Abstract
Ovarian cancer is the eighth most common cancer in women worldwide, and epithelial ovarian cancer (EOC) represents 90% of cases. Nerve growth factor (NGF) and its high affinity receptor tyrosine kinase A receptor (TRKA) have been associated with the development of several types of cancer, including EOC; both NGF and TRKA levels are elevated in this pathology. EOC presents high angiogenesis and several molecules have been reported to induce this process. NGF increases angiogenesis through its TRKA receptor on endothelial cells, and by indirectly inducing vascular endothelial growth factor expression. Other molecules controlled by NGF include ciclooxigenase-2, disintegrin and metalloproteinase domain-containing protein 17 (ADAM17) and calreticulin (CRT), proteins involved in crucial processes needed for EOC progression. These molecules could be modified through microRNA regulation, which could be regulated by NGF. MicroRNAs are the widest family of non-coding RNAs; they bind to 3′-UTR of mRNAs to inhibit their translation, to deadenilate or to degraded them. In EOC, a deregulation in microRNA expression has been described, including alterations of miR-200 family, cluster-17-92, and miR-23b, among others. Since the NGF-microRNA relationship in pathologies has not been studied, this review proposes that some microRNAs could be associated with NGF/TRKA activation, modifying protein levels needed for EOC progression.
1. Introduction
Ovarian cancer is a deadly disease that causes over 140,000 deaths every year [1]. Given the lack of specific symptoms and the poor efficacy of the currently available treatments, the survival rate remains below 50% overall 5-year [2]. Nerve growth factor (NGF) and its high affinity receptor, Tyrosine kinase A receptor (TRKA), are overexpressed in ovarian cancer and they have been associated with increased proliferation, survival and angiogenesis [3].
NGF, through TRKA activation, can alter the expression of several molecules associated with cancer development and progression [3]. There is also evidence to suggest that NGF could control the expression of microRNAs (miRs) [4,5]. miRs are small non-coding RNA molecules that downregulate gene expression by acting on mRNAs [6]. Several miRs have been linked to cancer, either through overexpression or downregulation, altering the levels of oncogenes or tumor suppressor genes [7].
In this review, an overview is given about ovarian cancer and its most common form, epithelial ovarian cancer (EOC). A summary of neurotrophins and miRs roles on EOC is also provided. Finally, a potential link between EOC, NGF and miRs is established.
2. Ovarian Cancer
Ovarian cancer remains major health problem worldwide, with over 225,000 new cases and 140,000 deaths reported annually [1]. Symptoms associated with ovarian cancer are often nonspecific [8]; therefore, the majority of patients are diagnosed at advanced stages of the disease [9], which increases treatment cost and diminishes survival rate [10].
It has been suggested that ovarian cancer is linked to an increased number of menstrual cycles [11]; early menarche, null parity, late menopause and not taking oral contraceptives are among its risk factors [12]. Other risk factors include smoking [13], obesity [14] and estrogen-based hormone replacement therapy after menopause [15].
A dualistic model categorizes ovarian tumors into two groups: type I and type II [16]. Type I tumors are part of a morphological continuum of tumor progression starting with benign tumors that develop into borderline tumors and finally into invasive tumors. These invasive tumors lose their differentiation along with their progression. Type II tumors, on the other hand, are aggressive high-grade, poorly differentiated carcinomas that are usually diagnosed at an advanced stage and have poor prognosis [16]. Clinically, ovarian cancer is classified according to the International Federation of Gynecology and Obstetrics (FIGO) criteria [17]. According to these guidelines, stage I consists of a tumor that is limited to the ovaries, while stage II tumors have expanded to the peritoneal space. In stage III, the tumor has spread through one or both ovaries, accompanied by primary peritoneal cancer and metastasis to the retroperitoneal lymph nodes. Lastly, stage IV consists of distant metastasis to extra-abdominal organs [17].
For ovarian cancer, the 5-year survival rate is low [2]. Because of the lack of efficient early diagnosis tools, ovarian cancer is mostly diagnosed at advanced stages, as has already been stated. This is unfortunate, given that early detection is the most effective means of reducing ovarian cancer mortality [18]. For example, currently 25% of ovarian cancer cases are detected at stage I or II [9]; however, if 75% of ovarian cancer cases were to be detected at an early stage of the disease, the number of deaths would be reduced by 50% [19]. At present, only 15% of patients are diagnosed when the disease is confined to the ovary at the time of diagnosis [9]; in most cases, at the time of diagnosis the disease has already spread through the ovary and into the peritoneal space. Under these conditions ovarian cancer responds poorly to therapy and the 5-year survival rate drops to between 17% and 30% [20]. However, if diagnosed at an early stage, when the disease is restricted to the ovary, about 90% of patients survive 5 years after treatment [21]. The late diagnosis that characterizes ovarian cancer is in part due to the lack of specific symptoms, which include abdominal pain, fatigue, indigestion, constipation, back pain, menstrual irregularities and changes in appetite [22].
The main treatment for ovarian cancer is surgery followed by chemotherapy [23]. The surgery consists of cytoreduction in order to remove the tumor, and then chemotherapy is applied to eliminate the remaining tumor mass [24]. First line chemotherapy involves the use of platinum-based therapy, including cisplatin and carboplatin, with an adjuvant therapy like paclitaxel [25]. Around 70% of patients respond to this combination, making ovarian cancer a highly chemotherapy-sensible cancer [26]. However, approximately 60% of patients develop recurrence, and in these patients the returning tumor is resistant to the first line treatment [27]. Second- and third-line treatments usually do not have a high response rate, decreasing the 5-year survival rate to 27% [28].
Because the late diagnosis and poor response to treatment, ovarian cancer ranks among the leading causes of cancer death, being the eighth most common cause of mortality in women due to cancer worldwide [29]. Even though extensive research has been done in order to better diagnose and treatment for this disease, ovarian carcinoma pathogenesis is not yet completely understood.
3. Epithelial Ovarian Cancer
Ovarian cancer consists of a malignant tumor that forms in the ovary. The ovary has three main cell types that can develop into a different type of tumor: germ cells can grow into germ cell tumors [30], stromal cells give rise to stromal sex cord tumors [31] and the origin of EOC is from the epithelial cells of the ovary [32]. Most malignant tumors are of epithelial origin, comprising around 90% of all ovarian cancers [33].
About 10% to 20% of EOC cases are of hereditary origin [34], and mutations of the breast cancer gene 1 (BRCA1) or the breast cancer gene 2 (BRCA2) are the most significant genetic mutations associated with an increase in overall ovarian cancer risk [35]. EOC is more common in older, postmenauposal women; therefore, this tumor is usually found in inactive ovaries that are no longer undergoing reproductive cycles [36].
While it was originally thought that EOC originated from the epithelial cells that can be found on the outer layer of tissue surrounding the inactive ovary or on the surface of ovarian cysts [32], there are presently several theories relating to the cell origin of EOC. Some investigators propose that EOC could develop from cysts located in the secondary Müllerian system [37]. According to this theory, the tumor would grow from these cysts and thus appear to have an ovarian origin. Also, recent research suggests that EOC arises at the fallopian epithelium and it spreads to the ovary from there [38].
In EOC, just like in any cancer, several signaling pathways are altered, resulting in uncontrolled growth, apoptosis avoidance and the acquisition of invasion capability, among other carcinogenic features [39]. One of the most studied altered mechanisms in EOC is angiogenesis, a necessary process given the large size that characterizes ovarian tumors [40]. Vascular endothelial growth factor (VEGF) is an angiogenic factor and potent mitogen for the vascular endothelium [41] and one of the most important factors in ovarian angiogenesis, both in normal [42] and in pathological tissues [43]. Bevacizumab, a monoclonal antibody targeting VEGF, has shown moderate success in clinical trials against ovarian cancer [44,45] and it has been approved in Europe against advanced or recurrent in this disease in combination with other chemotherapy medicines [46].
In addition to the importance of angiogenesis in tumor processes, cancer cells are characterized by a lack of cell growth control [39]. This occurs in part through signaling generated from a variety of growth factor receptors, including the ones belonging to the tyrosine kinase receptor family [47]. Neurotrophins can interact with these receptors, inducing pro carcinogenic responses [48].
4. Neurotrophins and Their Role in Ovarian Cancer
Neurotrophins are a family of small polypeptides growth factors that consists of five members: NGF, brain-derived neurotrophic factor (BDNF), neurotrophin 3 (NT-3), neurotrophin 4/5 (NT-4/5) and neurotrophin 6 (NT-6) [49]. Neurotrophins act by interacting with two different types of receptors: p75 and the TRK members of the tyrosine kinase receptor superfamily [50]. The p75 receptor is able to bind all neurotrophins with low affinity [51], while different TRK receptors bind to a specific neurotrophin with high affinity: NGF binds to TRKA; BDNF and NT4/5 bind to TRKB and NT-3 binds to TRKC, initiating an intracellular signaling response [52].
NGF was the first neurotrophin to be described [53]. It was discovered in the nervous system; however, it has been found to have functions in several other systems, including the cardiovascular [54], endocrine [55], immune [55] and reproductive systems [56]. Neurotrophins and their receptors are involved in the development and normal functioning of the ovary: NGF plays a role in early follicular development by allowing differentiation of primordial follicles into follicles [57]. This function is apparently due to NGF’s ability to act in granulosa and theca cells and to induce their proliferation [58,59]. NGF also participates in ovulation by inducing the release of prostaglandins, a necessary step in the ovulatory molecular cascade [60]. Besides, NGF is able to induce the expression of Follicle Stimulating Hormone Receptor (FSH-R) and estradiol in human granulosa cells [61].
Angiogenesis is a key process during the normal ovarian cycle, due to the need of new blood vessel formation in each ovulatory cycle [62]. In fact, the ovary is one of the few organs in which angiogenesis occurs in a cyclically controlled manner. During reproductive life, VEGF is one of the most important proangiogenic factors involved in cyclic angiogenesis, by being participant in the periodic growth of follicles and in the development [63] and maintenance of the corpeus luteus [64,65]. This growth factor expression is controlled by several factors, including FSH and Luteinizing Hormone (LH), through their receptors FSHR and LHR, respectively [66]. Both NGF and TRKA, its high affinity receptor, are expressed in the ovary [3]. Equal to VEGF, TRKA and NGF are expressed before ovulation, suggesting a role of these proteins in ovarian function and angiogenesis [3]. Interestingly, NGF can induce angiogenesis in endothelial cells [67]. Besides, NGF is able to induce a neovascularization process in the superior ganglion of newborn rats, which is accompanied by an increase of VEGF production [68]. In granulosa cell lines, NGF is able to induce VEGF expression through TRKA activation [69]. These findings suggest that NGF and TRKA are important in the regulation of the normal ovarian function.
In EOC, angiogenesis, which is tightly controlled during the regular cycle, becomes unregulated [70]. Because of the normal angiogenesis that occurs on the cyclic ovary during reproductive life, it is possible to suggest that ovarian cells keep their capability for angiogenesis. After malignant transformation, cancer cells could use their advantage of inducing angiogenesis as a mean to acquire nutrients and oxygen. In line with this, both NGF and TRKA have been involved in the angiogenesis of EOC through VEGF induction [71], whose production is the main component of angiogenesis in ovarian cancer [72] (Figure 1). The VEGF gene encodes five different protein isoforms (VEGF121, VEGF145, VEGF165, VEGF189 and VEGF206) that are generated by alternative splicing from a single gene [73]. While VEGF121 is efficiently secreted from the cell, VEGF165 is partially retained on the cell surface and the other VEGF isoforms are primarily retained on the surface [73]. In EOC explants, NGF induces an increase of VEGF121, VEGF165 and VEGF189 mRNA levels, as well the amount of VEGF protein secreted from the explants; these actions are mediated by TRKA [71].
