---|---|---|---|---|
| sAST | sALT | |||
| (−)-Hopeaphenol (1) | 100 | 6 | 92.2 ± 5.4 ** | 90.6 ± 6.5 ** |
| (+)-Isohopeaphenol (2) | 100 | 6 | 80.6 ± 4.1 ** | 79.8 ± 3.6 ** |
| (+)-α-Viniferin (8) | 100 | 6 | 70.1 ± 4.0 ** | 69.5 ± 6.2 ** |
| – | 200 | 6 | 75.3 ± 5.5 ** | 71.9 ± 2.6 ** |
| Trans-resveratrol 10-C-Glc (13) | 100 | 7 | 47.8 ± 4.8 | 43.9 ± 11.8 |
| – | 200 | 7 | 65.7 ± 3.4 ** | 67.5 ± 12.3 ** |
| Phayomphenol A1 (14) | 100 | 7 | 48.0 ± 6.0 * | 47.0 ± 6.5 * |
| Phayomphenol A2 (15) | 100 | 7 | 61.0 ± 8.1 ** | 64.7 ± 6.9 ** |
| Trans-resveratrol | 100 | 7 | 52.0 ± 9.0 * | 51.2 ± 7.9 * |
| – | 200 | 5 | 55.7 ± 9.9 * | 63.1 ± 5.0 ** |
| Curcumin [26,27] | 12.5 | 10 | 21.1 ± 20.0 | 24.0 ± 2.6 |
| – | 25 | 10 | 47.8 ± 16.0 | 50.9 ± 14.6 |
| – | 50 | 9 | 63.8 ± 9.1 * | 71.2 ± 7.1 * |
| Silybin [27] | 500 | 8 | 71.1 ± 6.8 ** | 71.9 ± 3.1 ** |
| Treatment | Inhibition (%) | ||||
|---|---|---|---|---|---|
| 0 µM | 3 µM | 10 µM | 30 µM | 100 µM | |
| (−)-Hopeaphenol (1) | 0.0 ± 0.6 | −5.8 ± 1.6 | −4.5 ± 1.3 | −5.0 ± 0.3 | −5.8 ± 1.5 |
| (+)-Isohopeaphenol (2) | 0.0 ± 1.4 | −4.9 ± 0.4 | −7.9 ± 1.2 | −10.2 ± 0.4 | −12.5 ± 0.9 |
| Hemsleyanol D (3) | 0.0 ± 1.3 | −7.2 ± 0.5 | −12.1 ± 0.5 | −7.8 ± 0.9 | −23.2 ± 0.5 |
| (−)-Ampelopsin H (4) | 0.0 ± 0.2 | −7.1 ± 0.7 | −7.1 ± 1.2 | −10.5 ± 1.3 | −17.3 ± 0.3 |
| Vaticanol A (5) | 0.0 ± 0.9 | 1.6 ± 0.5 | −1.3 ± 0.5 | 2.2 ± 1.1 | −2.0 ± 1.1 |
| Vaticanol E (6) | 0.0 ± 0.4 | −3.1 ± 0.7 | −3.0 ± 1.0 | −2.4 ± 0.6 | −0.3 ± 1.0 |
| Vaticanol G (7) | 0.0 ± 0.6 | −7.5 ± 0.7 | −7.0 ± 1.7 | −6.6 ± 1.7 | −12.7 ± 1.0 |
| (+)-α-Viniferin (8) | 0.0 ± 2.1 | −3.3 ± 1.9 | 10.1 ± 2.1 | 32.4 ± 4.0 ** | −29.9 ± 0.6 |
| Pauciflorol A (9) | 0.0 ± 1.4 | −1.5 ± 0.9 | −5.0 ± 0.7 | −6.1 ± 0.4 | −10.2 ± 0.4 |
| Hopeafuran (10) | 0.0 ± 1.4 | −6.7 ± 0.4 | 0.8 ± 1.4 | 13.0 ± 0.9 ** | −22.0 ± 0.6 |
| (−)-Balanocarpol (11) | 0.0 ± 1.5 | −6.4 ± 0.3 | −3.1 ± 2.0 | 11.8 ± 2.7 ** | 13.5 ± 0.8 ** |
