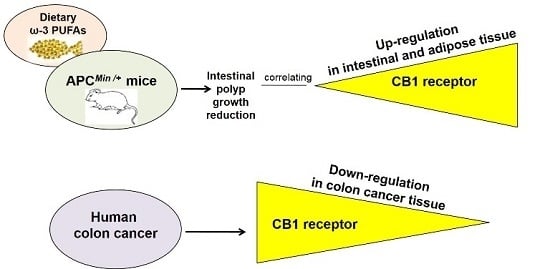
Dietary ω-3 Polyunsaturated Fatty Acids Inhibit Tumor Growth in Transgenic ApcMin/+ Mice, Correlating with CB1 Receptor Up-Regulation
Abstract
:1. Introduction
2. Results
3. Discussion
4. Material and Methods
4.1. Animals and Experimental Study Design
4.2. Patients
4.3. CB1 Receptor Gene Expression Analysis
4.4. Western Blotting
4.5. Statistical Analysis
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Kato, I.; Akhmedkhanov, A.; Koenig, K.; Toniolo, P.G.; Shore, R.E.; Riboli, E. Prospective study of diet and female colorectal cancer: The New York University Women’s Health Study. Nutr. Cancer 1997, 28, 276–281. [Google Scholar] [CrossRef] [PubMed]
- Dupertuis, Y.M.; Meguid, M.M.; Pichard, C. Colon cancer therapy: New perspectives of nutritional manipulations using polyunsaturated fatty acids. Curr. Opin. Clin. Nutr. Metab. Care 2007, 10, 427–432. [Google Scholar] [CrossRef] [PubMed]
- Han, Y.M.; Jeong, M.; Park, J.M.; Kim, M.Y.; Go, E.J.; Cha, J.Y.; Kim, K.J.; Haham, K.B. The ω-3 polyunsaturated fatty acids prevented colitis-associated carcinogenesis through blocking dissociation of β-catenin complex, inhibiting Cox-2 through repressing NF-κB, and inducing 15-prostaglandin dehydrogenase. Oncotarget 2016, 7, 63583–63595. [Google Scholar] [CrossRef] [PubMed]
- Barone, M.; Notarnicola, M.; Caruso, M.G.; Scavo, M.P.; Viggiani, M.T.; Tutino, V.; Polimeno, L.; Pesetti, B.; Di Leo, A.; Francavilla, A. Olive oil and ω-3 polyunsaturated fatty acids suppress intestinal polyp growth by modulating the apoptotic process in ApcMin/+ mice. Carcinogenesis 2014, 35, 1613–1619. [Google Scholar] [CrossRef] [PubMed]
- Notarnicola, M.; Messa, C.; Refolo, M.G.; Tutino, V.; Miccolis, A.; Caruso, M.G. Polyunsaturated fatty acids reduce Fatty Acid Synthase and Hydroxy-Methyl-Glutaryl CoA-Reductase gene expression and promote apoptosis in HepG2 cell line. Lipids Health Dis. 2011, 10, 10. [Google Scholar] [CrossRef] [PubMed]
- Notarnicola, M.; Messa, C.; Refolo, M.G.; Tutino, V.; Miccolis, A.; Caruso, M.G. Synergic effect of Eicosanpetaenoic acid and lovastatin on gene expression of HMGCoAreductase and LDL receptor in cultured HepG2 cells. Lipids Health Dis. 2010, 30, 135. [Google Scholar] [CrossRef] [PubMed]
- Notarnicola, M.; Tutino, V.; Caruso, M.G.; Francavilla, A. N-3-polyunsaturated fatty acids reverse the development of polyps in ApcMin/+ transgenic mice. Oncol. Rep. 2016, 35, 504–510. [Google Scholar] [CrossRef] [PubMed]
- Ligresti, A.; Bisogno, T.; Matias, I.; de Petrocellis, L.; Cascio, M.G.; Cosenza, V.; D’argenio, G.; Scaglione, G.; Bifulco, M.; Sorrentini, I.; et al. Possible endocannabinoid control of colorectal cancer growth. Gastroenterology 2003, 125, 677–687. [Google Scholar] [CrossRef]
- Linsalata, M.; Notarnicola, M.; Tutino, V.; Bifulco, M.; Santoro, A.; Laezza, C.; Messa, C.; Orlando, A.; Caruso, M.G. Effects of Anandamide on Polyamine Levels and cell growth in human colon cancer cells. Anticancer Res. 2010, 30, 2583–2590. [Google Scholar] [PubMed]