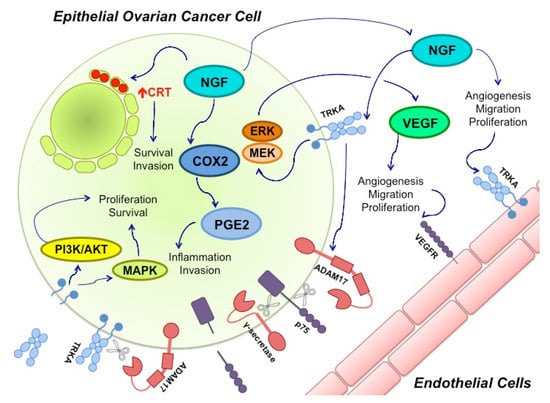
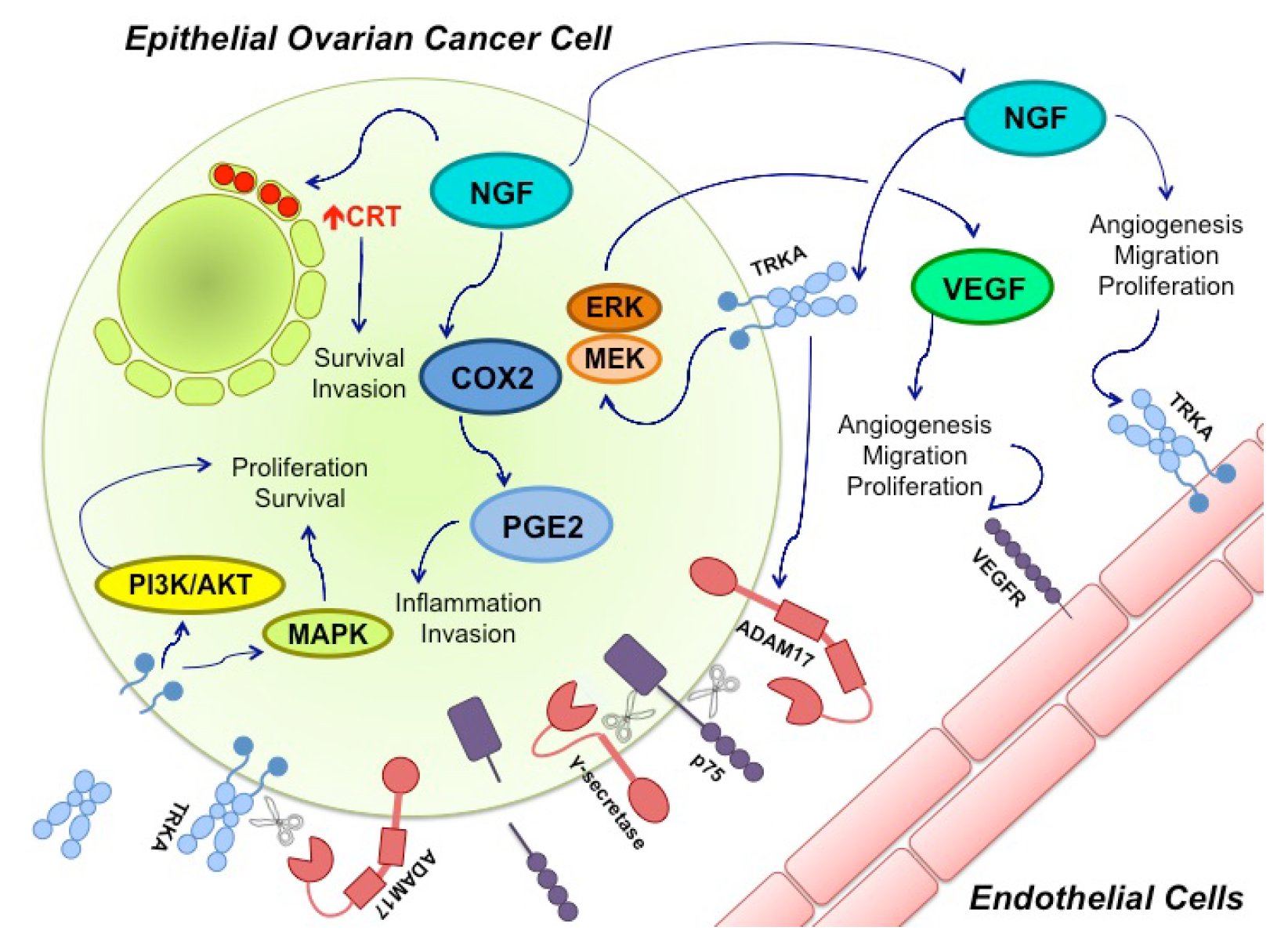
Figure 1.
Nerve growth factor (NGF) and its Tyrosine Kinase A receptor (TRKA) are involved in several signaling pathways in epithelial ovarian cancer (EOC). Ovarian cancer cells express and secrete NGF. Through TRKA activation, NGF induces angiogenesis by directly stimulating endothelial cell proliferation and migration. NGF also regulates angiogenesis in an indirect manner through vascular endothelial growth factor (VEGF) production by epithelial cancer cells. This mechanism involves TRKA activation and the Mitogen-activated protein kinase/Extracellular signal-regulated kinase (MEK/ERK) signaling pathway. Besides, NGF increases ciclooxigenase-2 (COX-2) levels, a proinflamatory molecule that induces protaglandine-2 (PGE-2) production; COX-2 and PGE-2 have been associated with invasion in cancer cells. NGF can also increase calreticulin (CRT) levels (Red Arrow), an endoplasmic reticulum resident whose levels have been found to be elevated in several cancers, where it participates in cell survival and invasion. Disintegrin and metalloproteinase domain-containing protein 17 (ADAM17), a proteinase, also seems to be regulated by NGF-TRKA activation. ADAM17 cleaves the p75 receptor, which is then cleaved a second time by γ-secretase. The biological effects of these cuts remain unknown. ADAM 17 can also cleave TRKA, leaving its intracellular domain active, which contributes to cancer progression through the MAPKs and PI3K/Akt pathways.
Neurotrophins have also been involved in other tumorigenic processes besides angiogenesis, including growth deregulation that normally occurs in cancer. Tyrosine kinase receptors are overexpressed in cancer, or have their activity altered [74,75]. As a result, there is an alteration of their intracellular signaling [76]. NGF and TRKA overexpression has been found in several cancer types, including thyroid [77], lung [78], esophagus [79], prostate [75], breast [74] and ovarian cancer [3]. In ovarian cancer, TRKA and its active form, p-TRKA, are overexpressed [80]. Interestingly, studies either have not found p-TRKA in normal tissues or it has only been found in a small number of samples [80]. Besides, NGF induces an increase of proliferation molecules and a decrease of apoptosis markers after NGF stimulation in EOC explants [81] (Figure 1).
NGF appears to be controlling other molecules involved in EOC tumorigenesis (Figure 1), such as ciclooxigenase-2 [82] a molecule that participates in inflammation through prostaglandine E2 production [83]. Disintegrin and metalloproteinase domain-containing protein 17 (ADAM17), a proteinase involved in metastasis and migration [84], also seems to be controlled by NGF [85]. ADAM17 is able to cause TRKA cleavage, facilitating cell survival and proliferation. Besides, upon TRKA activation through NGF binding, ADAM 17 cleaves p75 on its extracellular domain, and afterwards γ-secretase cleaves it on the intracellular domain [86]. The biological consequences of these processes are not completely understood. NGF also induces an increase of calreticulin levels [87], a chaperone that has been associated with survival and migration of cancer cells [88,89]. We are currently doing research to further elucidate these and other pathways in which NGF is involved with EOC carcinogenesis.
The evidence presented above shows that NGF alters the expression of several molecules that are involved in tumorigenic processes in ovarian cancer; however, this deregulation could be through different mechanisms: NGF could act at an epigenetic level, induce DNA mutations, induce chromosomal alterations, and/or alter post-transcriptional or post-translational regulations. Today, one of the most widely studied mechanisms for protein synthesis control is microRNA-dependent post-transcriptional regulation.
5. microRNAs and Cancer
miRs are the widest family of non-coding RNAs, their length is ~22 nucleotides and they regulate post-transcriptional mRNAs. In mammals, there have been ~2000 different miRs described, which are conserved in related species [90,91]. miRs are synthesized by RNApol II and they are cleaved, in the nucleus and cytoplasm, for their maturation. miR regulation is performed by the RNA-silencing inducing complex (RISC), composed by a multiprotein complex and the mature miR, where the mRNA target can be paired with partial or total complementarity with miR, leading to mRNA degradation, deadenilation or inhibition of its translation [92,93].
miRs have an important role regulating mRNA expression, which reflects on a modification in protein levels. Interestingly, one miR can regulate up to 30 mRNAs and one mRNA can be regulated by several miRs; then, an alteration of miR expression can regulate several processes. A deregulation of miR expression has been found in several pathologies, including glaucoma, neurodegenerative diseases [94], cardiovascular pathologies [95], metabolic diseases [96] and cancer [97], among others. Since deregulation in miR expression is crucial for cancer development [98,99], the roles of miR in this pathology are divided into two groups: oncomiRs and tumor suppressor miRs [100]. OncomiRs regulate mRNA of tumor suppressor genes, while tumor suppressor miRs regulate oncogenic gene mRNA expression. OncomiRs are found to be overexpressed, reducing tumor suppressor gene expression, at the same time that tumor suppressor miRs are found to be downregulated, increasing oncogenic gene expression (Figure 2) [101]. miR expression can be modified in different types of cancer regulating the same mRNAs and producing equal effects in neoplastic transformation. This is a very interesting phenomenon, since different types of cancer could have similar targets that could initiate tumorigenic development [99].