| (−)-Ampelopsin A (12) | 0.0 ± 1.8 | −2.9 ± 0.8 | −1.4 ± 1.2 | 22.5 ± 1.5 ** | 29.4 ± 1.0 ** |
| Trans-resveratrol 10-C-Glc (13) | 0.0 ± 2.0 | 7.1 ± 1.9 | 15.4 ± 2.5 ** | 12.8 ± 2.3 ** | 2.5 ± 0.8 |
| Phayomphenol A1 (14) | 0.0 ± 1.3 | 5.1 ± 6.8 | 12.2 ± 4.8 | 26.9 ± 3.2 ** | 42.7 ± 4.3 ** |
| Phayomphenol A2 (15) | 0.0 ± 2.2 | 1.7 ± 2.8 | 13.0 ± 1.9 * | 16.1 ± 3.0 ** | 33.9 ± 3.0 ** |
| Vaticanol B | 0.0 ± 1.4 | −1.5 ± 0.9 | −5.0 ± 0.7 | −6.1 ± 0.4 | −10.2 ± 0.4 |
| Vaticanol C | 0.0 ± 2.1 | −5.7 ± 2.0 | −4.3 ± 1.5 | −5.6 ± 2.2 | −12.0 ± 1.3 |
| Malibatol A | 0.0 ± 0.7 | −1.4 ± 0.9 | 4.2 ± 0.4 | 8.9 ± 1.3 * | 30.8 ± 2.9 ** |
| Malibatol B | 0.0 ± 0.9 | −0.8 ± 1.3 | −6.1 ± 0.6 | −10.9 ± 0.7 | −17.0 ± 0.4 |
| (+)-Parviflorol | 0.0 ± 1.1 | 9.1 ± 2.5 | 21.2 ± 0.6 ** | 23.8 ± 1.0 ** | −20.8 ± 0.2 |
| Cis-resveratrol 10-C-Glc | 0.0 ± 0.5 | 9.1 ± 2.0 | 20.9 ± 1.3 ** | 29.6 ± 2.6 ** | 33.6 ± 1.8 ** |
| Trans-piceid | 0.0 ± 3.3 | 1.3 ± 2.1 | 13.0 ± 2.6 | 19.9 ± 6.1 * | 33.9 ± 3.6 ** |
| 1′S-Dihydrophayomphenol A2 | 0.0 ± 1.9 | −0.8 ± 1.4 | −1.2 ± 1.1 | 14.4 ± 0.5 ** | 38.0 ± 4.8 ** |
| Trans-resveratrol | 0.0 ± 1.7 | 8.5 ± 0.4 | 14.1 ± 0.9 ** | 37.5 ± 3.7 ** | 57.3 ± 2.5 ** |
| Curcumin [22,23,25,27] | 0.0 ± 3.7 | 0.1 ± 3.8 | 1.1 ± 2.2 | −17.7 ± 1.3 | −44.3 ± 0.3 |
| Silybin [26,27,30,32] | 0.0 ± 0.3 | 4.8 ± 1.1 | 7.7 ± 0.7 | 45.2 ± 8.8 ** | 77.0 ± 5.5 ** |
| Treatment | Inhibition (%) | IC50 (μM) | ||||
|---|---|---|---|---|---|---|
| 0 μM | 3 μM | 10 μM | 30 μM | 100 μM | ||
| (−)-Hopeaphenol (1) | 0.0 ± 4.0 (100.0 ± 1.6) | 42.3 ± 2.5 ** (118.9 ± 3.1) | 64.9 ± 3.0 ** (119.1 ± 4.9) | 73.9 ± 1.5 ** (130.6 ± 4.5) | 80.5 ± 2.3 ** (111.5 ± 6.2) | 4.6 |
| (+)-Isohopeaphenol (2) | 0.0 ± 2.9 (100.0 ± 3.5) | 38.5 ± 4.6 ** (132.6 ± 1.1) | 30.7 ± 4.0 ** (129.6 ± 5.6) | 41.7 ± 6.3 ** (139.3 ± 4.4) | 95.7 ± 1.0 ** (35.2 ± 1.5 #) | 38.5 |
| Hemsleyanol D (3) | 0.0 ± 3.8 (100.0 ± 2.5) | −4.3 ± 3.7 (120.1 ± 2.5) | 8.4 ± 7.8 (135.3 ± 1.8) | 34.8 ± 4.4 ** (93.3 ± 1.3) | -34.0 ± 1.8 (32.2 ± 2.9 #) | |