- Notarnicola, M.; Messa, C.; Orlando, A.; Bifulco, M.; Laezza, C.; Gazzerro, P.; Caruso, M.G. Estrogenic induction of cannabinoid CB1 receptor in human colon cancer cell lines. Scand. J. Gastroenterol. 2008, 43, 66–72. [Google Scholar] [CrossRef] [PubMed]
- Refolo, M.G.; D’alessandro, R.; Malerba, N.; Laezza, C.; Bifulco, M.; Messa, C.; Caruso, M.G.; Notarnicola, M.; Tutino, V. Anti-proliferative and pro apoptotic effects of flavonoid quercetin are mediated by CB1 receptor in human colon cancer cell lines. J. Cell. Physiol. 2015, 230, 2973–2980. [Google Scholar] [CrossRef] [PubMed]
- Di Francesco, A.; Falconi, A.; Di Germanio, C.; Caramuta, S.; Del Carlo, M.; Compagnone, D.; Dainese, E.; Cifani, C.; Maccarone, M.; D’Addario, C.; et al. Extravirgin olive oil up-regulates CB1 tumor suppressor gene in human colon cancer cells and in rat colon via epigenetic mechanisms. J. Nutr. Biochem. 2015, 26, 250–258. [Google Scholar] [CrossRef] [PubMed]
- Laezza, C.; D’Alessandro, A.; Paladino, S.; Malfitano, A.M.; Proto, M.C.; Gazzero, P.; Pisanti, S.; Santoro, A.; Ciaglia, E.; Bifulco, M. Anandamide inhibits the Wnt/β-catenin signaling pathway in human breast cancer MDA MB 231 cells. Eur. J. Cancer 2012, 48, 3112–3122. [Google Scholar] [CrossRef] [PubMed]
- Sebio, A.; Kahn, M.; Lenz, H.-J. The potential of targeting Wnt/β-catenin in colon cancer. Expert Opin. Ther. Targets 2014, 18, 611–615. [Google Scholar] [CrossRef] [PubMed]
- Reya, T.; Clevers, H. Wnt signaling in stem cells and cancer. Nature 2005, 434, 843–850. [Google Scholar] [CrossRef] [PubMed]
- Baenke, F.; Peck, B.; Miess, H.; Schulze, A. Hooked on fat: The role of lipid synthesis in cancer metabolism and tumour development. Dis. Model. Mech. 2013, 6, 1353–1363. [Google Scholar] [CrossRef] [PubMed]
- Notarnicola, M.; Miccolis, A.; Tutino, V.; Lorusso, D.; Caruso, M.G. Low levels of lipogenic enzymes in peritumoral adipose tissue of colorectal cancer patients. Lipids 2012, 47, 59–63. [Google Scholar] [CrossRef] [PubMed]
- Bing, C.; Russel, S.; Becket, E.; Pope, M.; Tisdale, M.J.; Trayhurn, P.; Jenkins, J.R. Adipose atrophy in cancer cachexia: Morphologic and molecular analysis of adipose tissue in tumour-bearing mice. Br. J. Cancer 2006, 95, 1028–1037. [Google Scholar] [CrossRef] [PubMed]
- Riondino, S.; Roselli, M.; Palmirotta, R.; Della-Morte, D.; Ferroni, P.; Guadagni, F. Obesity and colorectal cancer: Role of adipokines in tumor initiation and progression. World J. Gastroenterol. 2014, 20, 5177–5190. [Google Scholar] [CrossRef] [PubMed]
- Schwartz, B.; Yehuda-Shnaidman, E. Putative role of adipose tissue in growth and metabolism of colon cancer cells. Front. Oncol. 2014, 4, 164. [Google Scholar] [CrossRef] [PubMed]
- Weisberg, S.P.; McCann, D.; Desai, M.; Rosenbaum, M.; Leibel, R.L.; Ferrante, A.W., Jr. Obesity is associated with macrophage accumulation in adipose tissue. J. Clin. Investig. 2003, 112, 1796–1808. [Google Scholar] [CrossRef] [PubMed]
- De Petrocellis, L.; Melck, D.; Palmisano, A.; Bisogno, T.; Laezza, C.; Bifulco, M.; Di Marzo, V. The endogenous cannabinoid anandamide inhibits human breast cancer cell proliferation. Proc. Natl. Acad. Sci. USA 1998, 95, 8375–8380. [Google Scholar] [CrossRef] [PubMed]