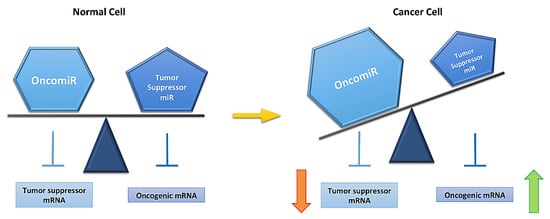
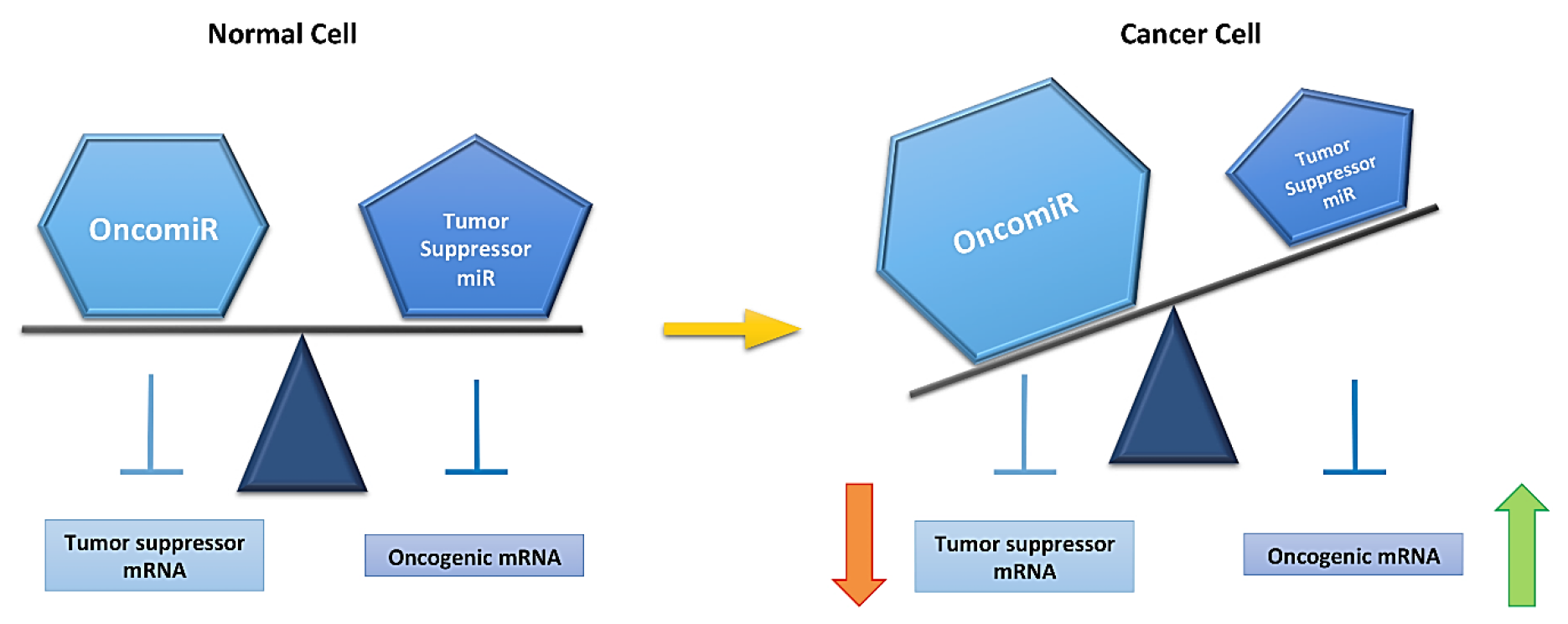
Figure 2.
MicroRNA (miR) expression is altered in a cancer cell. A normal cell has equilibrium between oncomiRs (overexpressed miRs in cancer) and tumor suppressor miRs (downregulated miRs in cancer), This equibrium is lost in a cancer cells (yellow arrow). This is reflected on mRNA expression, rising oncogenic and decreasing tumor suppressor mRNA levels, and allowing for cancer development.
Studies have been done in order to find a miR expression pattern in diverse types of cancer, which is being undertaken both in tissue samples, and in samples that require less invasive methods of extraction, like blood and serum [102]. Even though there is no consensus about miR expression patterns yet [103], some of those that are being studied are breast [104], ovarian [105], esophageal [106], renal [107] and bladder [108] cancers. In ovarian cancer [109], miRs could potentially be used as reliable markers for diagnosis, prognosis [110] and even as therapeutic targets [111].
6. Role of miRs in Ovarian Cancer
Several studies have been performed in order to evaluate the role of miRs in ovarian cancer; however, this information has not yet been used with a clinical approach. Some miRs that have been found altered in ovarian cancer include Let-7, miR-200 family, miR-17-92, miR-21, miR-145 and miR-23b [112,113,114]. Most of them have also been studied in other types of cancer, and they can have different roles depending on the cancer type.
In ovarian cancer, Let-7 is a family of tumor suppressor miR, inhibiting a downstream component of the EGFR signaling network (KRAS), regulating cancer-cell proliferation [115], protein that belongs to the non-histone chromosomal high-mobility group (HMGA2) associated with both malignant and benign tumor formation, as well as certain characteristic cancer-promoting mutations [116] and c-Myc [117]; then Let-7 has been identified as a potential maker for early diagnosis [118]. Concerning miR-200 family, it has been reported that miR-200c inhibits protein Zinc finger E-box-binding homeobox 1 (ZEB-1) and miR-200a inhibits ZEB-2 [119], proteins that are important transcription factors that allow epithelial–mesenquimal transition and associated with tumor progression. miR-141 inhibits Keap-1 [120] and miR-200c can regulate B-tubulin III expression [121]. Finally, if miR-200c levels are restored, there is an increase in overall free progression and survival, and it sensitizes cancer cells to cisplatin and paclitaxel therapies [122]. The miR-17-92 cluster, an oncomiR, presents a high frequency of genomic alterations [123] and it induces angiogenesis through inhibition of thrombospondin 1 [124]. miR-21, an oncomir, regulates Phosphatase and tensin homolog (PTEN), this enzyme acts as a tumor suppressor [125], it has an inverse relation with programmed cell death protein 4 (PDCD4) expression [126] and it exerts an inhibitory effect on cancer cell proliferation [127]. This miR has also been associated with recurrence-free survival, which will be lower if miR-21 levels are elevated [128]. miR-145 belongs to the miR143/145 cluster [129]; both miRs have been described as tumor suppressor miRs, and it has been proposed that the downregulation of miR-145 could be used as a predictive value for cancer. miR-145 has been implicated in angiogenesis, decreasing molecules that stimulate this process, like p79S6K1, which regulates hipoxic inducing factor-1a, an important growth factor that stimulates VEGF production, and also regulates directly to VEGF expression [130]. miR-145 also inhibits c-Myc [131], decreasing proliferation and inducing apoptosis. Most studies about miR-145 are focused on miR-145-5p, which is defined as the mature strand, while miR-145-3p is the passenger strand. Interestingly, it has recently been reported that both of these miRs could have biological activity, and it is very rare to find duplex miRs where both strands have biological functions [132]. miR-23b belongs to the miR-23b/27b/24 cluster, it has been described as a tumor suppressor, and some miR-23b targets include cyclin G1 [133] and the transcription factor RUNX2, involved in cellular survival, migration and invasion [134]. In ovarian cancer patients, miR-23b has been found to be downregulated and it has been associated with advanced tumor progression and poor prognosis [133].
7. NGF and miRs in Ovarian Cancer
NGF and miR roles in ovarian cancer are well described above, but NGF’s role in miR expression in ovarian cancer is far from well understood. There are some studies regarding miR alteration due to neutrophin action. For example, it was reported that miR-204 upregulation downregulates BDNF in order to enhance anoikis in EOC [135]. With respect to the action of NGF, most of these studies have not been done in EOC or even in cancer; but on neuronal models. What has been reported? Firstly, NGF signaling through ERK protein increases miR-222 and miR-221 levels [4]; secondly, NGF overexpresses miR-221 levels, with consequences in neuronal differentiation [5]; thirdly, NGF raises miR-21 levels, supporting its signaling and protecting cells from neuronal degeneration [136]. While all of these studies have been done on a PC12 cell line, there are also a couple of reports done in bladder [137] and bronchial epithelium [138].
In relation to the signaling pathways that could be involved in NGF-dependent miR expression levels, it has been reported that NGF can stimulate AKT and MAPK phosphorylation in an miR-21-dependent manner, increasing VEGF levels (which are raised with NGF stimulation in EOC). Therefore, miR-21 can be considered as a positive regulator of this neurothrophin signaling [136]. On the other hand, NGF can function as a positive regulator by increasing miR-221/222 through the activation of the ERK1/2 signaling pathway [4]. Another important protein for EOC metastasis is ADAM17, which is regulated by a metalloproteinase inhibitor (TIMP3). miR-222 targets TIMP3, decreasing its levels and allowing ADAM17 expression [139]. Ciclooxigenase-2 (COX-2), an important protein up-regulated in EOC, is regulated by miR-143 [140]. It has been published that this miR is downregulated in EOC [141]; besides, it decreases with NGF stimulation in a cellular line derived from a pheochromocytoma of the rat adrenal medulla (PC12) [5]. Therefore, a NGF-dependent decrease of miR-143 could increase COX-2 levels in EOC.
We are currently studying the downregulation of miR-23b in EOC [133]. As mentioned above, this downregulation coincides with the overexpression of NGF. miR-23b has several validated targets, some of which are shown in Table 1. The table focuses on those that are involved in different processes associated with EOC, NGF or both. miR-23b target genes related with NGF and EOC include proto-oncogene tyrosine-protein kinase (SRC) and a protein that is involved in controlling the activation of RAS/MAPK signaling (SOS1) and super oxide dismutase 1 (SOD1). SRC is a protein involved in TRK signal transduction, SOS1 is a guanine nucleotide exchange factor (GEF) that is also related to TRK transduction, and finally SOD1 is an enzyme that is in charge of converting superoxide radical into hydrogen peroxide, which could enhance DNA damage.

Table 1.
miR-23b validated targets from miRWalk 2.0.
The evidence presented above leads us to hypothesize that NGF could be regulating miR-23b downregulation, which is an important step for EOC development. To date, as shown in Figure 3, our results have indicated that miR-23b is downregulated during ovarian cancer progression: in tumor and cancer tissue samples miR-23b levels are lower when compared to inactive ovarian tissue samples (Figure 3A, p < 0.01). A comparison between basal conditions of A2780 cells (epithelial ovarian cancer cell line) and HOSE cells (epithelial cells from human inactive ovary) show that miR-23b levels are lower in the A2780 cell line (Figure 3B, p < 0.05), suggesting that the higher expression of NGF and TRKA in A2780 cell line could affect miR-23b levels. When we evaluated NGF effect on miR-23b expression in both cell lines, we found that stimulation of A2780 with NGF reduces miR-23b levels (Figure 3C, p < 0.05), and in HOSE cells a similar effect was found (Figure 3D, p < 0.05). Summarizing, these results show that: (a) miR-23b levels are down-regulated in ovarian cancer, as has been reported; and (b) NGF reduces miR-23b levels in HOSE and A2780 cell lines.