| (−)-Ampelopsin H (4) | 0.0 ± 5.4 (100.0 ± 2.4) | 29.2 ± 1.8 ** (102.1 ± 1.0) | 39.0 ± 2.8 ** (110.0 ± 4.0) | 52.0 ± 3.9 ** (139.0 ± 0.3) | 99.7 ± 0.5 ** (85.4 ± 0.9) | 18.6 |
| Vaticanol A (5) | 0.0 ± 5.0 (100.0 ± 1.3) | 30.3 ± 4.5 ** (122.1 ± 4.3) | 32.2 ± 5.5 ** (132.2 ± 2.7) | 14.5 ± 3.8 (138.4 ± 5.6) | 0.5 ± 4.7 (136.2 ± 10.9) | |
| Vaticanol E (6) | 0.0 ± 5.7 (100.0 ± 2.5) | -4.0 ± 8.9 (121.1 ± 2.9) | 14.7 ± 5.9 (111.7 ± 7.2) | 29.5 ± 5.3 ** (136.2 ± 4.0) | -21.7 ± 5.6 (122.1 ± 10.2) | |
| Vaticanol G (7) | 0.0 ± 3.0 (100.0 ± 2.9) | 8.2 ± 5.6 (130.2 ± 5.0) | 25.1 ± 2.5 ** (120.3 ± 3.0) | 26.7 ± 3.0 ** (123.3 ± 5.4) | 44.1 ± 2.3 ** (131.6 ± 9.5) | |
| (+)-α-Viniferin (8) | 0.0 ± 2.9 (100.0 ± 4.3) | 27.8 ± 5.7 ** (123.4 ± 8.8) | 46.3 ± 3.5 ** (98.8 ± 10.5) | 75.4 ± 1.3 ** (87.7 ± 2.9) | 97.9 ± 0.6 ** (36.5 ± 3.2 #) | 9.7 |
| Pauciflorol A (9) | 0.0 ± 4.1 (100.0 ± 4.5) | 38.3 ± 3.8 ** (120.7 ± 5.6) | 43.5 ± 3.2 ** (129.4 ± 3.8) | 53.2 ± 2.2 ** (119.6 ± 2.2) | 80.1 ± 1.4 ** (119.0 ± 2.4) | 17.8 |
| Hopeafuran (10) | 0.0 ± 5.5 (100.0 ± 1.0) | 30.6 ± 3.6 ** (125.7 ± 3.8) | 33.3 ± 1.8 ** (128.7 ± 3.9) | 33.6 ± 5.7 ** (128.9 ± 2.6) | 67.7 ± 3.7 ** (117.5 ± 14.1) | 45.9 |
| (−)-Balanocarpol (11) | 0.0 ± 7.2 (100.0 ± 6.5) | 26.1 ± 7.6 ** (129.8 ± 7.4) | 41.0 ± 5.2 ** (127.0 ± 4.8) | 41.9 ± 5.2 ** (89.6 ± 4.8) | 42.5 ± 2.7 ** (101.3 ± 2.9) | |
| (−)-Ampelopsin A (12) | 0.0 ± 6.8 (100.0 ± 4.4) | 16.5 ± 2.9 (127.3 ± 4.4) | 23.1 ± 10.3 (139.0 ± 6.4) | 32.0 ± 2.3 ** (128.3 ± 5.8) | 32.9 ± 7.5 ** (116.3 ± 6.1) | |
| Trans-resveratrol 10-C-Glc (13) | 0.0 ± 4.3 (100.0 ± 2.4) | 39.0 ± 5.1 ** (126.6 ± 4.4) | 43.6 ± 4.4 ** (125.2 ± 9.1) | 38.2 ± 4.3 ** (119.8 ± 4.1) | 45.4 ± 3.8 ** (113.0 ± 11.0) | |
| Phayomphenol A1 (14) | 0.0 ± 4.9 (100.0 ± 2.0) | 33.6 ± 6.0 ** (111.3 ± 1.1) | 40.7 ± 6.5 ** (108.0 ± 1.9) | 43.8 ± 4.2 ** (113.0 ± 2.1) | 40.3 ± 3.8 ** (107.5 ± 5.3) | |