- Micale, V.; Mazzola, C.; Drago, F. Endocannabinoids and neurodegenerative diseases. Pharmacol. Res. 2007, 56, 82–392. [Google Scholar] [CrossRef] [PubMed]
- D’Addario, C.; Di Francesco, A.; Pucci, M.; Finazzi Agrò, A.; Maccarone, M. Epigeneticmechanisms and endocannabinoidsignalling. FEBS J. 2013, 280, 1905–1907. [Google Scholar] [PubMed]
- Izzo, A.A.; Camilleri, M. Cannabinoids in intestinal inflammation and cancer. Pharmacol. Res. 2009, 60, 117–125. [Google Scholar] [CrossRef] [PubMed]
- Bifulco, M.; Di Marzo, V. Targeting the endocannabinoid system in cancer therapy: A call for further research. Nat. Med. 2002, 8, 547–550. [Google Scholar] [CrossRef] [PubMed]
- Guzman, M. Cannabinoids: Potential anticancer agents. Nat. Rev. Cancer 2003, 3, 745–755. [Google Scholar] [CrossRef] [PubMed]





| Parameters | Patients (n = 20) | |
|---|---|---|
| Age | 73.1 ± 12.4 (mean ± SD) | |
| Sex | Male | 11 |
| Female | 9 | |
| Tumor side 1 (R/L) | Right | 8 |
| Left | 12 | |
| Tumor stage 2 | Stage I | 2 |
| Stage II | 8 | |
| Stage III | 7 | |
| Stage IV | 3 | |
| Histological grading | Well differentiated | 2 |
| Moderately differentiated | 12 | |
| Poorly differentiated | 6 | |
| Gene | Primer | Sequence Primer |
|---|---|---|
| Human CB1-R | Sense | 5′-GGAGAACATCCAGTGTGGGG-3′ |
| Antisense | 5′-CATTGGGGCTGTCTTTACGG-3′ | |
| Human β-actin | Sense | 5′-AAAGACCTGTACGCCAACACAGTGCTGTCTGG-3′ |
| Antisense | 5′-CGTCATACTCCTGCTTGCTGATCCACATCTGC-3′ | |
| Mouse CB1-R | Sense | 5′-CCTGGGCTGGAACTGCAA-3′ |
| Antisense | 5′-CCGAAGACGTCATACACCATGA-3′ | |
| Mouse β-actin | Sense | 5′-GCCTCTGGTCGTACCACTGGC-3′ |
| Antisense | 5′-AGGGAGGAAGAGGATGCGGCA-3′ |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Notarnicola, M.; Tutino, V.; De Nunzio, V.; Dituri, F.; Caruso, M.G.; Giannelli, G. Dietary ω-3 Polyunsaturated Fatty Acids Inhibit Tumor Growth in Transgenic ApcMin/+ Mice, Correlating with CB1 Receptor Up-Regulation. Int. J. Mol. Sci. 2017, 18, 485. https://doi.org/10.3390/ijms18030485
Notarnicola M, Tutino V, De Nunzio V, Dituri F, Caruso MG, Giannelli G. Dietary ω-3 Polyunsaturated Fatty Acids Inhibit Tumor Growth in Transgenic ApcMin/+ Mice, Correlating with CB1 Receptor Up-Regulation. International Journal of Molecular Sciences. 2017; 18(3):485. https://doi.org/10.3390/ijms18030485
Chicago/Turabian StyleNotarnicola, Maria, Valeria Tutino, Valentina De Nunzio, Francesco Dituri, Maria Gabriella Caruso, and Gianluigi Giannelli. 2017. "Dietary ω-3 Polyunsaturated Fatty Acids Inhibit Tumor Growth in Transgenic ApcMin/+ Mice, Correlating with CB1 Receptor Up-Regulation" International Journal of Molecular Sciences 18, no. 3: 485. https://doi.org/10.3390/ijms18030485
APA StyleNotarnicola, M., Tutino, V., De Nunzio, V., Dituri, F., Caruso, M. G., & Giannelli, G. (2017). Dietary ω-3 Polyunsaturated Fatty Acids Inhibit Tumor Growth in Transgenic ApcMin/+ Mice, Correlating with CB1 Receptor Up-Regulation. International Journal of Molecular Sciences, 18(3), 485. https://doi.org/10.3390/ijms18030485