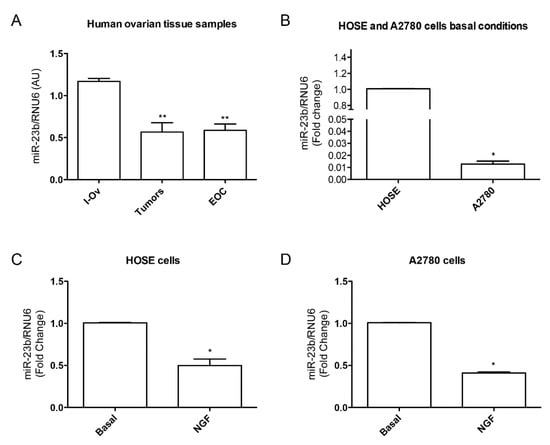
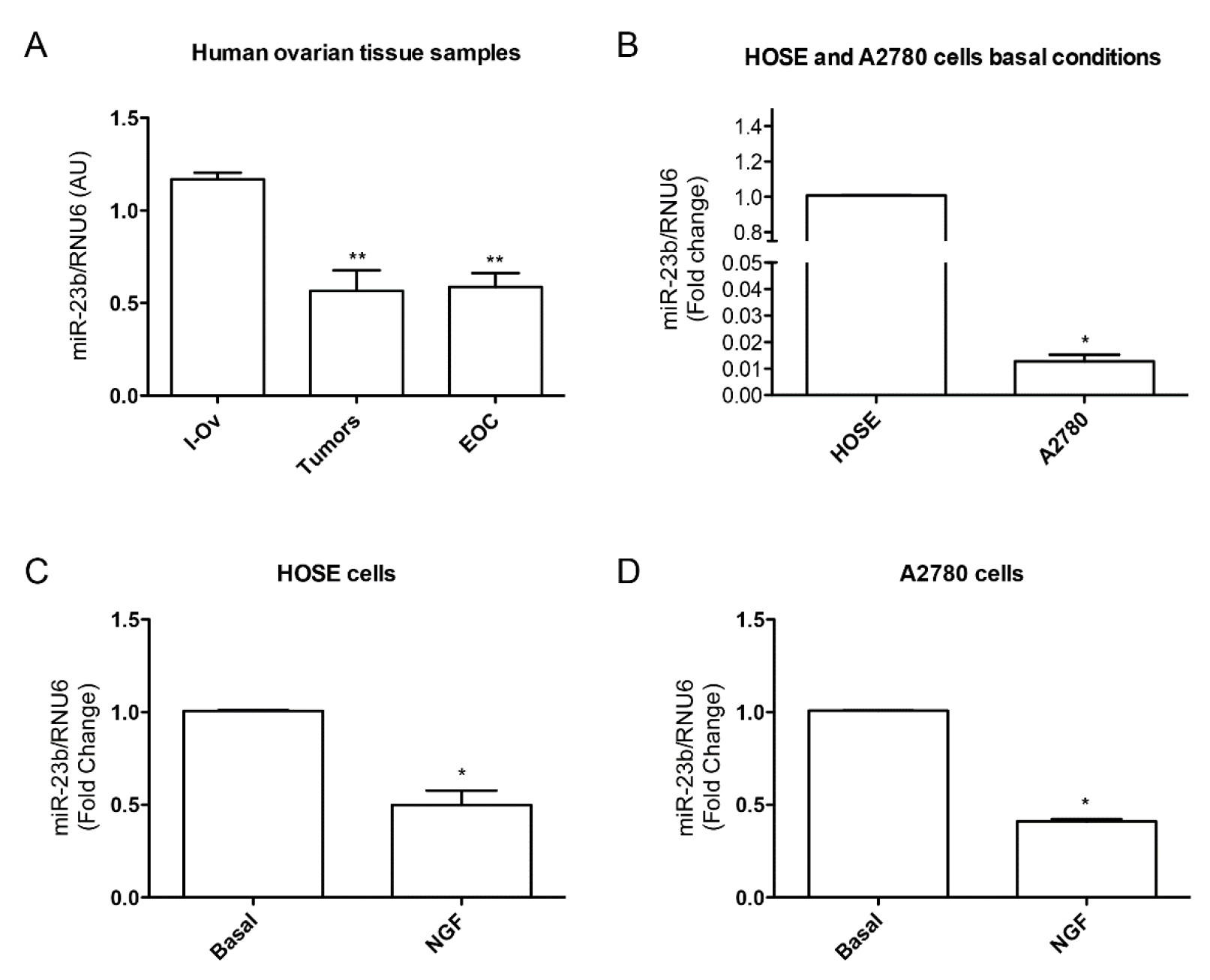
Figure 3.
miR-23b levels in epithelial ovarian cancer and its relationship with NGF in cell lines. (A) miR-23b levels in tissues from inactive ovary (I-Ov), tumor and EOC samples. miR-23b levels from tumor and EOC samples are significantly lower than I-Ov (** = p < 0.01); (B) miR-23b basal level comparision between HOSE and A2780 cell lines (* = p < 0.05); (C) NGF effect on miR-23b levels in HOSE cell line. NGF reduces miR-23b levels respect to basal condition (* = p < 0.05); (D) NGF effect on miR-23b levels in A2780 cell line. NGF reduces miR-23b levels respect to basal condition (* = p < 0.05). Stadistic: Kruskal–Wallis Test: A; Mann–Whitney Test: B, C and D.
The next step is to evaluate which proteins regulated by miR-23b in EOC could be enhancing carcinogenic processes in EOC, and if NGF is regulating expression of these proteins. One example of a miR-23b target and its contribution to EOC development is SRC, an oncogene deregulated in several solid tumors, including ovarian cancer. It is a serine/threonine kinase that activates three signaling pathways: STAT3/MYC, MAPKs and PI3K. While SRC is inactive in normal tissues (when miR-23b presents higher levels), it can be activated and overexpressed in cancer cells, inducing carcinogenic processes such as angiogenesis, proliferation, invasion, motility and chemoresistance. SRC has been found to be elevated in some serous EOC patients, making it a possible therapeutic target [142]. Another example of a mR-23b target is SOS1, which participates in cell migration, a process that requires the participation of proteins from the Rho-GTPase family, including Rac. Rac activity is regulated by Ras through SOS1, and it is responsible for the reorganization of actin cytoskeleton, a necessary process for cell migration. Besides, in ovarian cancer patients, the expression of proteins involved in epithermal growth factor receptor (EGFR) such as a tri-complex SOS1 and Epithermal growth factor receptor Pathway Substrate 8 and an adapter protein ABI-1 (SOS1/EPS8/ABI1) This complex is correlated with advanced clinical stage. In addition, this complex is only present in metastatic ovarian cancer cells, while it is absent in non-metastatic cells [143]. Another molecule involved in EOC development is the transcription factor c-Myc. This factor increases when ovarian cancer cells are stimulated with NGF [81]. Interestingly, it is known that c-Myc decreases miR-23b expression in myeloma [144]. Thus, the negative regulation of miR-23b by c-Myc through NGF could favor EOC progression. The perspectives of these results and analyses are to evaluate the role of NGF in miR-23b regulation in EOC-involved targets.
8. Conclusions and Perspectives
NGF is a relevant molecule that stimulates epithelial ovarian cancer cell proliferation, migration, invasion and angiogenesis; processes that are crucial for ovarian cancer development. On the other hand, the post-transcriptional regulation of miRs is altered in several pathologies, including EOC, and this deregulation is involved in different characteristics that initiate neoplastic transformation. With respect to how NGF and miR are involved in EOC progression, it is important to evaluate its relationship in this pathology. NGF could modify some miR levels, oncomiRs and tumor suppressor miRs, in order to increase levels of VEGF, COX2, ADAM17, and other proteins (Figure 4). As reported above, it is known that NGF regulates some miR expression in EOC, but it is necessary to further investigate in order to know which miR could be used as an early diagnosis tool and/or for therapy development.
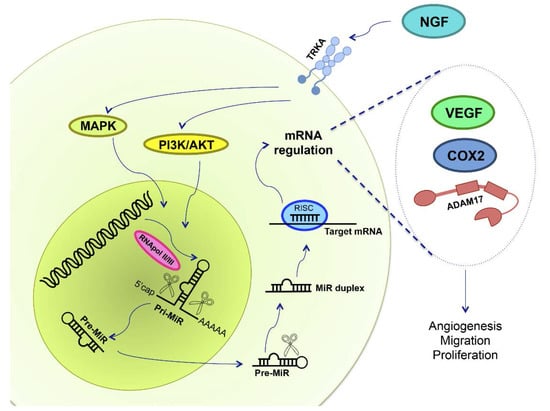
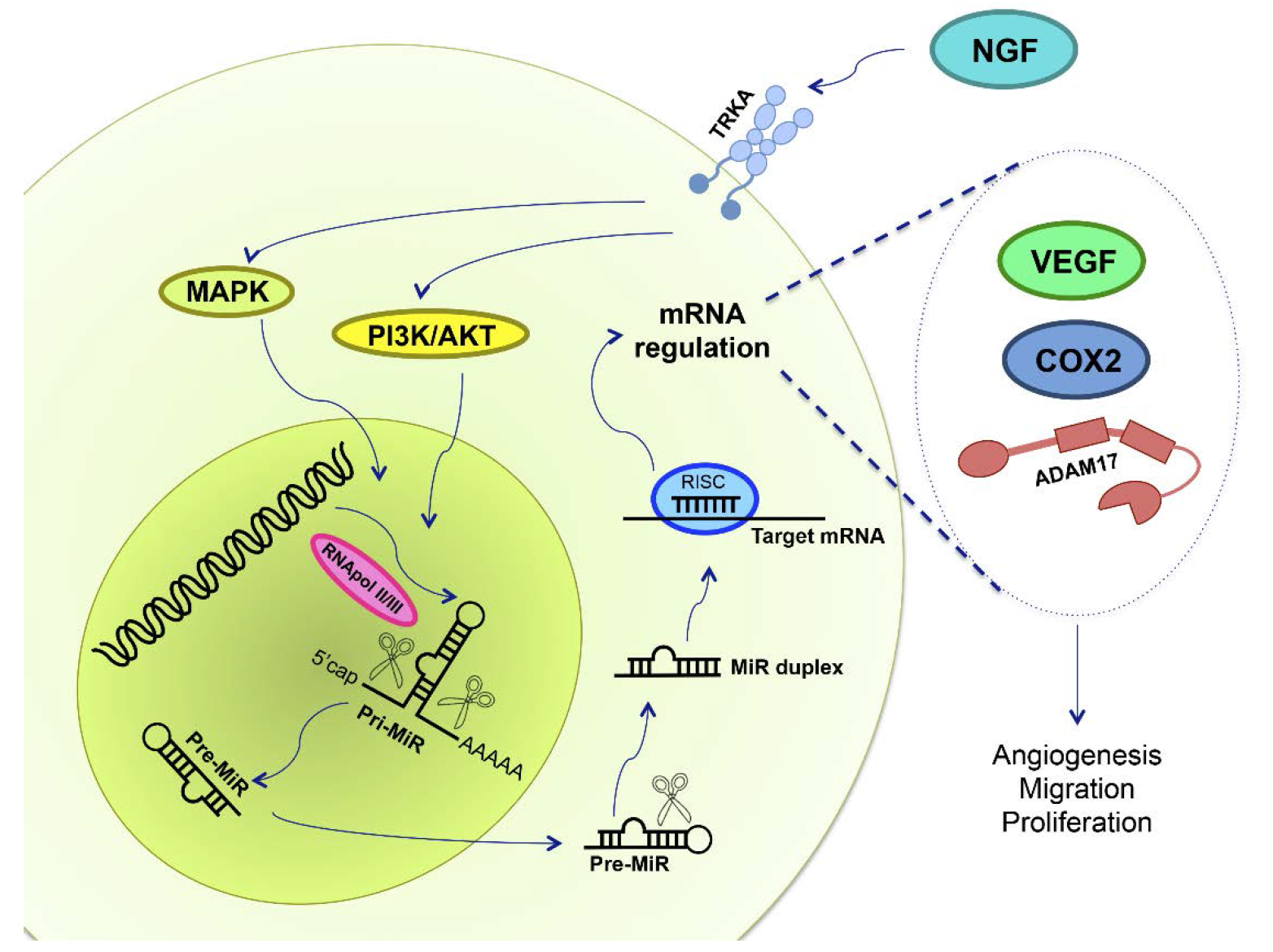
Figure 4.