| Phayomphenol A2 (15) | 0.0 ± 2.0 (100.0 ± 6.4) | 35.2 ± 4.2 ** (102.0 ± 3.8) | 38.1 ± 1.4 ** (102.9 ± 7.3) | 42.4 ± 1.3 ** (120.9 ± 7.7) | 45.4 ± 4.8 ** (104.7 ± 5.6) | |
| Vaticanol B | 0.0 ± 5.4 (100.0 ± 2.0) | 28.6 ± 2.6 ** (133.8 ± 2.7) | 31.0 ± 4.8 ** (111.5 ± 7.1) | 51.9 ± 1.7 ** (99.6 ± 5.1) | 75.7 ± 1.0 ** (56.5 ± 2.8 #) | 26.8 |
| Vaticanol C | 0.0 ± 3.1 (100.0 ± 2.7) | 35.4 ± 5.6 ** (124.4 ± 9.7) | 45.3 ± 4.2 ** (105.4 ± 8.0) | 59.2 ± 2.2 ** (94.2 ± 4.0) | 96.5 ± 2.3 ** (11.8 ± 0.8 #) | 14.5 |
| Malibatol A | 0.0 ± 4.8 (100.0 ± 2.6) | 28.5 ± 7.9 ** (133.8 ± 3.7) | 42.5 ± 8.6 ** (131.6 ± 3.8) | 47.8 ± 4.1 ** (106.3 ± 4.6) | 77.3 ± 2.1 ** (96.0 ± 5.2) | 23.0 |
| Malibatol B | 0.0 ± 5.7 (100.0 ± 41) | 39.0 ± 5.7 ** (119.1 ± 3.4) | 44.4 ± 4.8 ** (122.3 ± 2.3) | 49.7 ± 2.3 ** (105.8 ± 7.0) | 80.5 ± 3.3 ** (91.9 ± 4.6) | 18.5 |
| (+)-Parviflorol | 0.0 ± 8.0 (100.0 ± 6.4) | 34.8 ± 2.2 ** (122.8 ± 7.4) | 48.6 ± 1.7 ** (130.1 ± 7.4) | 49.7 ± 1.6 ** (92.7 ± 8.6) | 50.9 ± 1.7 ** (95.7 ± 10.5) | 40.9 |
| Cis-resveratrol 10-C-Glc | 0.0 ± 2.0 (100.0 ± 3.6) | 26.5 ± 2.0 ** (102.9 ± 4.4) | 35.5 ± 2.4 ** (109.6 ± 2.9) | 47.0 ± 2.8 ** (99.6 ± 5.6) | 46.5 ± 2.4 ** (91.8 ± 0.7) | |
| Trans-piceid | 0.0 ± 3.5 (100.0 ± 1.8) | 38.6 ± 3.1 ** (91.9 ± 7.7) | 41.0 ± 2.7 ** (87.8 ± 5.5) | 48.8 ± 2.1 ** (81.5 ± 3.3) | 51.4 ± 5.3 ** (66.1 ± 4.8 #) | 59.8 |
| 1'S-Dihydrophayomphenol A2 | 0.0 ± 2.7 (100.0 ± 3.0) | 40.1 ± 2.3 ** (117.3 ± 4.0) | 40.6 ± 1.0 ** (115.6 ± 5.5) | 48.6 ± 1.8 ** (109.9 ± 3.5) | 49.4 ± 3.6 ** (113.5 ± 1.8) | |
| Trans-resveratrol | 0.0 ± 2.6 (100.0 ± 1.4) | 38.2 ± 6.3 ** (120.4 ± 1.7) | 45.6 ± 1.3 ** (128.7 ± 3.6) | 78.8 ± 1.1 ** (96.7 ± 10.1) | 88.8 ± 1.0 ** (84.4 ± 3.0) | 17.8 |
| l-NMMA [26,27,30] | 0.0 ± 3.1 (100.0 ± 0.9) | 1.4 ± 2.8 (101.1 ± 5.7) | 19.9 ± 2.8 ** (100.7 ± 6.2) | 43.0 ± 2.1 ** (102.6 ± 4.2) | 70.9 ± 1.6 ** (106.4 ± 4.6) | 36.0 |
| CAPE [26,27,30] | 0.0 ± 2.1 (100.0 ± 1.5) | 5.9 ± 5.2 (95.4 ± 0.7) | 44.4 ± 3.2 ** (70.0 ± 4.0 #) | 86.2 ± 1.1 ** (71.4 ± 6.0 #) | 99.6 ± 0.1 ** (53.0 ± 1.4 #) | 11.0 |