NGF and miRs in epithelial ovarian cancer. NGF binds to its high affinity receptor TRKA, activating MAPK and PI3K/AKT signaling pathways. Deregulation in miR expression has been reported in EOC, and NGF could regulate primary-miR (pri-MiR) synthesis. RNApol II/III synthesizes miRs, and afterwards proteins cleave both pri-miR and precursor miRs (pre-miR), in the nucleus and in the cytoplasm, respectively, leading to miR maturation. mRNA repression is done by RNA-silencing inducing complex (RISC), a multiprotein complex, and by the mature miR, regulating mRNA expression through its degradation, deadenilation or inhibiting its translation. Therefore, we hypothesized that NGF/TRKA modifies miR expression in order to regulate VEGF, COX2 and ADAM17 protein levels. An increase of VEGF, COX2 and ADAM17 are related with changes in angiogenesis, migration and proliferation, processes needed for EOC development.
Acknowledgments
This review was supported by grants #1110372 and #1160139 from Fondo Nacional de Desarrollo Científico y Tecnológico, Chile (to CR). Conicyt-Fondap 15130011 (ACCDiS).
Conflicts of Interest
The authors declare no conflict of interest.
Abbreviations
| EOC | Epithelial ovarian cancer |
| NGF | Nerve growth factor |
| VEGF | Vascular endothelial growth factor |
| miR | MicroRNA |
| TRKA | Tyrosine kinase A receptor |
| ADAM17 | Disintegrin and metalloproteinase domain-containing protein 17 |
References
- Ferlay, J.; Shin, H.R.; Bray, F.; Forman, D.; Mathers, C.; Parkin, D.M. Estimates of worldwide burden of cancer. Int. J. Cancer. 2008, 127, 2893–2917. [Google Scholar] [CrossRef] [PubMed]
- Siegel, R.; Ward, E.; Brawley, O.; Jemal, A. Cancer statistics, 2011: The impact of eliminating socioeconomic and racial disparities on premature cancer deaths. CA Cancer J. Clin. 2011, 61, 212–236. [Google Scholar] [CrossRef] [PubMed]
- Vera, C.; Tapia, V.; Vega, M.; Romero, C. Role of nerve growth factor and its TRKA receptor in normal ovarian and epithelial ovarian cancer angiogenesis. J. Ovarian Res. 2014, 7, 82. [Google Scholar] [CrossRef] [PubMed]
- Terasawa, K.; Ichimura, A.; Sato, F.; Shimizu, K.; Tsujimoto, G. Sustained activation ERK1/2 by NGF induces microRNA-221 and 222 in PC12 cells. FEBS J. 2009, 276, 3269–3276. [Google Scholar] [CrossRef] [PubMed]
- Hamada, N.; Fujita, Y.; Kojima, T.; Kitamoto, A.; Akao, Y.; Nozawa, Y.; Ito, M. MicroRNA expression profiling of NGF-treated PC12 cells revealed a critical role for miR-221 in neuronal differentiation. Neurochem. Int. 2012, 60, 743–750. [Google Scholar] [CrossRef] [PubMed]
- Ruvkun, G. Molecular biology: Glimpses of a tiny RNA world. Science 2001, 294, 797–799. [Google Scholar] [CrossRef] [PubMed]
- Jansson, M.D.; Lund, A.H. MicroRNA and cancer. Mol. Oncol. 2012, 6, 590–610. [Google Scholar] [CrossRef] [PubMed]
- Smith, L.K.; Pope, C.; Botha, J.L. Patients’ help-seeking experiences and delay in cancer presentation: A qualitative synthesis. Lancet 2005, 366, 3–9. [Google Scholar] [CrossRef]
- Howlader, N.; Noone, A.M.; Krapcho, M.; Miller, D.; Bishop, K.; Altekruse, S.F.; Kosary, C.L.; Yu, M.; Ruhl, J.; Tatalovich, Z.; et al. SEER Cancer Statistics Review, 1975-2013; National Cancer Institute: Bethesda, MD, USA, 2016. [Google Scholar]
- Brown, P.O.; Palmer, C. The preclinical natural history of serous ovarian cancer: Defining the target for early detection. PLoS Med. 2009, 6, e1000114. [Google Scholar] [CrossRef] [PubMed]
- Schildkraut, J.M.; Bastos, E.; Berchuck, A. Relationship between lifetime ovulatory cycles and overexpression of mutant p53 in epithelial ovarian cancer. J. Natl. Cancer Inst. 1997, 89, 932–938. [Google Scholar] [CrossRef] [PubMed]
- Hunn, J.; Rodriguez, G. Ovarian cancer: Etiology, risk factors, and epidemiology. Clin. Obstet. Gynecol. 2012, 55, 3–23. [Google Scholar] [CrossRef] [PubMed]
- Faber, M.T.; Kjær, S.K.; Dehlendorff, C.; Chang-Claude, J.; Andersen, K.K.; Høgdall, E.; Webb, P.; Jordan, S.; Rossing, M.A.; Doherty, J.A.; et al. Cigarette smoking and risk of ovarian cancer: A pooled analysis of 21 case-control studies. Cancer Cause Control 2013, 24, 989–1004. [Google Scholar] [CrossRef] [PubMed]
- Olsen, C.M.; Nagle, C.M.; Whiteman, D.C.; Ness, R.; Pearce, C.L.; Pike, M.C.; Rossing, M.A.; Terry, K.L.; Wu, A.H.; Australian Cancer Study (Ovarian Cancer); et al. Obesity and risk of ovarian cancer subtypes: Evidence from the ovarian cancer association consortium. Endocr. Related Cancer 2013, 20, 251–262. [Google Scholar] [CrossRef] [PubMed]
- Hein, A.; Thiel, F.C.; Bayer, C.M.; Fasching, P.A.; Häberle, L.; Lux, M.P.; Renner, S.P.; Jud, S.M.; Schrauder, M.G.; Müller, A.; et al. Hormone replacement therapy and prognosis in ovarian cancer patients. Eur. J. Cancer Prev. 2013, 22, 52–58. [Google Scholar] [CrossRef] [PubMed]
- Kurman, R.J.; Shih, I.M. The dualistic model of ovarian carcinogenesis: Revisited, revised, and expanded. Am. J. Pathol. 2016, 186, 733–747. [Google Scholar] [CrossRef] [PubMed]
- Zeppernick, F.; Meinhold-Heerlein, I. The new FIGO staging system for ovarian, fallopian tube, and primary peritoneal cancer. Archiv. Gynecol. Obstet. 2014, 290, 839–842. [Google Scholar] [CrossRef] [PubMed]
- Gilbert, L.; Basso, O.; Sampalis, J.; Karp, I.; Martins, C.; Feng, J.; Piedimonte, S.; Quintal, L.; Ramanakumar, A.V.; Takefman, J. Assessment of symptomatic women for early diagnosis of ovarian cancer: Results from the prospective DOvE pilot project. Lancet Oncol. 2012, 13, 285–291. [Google Scholar] [CrossRef]
- Das, P.M.; Bast, R.B. Early detection of ovarian cancer. Biomark. Med. 2008, 2, 291–303. [Google Scholar] [CrossRef] [PubMed]
- Chi, D.S.; Eisenhauer, E.L.; Lang, J.; Huh, J.; Haddad, L.; Abu-Rustum, N.R.; Sonoda, Y.; Levine, D.A.; Hensley, M.; Barakat, R.R. What is the optimal goal of primary cytoreductive surgery for bulky stage IIIC epithelial ovarian carcinoma (EOC)? Gynecol. Oncol. 2006, 103, 559–564. [Google Scholar] [CrossRef] [PubMed]
- Seidman, J.D.; Zhao, P.; Yemelyanova, A. “Primary peritoneal” high-grade serous carcinoma is very likely metastatic from serous tubal intraepithelial carcinoma: Assessing the new paradigm of ovarian and pelvic serous carcinogenesis and its implications for screening for ovarian cancer. Gynecol. Oncol. 2011, 120, 470–473. [Google Scholar] [CrossRef] [PubMed]
- National Institute for Health and Care Excellence (NICE). Ovarian Cancer: The Recognition and Initial Management of Ovarian Cancer. Available online: http://www.nice.org.uk/guidance/CG122 (accessed on 20 December 2016).
- Van der Burg, M.E.; van Lent, M.; Buyse, M.; Kobierska, A.; Colombo, N.; Favalli, G.; Lacave, A.J.; Nardi, M.; Renard, J.; Pecorelli, S. The effect of debulking surgery after induction chemotherapy on the prognosis in advanced epithelial ovarian cancer. N. Engl. J. Med. 1995, 332, 629–634. [Google Scholar] [CrossRef] [PubMed]
- Banerjee, S.; Kaye, S.B. New strategies in the treatment of ovarian cancer: Current clinical perspectives and future potential. Clin. Cancer Res. 2012, 19, 961–968. [Google Scholar] [CrossRef] [PubMed]
- Katsumata, N.; Yasuda, M.; Takahashi, F.; Isonishi, S.; Jobo, T.; Aoki, D.; Tsuda, H.; Sugiyama, T.; Kodama, S.; Kimura, E.; et al. Dose-dense paclitaxel once a week in combination with carboplatin every 3 weeks for advanced ovarian cancer: A phase 3, open-label, randomised controlled trial. Lancet 2009, 374, 1331–1338. [Google Scholar] [CrossRef]
- Jelovac, D.; Armstrong, D.K. Recent progress in the diagnosis and treatment of ovarian cancer. CA Cancer J. Clin. 2011, 61, 183–203. [Google Scholar] [CrossRef] [PubMed]
- Jayson, G.C.; Kohn, E.C.; Kitchener, H.C.; Ledermann, J.A. Ovarian cancer. Lancet 2014, 384, 1376–1388. [Google Scholar] [CrossRef]
- Markman, M.; Markman, J.; Webster, K.; Zanotti, K.; Kulp, B.; Peterson, G.; Belinson, J. Duration of response to second-line, platinum-based chemotherapy for ovarian cancer: Implications for patient management and clinical trial design. J. Clin. Oncol. 2004, 22, 3120–3125. [Google Scholar] [CrossRef] [PubMed]
- Torre, L.A.; Bray, F.; Siegel, R.L.; Ferlay, J.; Lortet-Tieulent, J.; Jemal, A. Global cancer statistics, 2012. CA Cancer J. Clin. 2015, 65, 87–108. [Google Scholar] [CrossRef] [PubMed]
- Low, J.; Ilancheran, A.; Ng, J.S. Malignant ovarian germ-cell tumours. Best. Pract. Res. Clin. Obstet. 2012, 26, 347–355. [Google Scholar] [CrossRef] [PubMed]
- Garbi, A.; Achilarre, M.T.; Colombo, N. Ovarian Sex-Cord Tumors. In Ovarian Cancers; Pujade-Lauraine, E., Ray-Coquard, I., Lécuru, F., Eds.; Springer International Publishing: Cham, Switzerland, 2016; pp. 261–279. [Google Scholar]
- Auersperg, N.; Wong, A.S.; Choi, K.C.; Kang, S.K.; Leung, P.C. Ovarian surface epithelium: Biology, endocrinology, and pathology. Endocr. Rev. 2001, 22, 255–288. [Google Scholar] [CrossRef] [PubMed]
- Matz, M.; Coleman, M.P.; Carreira, H.; Salmerón, D.; Chirlaque, M.D.; Allemani, C. The histology of ovarian cancer: Worldwide distribution and implications for international survival comparisons (CONCORD-2). Ginecol. Oncol. 2016. [Google Scholar] [CrossRef] [PubMed]
- Pharoah, P.D.; Ponder, B.A. The genetics of ovarian cancer. Best Pract. Res. Clin. Obstet. Gynaecol. 2002, 16, 449–468. [Google Scholar] [CrossRef] [PubMed]
- Petrucelli, N.; Daly, M.B.; Pal, T. BRCA1 and BRCA2 Associated Hereditary Breast and Ovarian Cancer. In GeneReviews®; Pagon, R.A., Adam, M.P., Ardinger, H.H., Wallace, S.E., Amemiya, A., Bean, L.J.H., Bird, T.D., Ledbetter, N., Mefford, H.C., Smith, R.J.H., Eds.; University of Washington: Seattle, WA, USA, 1998. [Google Scholar]
- Lowe, K.A.; Chia, V.M.; Taylor, A.; O’Malley, C.; Kelsh, M.; Mohamed, M.; Mowat, F.S.; Goff, B. An international assessment of ovarian cancer incidence and mortality. Gynecol. Oncol. 2013, 130, 107–114. [Google Scholar] [CrossRef] [PubMed]
- Dubeau, L.; Drapkin, R. Coming into focus: The nonovarian origins of ovarian cancer. Ann. Oncol. 2013, 24, 28–35. [Google Scholar] [CrossRef] [PubMed]
- Erickson, B.K.; Conner, M.G.; Landen, C.N. The role of the fallopian tube in the origin of ovarian cancer. Am. J. Obstet. Gynecol. 2013, 209, 409–414. [Google Scholar] [CrossRef] [PubMed]
- Hanahan, D.; Weinberg, R.A. Hallmarks of cancer: The next generation. Cell 2011, 144, 646–674. [Google Scholar] [CrossRef] [PubMed]
- Carmeliet, P.; Jain, R.K. Angiogenesis in cancer and other diseases. Nature 2000, 407, 249–257. [Google Scholar] [CrossRef] [PubMed]
- Carmeliet, P. VEGF as a key mediator of angiogenesis in cancer. Oncology 2005, 69, 4–10. [Google Scholar] [CrossRef] [PubMed]
- Ferrara, N.; Frantz, G.; LeCouter, J.; Dillard-Telm, L.; Pham, T.; Draksharapu, A.; Giordano, T.; Peale, F. Differential expression of the angiogenic factor genes vascular endothelial growth factor (VEGF) and endocrine gland-derived VEGF in normal and polycystic human ovaries. Am. J. Pathol. 2003, 162, 1881–1893. [Google Scholar] [CrossRef]
- Wong, C.; Wellman, T.L.; Lounsbury, K.M. VEGF and HIF-1α expression are increased in advanced stages of epithelial ovarian cancer. Gynecol. Oncol. 2003, 91, 513–517. [Google Scholar] [CrossRef] [PubMed]
- Pujade-Lauraine, E.; Hilpert, F.; Weber, B.; Reuss, A.; Poveda, A.; Kristensen, G.; Sorio, R.; Vergote, I.; Witteveen, P.; Bamias, A. Bevacizumab combined with chemotherapy for platinum-resistant recurrent ovarian cancer: The AURELIA open-label randomized phase III trial. J. Clin. Oncol. 2014, 32, 1302–1308. [Google Scholar] [CrossRef] [PubMed]
- Oza, A.M.; Cook, A.D.; Pfisterer, J.; Embleton, A.; Ledermann, J.A.; Pujade-Lauraine, E.; Kristensen, G.; Carey, M.S.; Beale, P.; Cervantes, A. Standard chemotherapy with or without bevacizumab for women with newly diagnosed ovarian cancer (ICON7): Overall survival results of a phase 3 randomised trial. Lancet Oncol. 2015, 16, 928–936. [Google Scholar] [CrossRef]
- The Electronic Medicines Compendium. Avastin 25 mg/mL Concentrate for Solution for Infusion. Available online: https://www.medicines.org.uk/emc/medicine/15748/SPC/Avastin+25mg+ml+concentrate+for+solution+for+infusion/ (accessed on 16 December 2016).
- Gschwind, A.; Fischer, O.M.; Ullrich, A. The discovery of receptor tyrosine kinases: Targets for cancer therapy. Nat. Rev. Cancer 2004, 4, 361–370. [Google Scholar] [CrossRef] [PubMed]
- Nakagawara, A. TRK receptor tyrosine kinases: A bridge between cancer and neural development. Cancer Lett. 2001, 169, 107–114. [Google Scholar] [CrossRef]
- Lewin, G.R.; Barde, Y.A. Physiology of the neurotrophins. Annu. Rev. Neurosci. 1996, 19, 289–317. [Google Scholar] [CrossRef] [PubMed]
- Chao, M.V.; Hempstead, B.L. p75 and TRK: A two-receptor system. Trends Neurosci. 1995, 18, 321–326. [Google Scholar] [CrossRef]
- Chao, M.V. The p75 neurotrophin receptor. J. Neurobiol. 1994, 25, 1373–1385. [Google Scholar] [CrossRef] [PubMed]
- Patapoutian, A.; Reichardt, L.F. TRK receptors: Mediators of neurotrophin action. Curr. Opin. Neurobiol. 2001, 11, 272–280. [Google Scholar] [CrossRef]
- Aloe, L. Rita Levi-Montalcini and the discovery of NGF, the first nerve cell growth factor. Arch. Ital. Biol. 2011, 149, 175–181. [Google Scholar] [PubMed]
- Hein, L. The Neuroendocrine Adrenergic System and Cardiovascular Function. In The Cardiovascular Adrenergic System; Springer International Publishing: Cham, Switzerland, 2015; pp. 117–132. [Google Scholar]
- Procaccini, C.; Pucino, V.; de Rosa, V.; Marone, G.; Matarese, G. Neuro-endocrine networks controlling immune system in health and disease. Front. Immunol. 2014, 5, 143. [Google Scholar] [CrossRef] [PubMed]
- Streiter, S.; Fisch, B.; Sabbah, B.; Ao, A.; Abir, R. The importance of neuronal growth factors in the ovary. Mol. Hum Reprod. 2015. [Google Scholar] [CrossRef] [PubMed]
- Chaves, R.N.; Alves, A.M.; Lima, L.F.; Matos, H.M.; Rodrigues, A.P.; Figueiredo, J.R. Role of nerve growth factor (NGF) and its receptors in folliculogenesis. Zygote 2013, 21, 187–197. [Google Scholar] [CrossRef] [PubMed]
- Dissen, G.A.; Parrott, J.A.; Skinner, M.K.; Hill, D.F.; Costa, M.E.; Ojeda, S.R. Direct effects of nerve growth factor on thecal cells from antral ovarian follicles. Endocrinology 2000, 141, 4736–4750. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Liu, W.; Du, J.; Yu, Y.; Liang, N.; Liang, M.; Yao, G.; Cui, S.; Huang, H.; Sun, F. NGF promotes mouse granulosa cell proliferation by inhibiting ESR2 mediated down-regulation of CDKN1A. Mol. Cell. Endocrinol. 2015, 406, 68–77. [Google Scholar] [CrossRef] [PubMed]
- Dissen, G.A.; Hill, D.F.; Costa, M.E.; Les Dees, C.W.; Lara, H.E.; Ojeda, S.R. A role for TRKA nerve growth factor receptors in mammalian ovulation. Endocrinology 1996, 137, 198–209. [Google Scholar] [PubMed]
- Romero, C.; Paredes, A.; Dissen, G.A.; Ojeda, S.R. Nerve growth factor induces the expression of functional FSH receptors in newly formed follicles of the rat ovary. Endocrinology 2002, 143, 1485–1494. [Google Scholar] [CrossRef] [PubMed]
- Goede, V.; Schmidt, T.; Kimmina, S.; Kozian, D.; Augustin, H.G. Analysis of blood vessel maturation processes during cyclic ovarian angiogenesis. Lab. Investig. 1998, 78, 1385–1394. [Google Scholar] [PubMed]
- Zimmermann, RC.; Xiao, E.; Husami, N.; Sauer, M.V.; Lobo, R.; Kitajewski, J.; Ferin, M. Short-term administration of antivascular endothelial growth factor antibody in the late follicular phase delays follicular development in the rhesus monkey. J. Clin. Endocrinol. Metab. 2001, 86, 768–772. [Google Scholar] [CrossRef] [PubMed]
- Ferrara, N.; Chen, H.; Davis-Smyth, T.; Gerber, H.-P.; Nguyen, T.-N.; Peers, D.; Chisholm, V.; Hillan, K.J.; Schwall, R.H. Vascular endothelial growth factor is essential for corpus luteum angiogenesis. Nat. Med. 1998, 4, 336–340. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, S.; Konishi, I.; Tsuruta, Y.; Nanbu, K.; Mandai, M.; Kuroda, H.; Matsushita, K.; Hamid, A.A.; Yura, Y.; Mori, T. Expression of vascular endothelial growth factor (VEGF) during folliculogenesis and corpus luteum formation in the human ovary. Gynecol. Endocrinol. 1997, 11, 371–381. [Google Scholar] [CrossRef] [PubMed]
- Hillier, S.G. Gonadotropic control of ovarian follicular growth and development. Mol. Cel. Endocrinol. 2001, 179, 39–46. [Google Scholar] [CrossRef]
- Cantarella, G.; Lempereur, L.; Presta, M.; Ribatti, D.; Lombardo, G.; Lazarovici, P.; Zappala, G.; Pafumi, C.; Bernardini, R. Nerve growth factor-endothelial cell interaction leads to angiogenesis in vitro and in vivo. FASEB J. 2002, 16, 1307–1309. [Google Scholar] [CrossRef] [PubMed]
- Calza, L.; Giardino, L.; Giuliani, A.; Aloe, L.; Levi-Montalcini, R. Nerve growth factor control of neuronal expression of angiogenic and vasoactive factors. Proc. Natl. Acad. Sci. USA 2001, 98, 4160–4165. [Google Scholar] [CrossRef] [PubMed]
- Salas, C.; Julio-Pieper, M.; Valladares, M.; Pommer, R.; Vega, M.; Mastronardi, C.; Kerr, B.; Ojeda, S.R.; Lara, H.E.; Romero, C. Nerve growth factor-dependent activation of TRKA receptors in the human ovary results in synthesis of follicle-stimulating hormone receptors and estrogen secretion. J. Clin. Endocrinol. Metab. 2006, 91, 2396–2403. [Google Scholar] [CrossRef] [PubMed]
- Bast, R.C.; Hennessy, B.; Mills, G.B. The biology of ovarian cancer: New opportunities for translation. Nat. Rev. Cancer 2009, 9, 415–428. [Google Scholar] [CrossRef] [PubMed]
- Campos, X.; Muñoz, Y.; Selman, A.; Yazigi, R.; Moyano, L.; Weinstein-Oppenheimer, C.; Lara, H.E.; Romero, C. Nerve growth factor and its high-affinity receptor TRKA participate in the control of vascular endothelial growth factor expression in epithelial ovarian cancer. Gynecol. Oncol. 2007, 104, 168–175. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Wang, L.; Zhang, W.; Tang, B.; Zhang, J.; Song, H.; Yao, D.; Tang, Y.; Chen, X.; Yang, Z.; et al. Correlation of serum VEGF levels with clinical stage, therapy efficacy, tumor metastasis and patient survival in ovarian cancer. Anticancer Res. 2004, 24, 1973–1979. [Google Scholar] [PubMed]
- Ferrara, N.; Gerber, H.P.; LeCouter, J. The biology of VEGF and its receptors. Nat. Med. 2003, 9, 669–676. [Google Scholar] [CrossRef] [PubMed]
- Adriaenssens, E.; Vanhecke, E.; Saule, P.; Mougel, A.; Page, A.; Romon, R.; Nurcombe, V.; Le Bourhis, X.; Hondermarck, H. Nerve growth factor is a potential therapeutic target in breast cancer. Cancer Res. 2008, 68, 346–351. [Google Scholar] [CrossRef] [PubMed]
- Walch, E.T.; Marchetti, D. Role of neurotrophins and neurotrophin receptors in the in vitro invasion and heparanase production of human prostate cancer cells. Clin. Exp. Metastasis 1999, 17, 307–314. [Google Scholar] [CrossRef] [PubMed]
- Dollé, L.; Adriaenssens, E.; Yazidi-Belkoura, I.E.; Bourhis, X.L.; Nurcombe, V.; Hondermarck, H. Nerve growth factor receptors and signaling in breast cancer. Curr. Cancer Drug Targets 2004, 4, 463–470. [Google Scholar] [CrossRef] [PubMed]
- McGregor, L.M.; McCune, B.K.; Graff, J.R.; McDowell, P.R.; Romans, K.E.; Yancopoulos, G.D.; Ball, D.W.; Baylin, S.B.; Nelkin, B.D. Roles of TRK family neurotrophin receptors in medullary thyroid carcinoma development and progression. Proc. Natl. Acad. Sci. USA 1999, 96, 4540–4545. [Google Scholar] [CrossRef] [PubMed]
- Ricci, A.; Greco, S.; Mariotta, S.; Felici, L.; Bronzetti, E.; Cavazzana, A.; Barbolini, G. Neurotrophins and neurotrophin receptors in human lung cancer. Am. J. Respir. Cell Mol. Biol. 2001, 25, 439–446. [Google Scholar] [CrossRef] [PubMed]
- Tsunoda, S.; Okumura, T.; Ito, T.; Mori, Y.; Soma, T.; Watanabe, G.; Kaganoi, J.; Itami, A.; Sakai, Y.; Shimada, Y. Significance of nerve growth factor overexpression and its autocrine loop in oesophageal squamous cell carcinoma. Br. J. Cancer 2006, 95, 322–330. [Google Scholar] [CrossRef] [PubMed]
- Tapia, V.; Gabler, F.; Muñoz, M.; Yazigi, R.; Paredes, A.; Selman, A.; Vega, M.; Romero, C. Tyrosine kinase A receptor (TRKA): A potential marker in epithelial ovarian cancer. Gynecol. Oncol. 2011, 121, 13–23. [Google Scholar] [CrossRef] [PubMed]
- Urzúa, U.; Tapia, V.; Geraldo, M.P.; Selman, A.; Vega, M.; Romero, C. Nerve growth factor stimulates cellular proliferation of human epithelial ovarian cancer. Horm. Metab. Res. 2012, 44, 656–661. [Google Scholar] [CrossRef] [PubMed]
- Romero, C.; Hurtado, I.; Garrido, M.; Selman, A.; Vega, M. The expression of coclooxigenase-2 is increased by nerve growth factor in epithelial ovarian cancer. In Proceedings of the 24th Biennial Congress of the European Associtaion for Cancer Research, Manchester, UK, 9–12 July 2016.