| Treatment | Inhibition (%) | IC50 (µM) | ||||
|---|---|---|---|---|---|---|
| 0 µM | 3 µM | 10 µM | 30 µM | 100 µM | ||
| (−)-Hopeaphenol (1) | 0.0 ± 0.2 | 3.8 ± 1.5 | 28.9 ± 0.2 ** | −1.8 ± 0.7 | −6.7 ± 0.8 | |
| (+)-Isohopeaphenol (2) | 0.0 ± 0.5 | −0.6 ± 1.1 | 0.3 ± 1.0 | 69.8 ± 1.4 ** | −6.2 ± 0.5 | |
| Hemsleyanol D (3) | 0.0 ± 1.1 | 6.7 ± 1.8 | 17.6 ± 1.7 ** | 91.5 ± 2.1 ** | 10.0 ± 1.8 | |
| (−)-Ampelopsin H (4) | 0.0 ± 0.8 | 1.0 ± 2.1 | −10.7 ± 0.3 | −16.4 ± 0.4 | −18.9 ± 0.3 | |
| Vaticanol A (5) | 0.0 ± 0.3 | 3.6 ± 0.4 | 4.0 ± 1.2 | 7.8 ± 1.5 | 28.9 ± 1.2 ** | |
| Vaticanol E (6) | 0.0 ± 0.4 | 6.7 ± 1.1 | 9.4 ± 0.6 | 3.7 ± 0.8 | 4.9 ± 0.5 | |
| Vaticanol G (7) | 0.0 ± 0.4 | 7.9 ± 0.8 | 9.7 ± 0.7 | 12.7 ± 0.6 * | 57.6 ± 4.2 ** | 86.6 |
| (+)-α-Viniferin (8) | 0.0 ± 1.2 | 12.2 ± 1.2 * | 38.3 ± 0.6 ** | 84.4 ± 2.5 ** | 78.5 ± 1.9 ** | 15.0 |
| Pauciflorol A (9) | 0.0 ± 1.0 | 8.2 ± 0.9 | 16.1 ± 0.7 ** | 54.6 ± 5.0 ** | 94.7 ± 4.6 ** | 26.7 |
| Hopeafuran (10) | 0.0 ± 0.7 | 8.3 ± 1.3 | 17.2 ± 1.3 ** | 66.4 ± 1.5 ** | 85.1 ± 1.2 ** | 22.0 |
| (−)-Balanocarpol (11) | 0.0 ± 1.1 | 4.5 ± 0.8 | 7.5 ± 1.7 | 10.4 ± 0.9 * | 17.7 ± 3.2 ** | |
| (−)-Ampelopsin A (12) | 0.0 ± 1.1 | 4.1 ± 1.5 | 8.4 ± 1.1 | 14.6 ± 2.1 * | 35.5 ± 1.5 ** | |
| Trans-resveratrol 10-C-Glc (13) | 0.0 ± 1.4 | 4.5 ± 2.2 | 4.4 ± 1.9 | 6.6 ± 1.6 | 6.6 ± 2.3 | |
| Phayomphenol A1 (14) | 0.0 ± 0.7 | 0.0 ± 0.6 | 1.8 ± 0.6 | 1.9 ± 0.6 | 1.5 ± 0.4 | |
| Phayomphenol A2 (15) | 0.0 ± 0.8 | 0.7 ± 0.7 | 1.4 ± 0.9 | 0.6 ± 0.5 | −3.1 ± 0.7 | |
| Vaticanol B | 0.0 ± 0.7 | 0.0 ± 0.7 | 12.1 ± 1.1 * | 7.9 ± 0.4 | −9.8 ± 0.5 | |
| Vaticanol C | 0.0 ± 0.6 | 9.3 ± 0.3 | 14.4 ± 1.0 ** | 94.3 ± 2.6 ** | −8.2 ± 0.2 | 18.2 |
| Malibatol A | 0.0 ± 0.5 | 8.1 ± 0.9 | 30.7 ± 3.8 ** | 86.9 ± 2.9 ** | 81.8 ± 4.3 ** | 12.3 |
| Malibatol B | 0.0 ± 1.2 | 16.8 ± 0.6 ** | 37.7 ± 3.3 ** | 90.7 ± 0.3 ** | 77.2 ± 2.9 ** | 10.2 |