- Tordjman, C.; Coge, F.; Andre, N.; Rique, H.; Spedding, M.; Bonnet, J. Characterisation of cyclooxygenase 1 and 2 expression in mouse resident peritoneal macrophages in vitro; interactions of non steroidal anti-inflammatory drugs with COX2. Biochim. Biophys. Acta 1995, 1256, 249–256. [Google Scholar] [CrossRef]
- Shen, H.; Li, L.; Zhou, S.; Yu, D.; Yang, S.; Chen, X.; Wang, D.; Zhong, S.; Zhao, J.; Tang, J. The role of ADAM17 in tumorigenesis and progression of breast cancer. Tumour Biol. 2016. [Google Scholar] [CrossRef] [PubMed]
- Girardi, S.; Tapia, V.; Kohan, K.; Contreras, H.; Gabler, F.; Selman, A.; Vega, M.; Romero, C. ADAM17 and TRKA receptor are involved in epithelial ovarian cancer progression. In Proceedings of the 17th World Congress on Advances in Oncology and 15th International Symposium on Molecular Medicine, Hersonissos, Greece, 11–13 October 2012.
- Romero, C.; Vallejos, C.; Gabler, F.; Selman, A.; Vega, M. Activation of TRKA receptor by nerve growth factor induces shedding of p75 receptor related with progression of epithelial ovarian cancer. In Proceedings of the 23rd Biennial Congress of the European Association for Cancer Research, Munich, Germany, 5–8 July 2014; pp. 5119–5120.
- Vera, C.; Tapia, V.; Kohan, K.; Gabler, F.; Ferreira, A.; Selman, A.; Vega, M.; Romero, C. Nerve growth factor induces the expression of chaperone protein calreticulin in human epithelial ovarian cells. Horm. Metab. Res. 2012, 44, 639–643. [Google Scholar] [CrossRef] [PubMed]
- Zamanian, M.; Qader Hamadneh, L.A.; Veerakumarasivam, A.; Abdul Rahman, S.; Shohaimi, S.; Rosli, R. Calreticulin mediates an invasive breast cancer phenotype through the transcriptional dysregulation of p53 and MAPK pathways. Cancer Cell. Int. 2016, 16, 56. [Google Scholar] [CrossRef] [PubMed]
- Sheng, W.; Chen, C.; Dong, M.; Zhou, J.; Liu, Q.; Dong, Q.; Li, F. Overexpression of calreticulin contributes to the development and progression of pancreatic cancer. J. Cell. Physiol. 2014, 229, 887–897. [Google Scholar] [CrossRef] [PubMed]
- Iwakawa, H.O.; Tomari, Y. The functions of microRNAs: mRNA decay and translational repression. Trends Cell Biol. 2015, 25, 651–665. [Google Scholar] [CrossRef] [PubMed]
- Zlotorynski, E. Small RNAs: New microRNA-like molecules. Nat. Rev. Mol. Cell Biol. 2016, 17, 396. [Google Scholar] [CrossRef] [PubMed]
- Winter, J.; Jung, S.; Keller, S.; Gregory, R.I.; Diederichs, S. Many roads to maturity: microRNA biogenesis pathways and their regulation. Nat. Cell Biol. 2009, 11, 228–234. [Google Scholar] [CrossRef] [PubMed]
- Ha, M.; Kim, V.N. Regulation of microRNA biogenesis. Nat. Rev. Mol. Cell Biol. 2014, 15, 509–524. [Google Scholar] [CrossRef] [PubMed]
- Molasy, M.; Walczak, A.; Szaflik, J.; Szaflik, J.P.; Majsterek, I. MicroRNAs in glaucoma and neurodegenerative diseases. J. Hum. Genet. 2016. [Google Scholar] [CrossRef] [PubMed]
- Barwari, T.; Joshi, A.; Mayr, M. MicroRNAs in cardiovascular disease. J. Am. Coll. Cardiol. 2016, 68, 2577–2584. [Google Scholar] [CrossRef] [PubMed]
- Vienberg, S.; Geiger, J.; Madsen, S.; Dalgaard, L.T. MicroRNAs in metabolism. Acta Physiol. 2016. [Google Scholar] [CrossRef] [PubMed]
- Reddy, K.B. MicroRNA (miRNA) in cancer. Cancer Cell Int. 2015. [Google Scholar] [CrossRef] [PubMed]
- Ruan, K.; Fang, X.; Ouyang, G. MicroRNAs: Novel regulators in the hallmarks of human cancer. Cancer Lett. 2009, 285, 116–126. [Google Scholar] [CrossRef] [PubMed]
- Di Leva, G.; Croce, C.M. Roles of small RNAs in tumor formation. Trends Mol. Med. 2010, 16, 257–267. [Google Scholar] [CrossRef] [PubMed]
- Svoronos, A.A.; Engelman, D.M.; Slack, F.J. OncomiR or tumor suppressor? The duplicity of microRNAs in cancer. Cancer Res. 2016, 76, 3666–3670. [Google Scholar] [CrossRef] [PubMed]
- Brian, A.; Kasinski, A.; Slack, F. Aberrant regulation and function of microRNAs in cancer. Curr. Biol. 2014, 24, 762–776. [Google Scholar]
- Zhang, Y.; Zhang, D.; Wang, D.; Xu, D.; Guo, Y.; Cui, W. Serum miRNAs panel (miR-16–2*, miR-195, miR-2861, miR-497) as novel non-invasive biomarkers for detection of cervical cancer. Sci. Rep. 2015, 14, 17942. [Google Scholar] [CrossRef] [PubMed]
- Lu, J.; Getz, G.; Miska, E.A.; Alvarez-Saavedra, E.; Lamb, J.; Peck, D.; Sweet-Cordero, A.; Ebert, B.L.; Mak, R.H.; Ferrando, A.A.; et al. MicroRNA expression profiles classify human cancers. Nature 2005, 435, 834–838. [Google Scholar] [CrossRef] [PubMed]
- Taslim, C.; Weng, D.Y.; Brasky, T.M.; Dumitrescu, R.G.; Huang, K.; Kallakury, B.V.; Krishnan, S.; Llanos, A.A.; Marian, C.; McElroy, J.; et al. Discovery and replication of microRNAs for breast cancer risk using genome-wide profiling. Oncotarget 2016. [Google Scholar] [CrossRef] [PubMed]
- Iorio, M.V.; Visone, R.; Di Leva, G.; Donati, V.; Petrocca, F.; Casalini, P.; Taccioli, C.; Volinia, S.; Liu, C.G.; Alder, H.; et al. MicroRNA signatures in human ovarian cancer. Cancer Res. 2007, 67, 8699–8707. [Google Scholar] [CrossRef] [PubMed]
- Zhang, K.; Wu, X.; Wang, J.; Lopez, J.; Zhou, W.; Yang, L.; Wang, S.E.; Raz, D.J.; Kim, J.Y. Circulating miRNA profile in esophageal adenocarcinoma. Am. J. Cancer Res. 2016, 6, 2713–2721. [Google Scholar] [PubMed]
- Shu, X.; Hildebrandt, M.A.; Gu, J.; Tannir, N.M.; Matin, S.F.; Karam, J.A.; Wood, C.G.; Wu, X. MicroRNA profiling in clear cell renal cell carcinoma tissues potentially links tumorigenesis and recurrence with obesity. Br. J. Cancer 2016. [Google Scholar] [CrossRef] [PubMed]
- Urquidi, V.; Netherton, M.; Gomes-Giacoia, E.; Serie, D.J.; Eckel-Passow, J.; Rosser, C.J.; Goodison, S. A microRNA biomarker panel for the non-invasive detection of bladder cancer. Oncotarget 2016. [Google Scholar] [CrossRef] [PubMed]
- Gadducci, A.; Sergiampietri, C.; Lanfredini, N.; Guiggi, I. Micro-RNAs and ovarian cancer: The state of art and perspectives of clinical research. Gynecol. Endocrinol. 2014, 30, 266–271. [Google Scholar] [CrossRef] [PubMed]
- Pal, M.K.; Jaiswar, S.P.; Dwivedi, V.N.; Tripathi, A.K.; Dwivedi, A.; Sankhwar, P. MicroRNA: A new and promising potential biomarker for diagnosis and prognosis of ovarian cancer. Cancer Biol. Med. 2015, 12, 328–341. [Google Scholar] [PubMed]
- Cheng, G. Circulating miRNAs: Roles in cancer diagnosis, prognosis and therapy. Adv. Drug Deliv. Rev. 2015, 81, 75–93. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Lu, Z.; Unruh, A.K.; Ivan, C.; Baggerly, K.A.; Calin, G.A.; Li, Z.; Bast, R.C., Jr.; Le, X.F. Clinically relevant microRNAs in ovarian cancer. Mol. Cancer Res. 2015, 13, 393–401. [Google Scholar] [CrossRef] [PubMed]
- Katz, B.; Tropé, C.G.; Reich, R.; Davidson, B. MicroRNAs in ovarian cancer. Hum. Pathol. 2015, 46, 1245–1256. [Google Scholar] [CrossRef] [PubMed]
- Kinose, Y.; Sawada, K.; Nakamura, K.; Kimura, T. The role of microRNAs in ovarian cancer. Biomed. Res. Int. 2014, 2014, 249393. [Google Scholar] [CrossRef] [PubMed]
- Johnson, S.M.; Grosshans, H.; Shingara, J.; Byrom, M.; Jarvis, R.; Cheng, A.; Labourier, E.; Reinert, K.L.; Brown, D.; Slack, F.J. RAS is regulated by the let-7 microRNA family. Cell 2005, 120, 635–647. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Wei, J.J. HMGA2 and high-grade serous ovarian carcinoma. J. Mol. Med. 2013, 91, 1155–1165. [Google Scholar] [CrossRef] [PubMed]
- Helland, Å.; Anglesio, M.S.; George, J.; Cowin, P.A.; Johnstone, C.N.; House, C.M.; Sheppard, K.E.; Etemadmoghadam, D.; Melnyk, N.; Rustgi, A.K.; et al. Deregulation of MYCN, LIN28B and LET7 in a molecular subtype of aggressive high-grade serous ovarian cancers. PLoS ONE 2011, 6, e18064. [Google Scholar] [CrossRef] [PubMed]
- Zheng, H.; Zhang, L.; Zhao, Y.; Yang, D.; Song, F.; Wen, Y.; Hao, Q.; Hu, Z.; Zhang, W.; Chen, K. Plasma miRNAs as diagnostic and prognostic biomarkers for ovarian cancer. PLoS ONE 2013, 8, e77853. [Google Scholar] [CrossRef] [PubMed]
- Wu, Q.; Guo, R.; Lin, M.; Zhou, B.; Wang, Y. MicroRNA-200a inhibits CD133/1+ ovarian cancer stem cells migration and invasion by targeting E-cadherin repressor ZEB2. Gynecol. Oncol. 2011, 122, 149–154. [Google Scholar] [CrossRef] [PubMed]
- Van Jaarsveld, M.T.; Helleman, J.; Boersma, A.W.; van Kuijk, P.F.; van Ijcken, W.F.; Despierre, E.; Vergote, I.; Mathijssen, R.H.; Berns, E.M.; Verweij, J.; et al. miR-141 regulates KEAP1 and modulates cisplatin sensitivity in ovarian cancer cells. Oncogene 2013, 32, 4284–4293. [Google Scholar] [CrossRef] [PubMed]
- Cochrane, D.R.; Spoelstra, N.S.; Howe, E.N.; Nordeen, S.K.; Richer, J.K. MicroRNA-200c mitigates invasiveness and restores sensitivity to microtubule-targeting chemotherapeutic agents. Mol. Cancer Ther. 2009, 8, 1055–1066. [Google Scholar] [CrossRef] [PubMed]
- Cittelly, D.M.; Dimitrova, I.; Howe, E.N.; Cochrane, D.R.; Jean, A.; Spoelstra, N.S.; Post, M.D.; Lu, X.; Broaddus, R.R.; Spillman, M.A.; et al. Restoration of miR-200c to ovarian cancer reduces tumor burden and increases sensitivity to paclitaxel. Mol. Cancer Ther. 2012, 11, 2556–2565. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Huang, J.; Yang, N.; Greshock, J.; Megraw, M.S.; Giannakakis, A.; Liang, S.; Naylor, T.L.; Barchetti, A.; Ward, M.R.; et al. microRNAs exhibit high frecuency genomic alteration in human cancer. Proc. Natl. Acad. Sci. USA 2006, 103, 9136–9141. [Google Scholar] [CrossRef] [PubMed]
- Italiano, A.; Thomas, R.; Breen, M.; Zhang, L.; Crago, A.M.; Singer, S.; Khanin, R.; Maki, R.G.; Mihailovic, A.; Hafner, M.; et al. The miR-17–92 cluster and its target THBS1 are differentially expressed in angiosarcomas dependent on MYC amplification. Genes Chromosomes Cancer 2012, 51, 569–578. [Google Scholar] [CrossRef] [PubMed]
- Lou, Y.; Yang, X.; Wang, F.; Cui, Z.; Huang, Y. MicroRNA-21 promotes the cell proliferation, invasion and migration abilities in ovarian epithelial carcinomas through inhibiting the expression of PTEN protein. Int. J. Mol. Med. 2010, 26, 819–827. [Google Scholar] [CrossRef] [PubMed]
- Cappellesso, R.; Tinazzi, A.; Giurici, T.; Simonato, F.; Guzzardo, V.; Ventura, L.; Crescenzi, M.; Chiarelli, S.; Fassina, A. Programmed cell death 4 and miR-21 inverse expression is maintained in cells and exosomes from ovarian serous carcinoma effusions. Cancer Cytopathol. 2014, 122, 685–693. [Google Scholar] [CrossRef] [PubMed]
- Báez-Vega, P.M.; Echevarría Vargas, I.M.; Valiyeva, F.; Encarnación-Rosado, J.; Roman, A.; Flores, J.; Marcos-Martínez, M.J.; Vivas-Mejía, P.E. Targeting miR-21–3p inhibits proliferation and invasion of ovarian cancer cells. Oncotarget 2016, 7, 36321–36337. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.Z.; Xi, Q.H.; Ge, W.L.; Zhang, X.Q. Identification of serum microRNA-21 as a biomarker for early detection and prognosis in human epithelial ovarian cancer. Asian Pac. J. Cancer Prev. 2013, 14, 1057–1060. [Google Scholar] [CrossRef] [PubMed]
- Kent, O.A.; McCall, M.N.; Cornish, T.C.; Halushka, M.K. Lessons from miR-143/145: The importance of cell-type localization of miRNAs. Nucleic Acids Res. 2014, 42, 7528–7538. [Google Scholar] [CrossRef] [PubMed]
- Xu, Q.; Liu, L.Z.; Qian, X.; Chen, Q.; Jiang, Y.; Li, D.; Lai, L.; Jiang, B.H. MiR-145 directly targets p70S6K1 in cancer cells to inhibit tumor growth angiogenesis. Nucleic Acids Res. 2012, 40, 761–774. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Wang, Q.; Yu, M.; Wu, N.; Wang, H. MicroRNA-145 function as a cell growth repressor by directly targeting c-Myc in human ovarian cancer. Technol. Cancer Res. Treat. 2014, 13, 161–168. [Google Scholar] [CrossRef] [PubMed]
- Mataki, H.; Seki, N.; Mizuno, K.; Nohata, N.; Kamikawaji, K.; Kumamoto, T.; Koshizuka, K.; Goto, Y.; Inoue, H. Dual-strand tumor-suppressor microRNA-145 (miR-145–5p and miR-145–3p) coordinately targeted MTDH in lung squamous cell carcinoma. Oncotarget 2016, 7, 72085–72098. [Google Scholar] [CrossRef] [PubMed]
- Yan, J.; Jiang, J.Y.; Meng, X.N.; Xiu, Y.L.; Zong, Z.H. MiR-23b targets cyclin G1 and suppresses ovarian cancer tumorigenesis and progression. J. Exp. Clin. Cancer Res. 2016, 35, 31. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Liu, Z.; Chen, L.; Zhou, L.; Yao, Y. MicroRNA-23b is an independent prognostic marker and suppresses ovarian cancer progression by targeting runt-related transcription factor-2. FEBS Lett. 2014, 588, 1608–1615. [Google Scholar] [CrossRef] [PubMed]
- Yan, H.; Wu, W.; Ge, H.; Li, P.; Wang, Z. Up-regulation of miR-204 enhaces anoikis sensitivity in ephitehlial ovarian cancer cell line via brain-derived neurotrophic factor pathway in vitro. Int. J. Gynecol. Cancer 2015, 25, 944–952. [Google Scholar] [CrossRef] [PubMed]
- Montalban, E.; Mattugini, N.; Ciarapica, R.; Provenzano, C.; Savino, M.; Scagnoli, F.; Prosperini, G.; Carissimi, C.; Fulci, V.; Matrone, C.; et al. MiR-21 is an NGF-modulated microRNA that supports NGF signaling and regulates neuronal degeneration in PC12 cells. Neuromol. Med. 2014, 16, 415–430. [Google Scholar] [CrossRef] [PubMed]
- Kashyap, M.; Pore, S.; Chancellor, M.; Yoshimura, N.; Tyagi, P. Bladder overactivity involves overexpression of microRNA 132 and nerve growth factor. Life Sci. 2016, 167, 98–104. [Google Scholar] [CrossRef] [PubMed]
- Othumpangat, S.; Walton, C.; Piedimonte, G. MicroRNA-221 modulates RSV replication in human bronchial epithelial by targeting NGF expression. PLoS ONE 2012, 7, e30030. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.; Roy, S.; Nuovo, G.; Ramaswamy, B.; Miller, T.; Shapiro, C.; Jacob, S.T.; Majumder, S. Anti-microRNA-222 (anti-miR-222) and -181B suppress growth of tamixofen-resistant xenografts in mouse by targeting TIMP3 protein and modulating mitogenic signal. J. Biol. Chem. 2011, 286, 42292–42302. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.L.; Cheng, B.; Li, P.Y.; Huang, H.J.; Zhao, Q.; Dan, Z.L.; Tian, D.A.; Zhang, P. MicroRNA-143 suppresses gastric cancer cells growth and induces apoptosis by targeting COX2. World J. Gastroenterol. 2013, 19, 7758–7765. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; He, J.; Xu, H.; Xu, L.; Li, N. MiR-143 targets CTCF and exerts tumor-suppressing functions in epithelial ovarian cancer. Am. J. Transl. Res. 2016, 8, 2716–2726. [Google Scholar] [PubMed]
- Manek, R.; Pakzamir, E.; Mhawech-Fauceglia, P.; Pejovic, T.; Sowter, H.; Gayther, S.A.; Lawrenson, K. Targeting SRC in endometriosis-associated ovarian cancer. Oncogenesis 2016, 5, e251. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Wu, X.; Pan, Z.K.; Huang, S. Integrity of SOS1/EPS8/ABI1 TRI-complex determines ovarian cancer metastasis. Cancer Res. 2016, 70, 9979–9990. [Google Scholar] [CrossRef] [PubMed]
- Fulciniti, M.; Amodio, N.; Bandi, R.L.; Cagnetta, A.; Samur, M.K.; Acharya, C.; Prabhala, R.; D’Aquila, P.; Bellizzi, D.; Passarino, G. miR-23b/SP1/c-Myc forms a feed-forward loop supporting multiple myeloma cell growth. Blood Cancer J. 2016, 15, e380. [Google Scholar] [CrossRef] [PubMed]
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license ( http://creativecommons.org/licenses/by/4.0/).