| (+)-Parviflorol | 0.0 ± 0.8 | 4.5 ± 0.9 | 9.4 ± 2.2 | 13.0 ± 0.4 ** | 24.3 ± 0.9 ** | |
| Cis-resveratrol 10-C-Glc | 0.0 ± 0.7 | 4.8 ± 0.2 | 9.7 ± 1.2 | 14.5 ± 1.7 * | 31.6 ± 1.5 ** | |
| Trans-piceid | 0.0 ± 0.9 | 3.4 ± 1.4 | 6.7 ± 2.6 | 3.6 ± 1.8 | 1.6 ± 1.7 | |
| 1′S-Dihydrophayomphenol A2 | 0.0 ± 0.3 | 2.0 ± 0.4 | 3.8 ± 0.5 | 2.5 ± 1.2 | 4.7 ± 0.5 | |
| Trans-resveratrol | 0.0 ± 1.4 | 1.4 ± 0.5 | 1.8 ± 0.8 | 2.1 ± 1.2 | 5.3 ± 0.6 | |
| Silybin [27,36] | 0.0 ± 2.6 | 5.3 ± 2.8 | 22.0 ± 3.8 ** | 48.0 ± 4.1 ** | 50.8 ± 3.9 ** | 60.4 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ninomiya, K.; Chaipech, S.; Kunikata, Y.; Yagi, R.; Pongpiriyadacha, Y.; Muraoka, O.; Morikawa, T. Quantitative Determination of Stilbenoids and Dihydroisocoumarins in Shorea roxburghii and Evaluation of Their Hepatoprotective Activity. Int. J. Mol. Sci. 2017, 18, 451. https://doi.org/10.3390/ijms18020451
Ninomiya K, Chaipech S, Kunikata Y, Yagi R, Pongpiriyadacha Y, Muraoka O, Morikawa T. Quantitative Determination of Stilbenoids and Dihydroisocoumarins in Shorea roxburghii and Evaluation of Their Hepatoprotective Activity. International Journal of Molecular Sciences. 2017; 18(2):451. https://doi.org/10.3390/ijms18020451
Chicago/Turabian StyleNinomiya, Kiyofumi, Saowanee Chaipech, Yusuke Kunikata, Ryohei Yagi, Yutana Pongpiriyadacha, Osamu Muraoka, and Toshio Morikawa. 2017. "Quantitative Determination of Stilbenoids and Dihydroisocoumarins in Shorea roxburghii and Evaluation of Their Hepatoprotective Activity" International Journal of Molecular Sciences 18, no. 2: 451. https://doi.org/10.3390/ijms18020451
APA StyleNinomiya, K., Chaipech, S., Kunikata, Y., Yagi, R., Pongpiriyadacha, Y., Muraoka, O., & Morikawa, T. (2017). Quantitative Determination of Stilbenoids and Dihydroisocoumarins in Shorea roxburghii and Evaluation of Their Hepatoprotective Activity. International Journal of Molecular Sciences, 18(2), 451. https://doi.org/10.3390/ijms18020451