Role of uL3 in Multidrug Resistance in p53-Mutated Lung Cancer Cells
Abstract
:1. Introduction
2. Results
2.1. Production of the MDR Resistant p53-Mutated Lung Cancer Cell Line
2.2. Cross-Resistance Profiles of rCalu-6 Cells
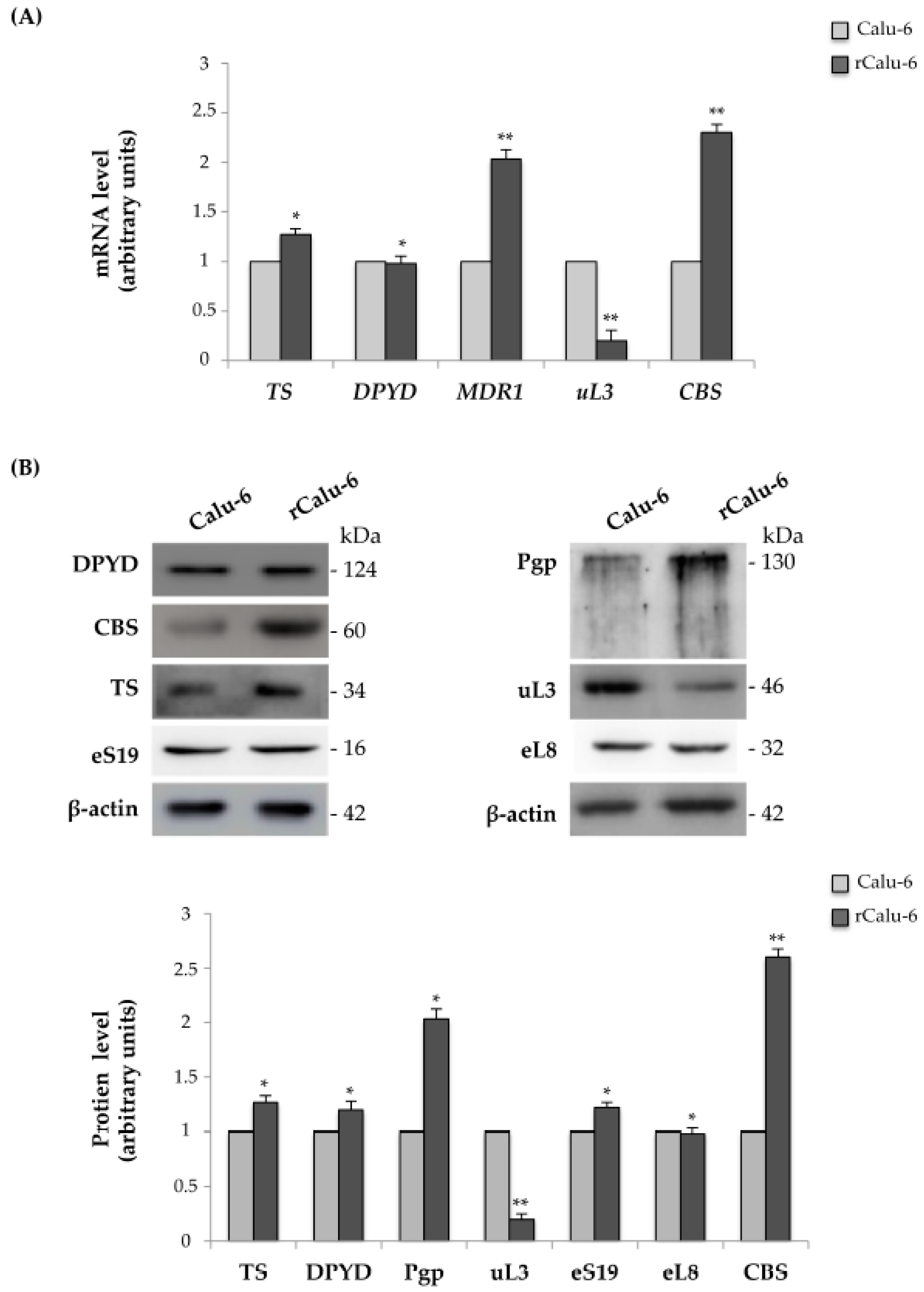
2.3. Expression Analysis of Genes Related to Drug Resistance in rCalu-6 Cells
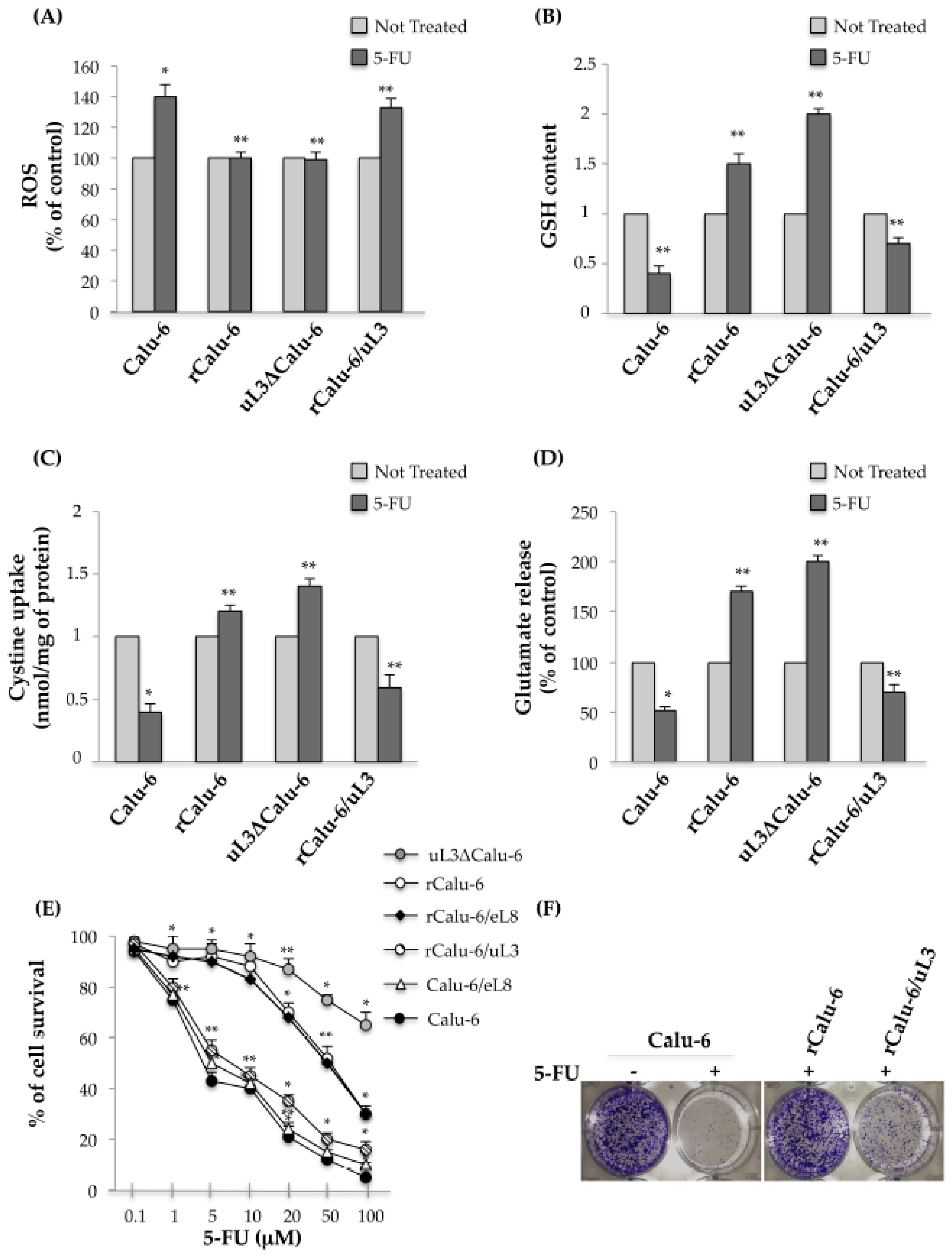
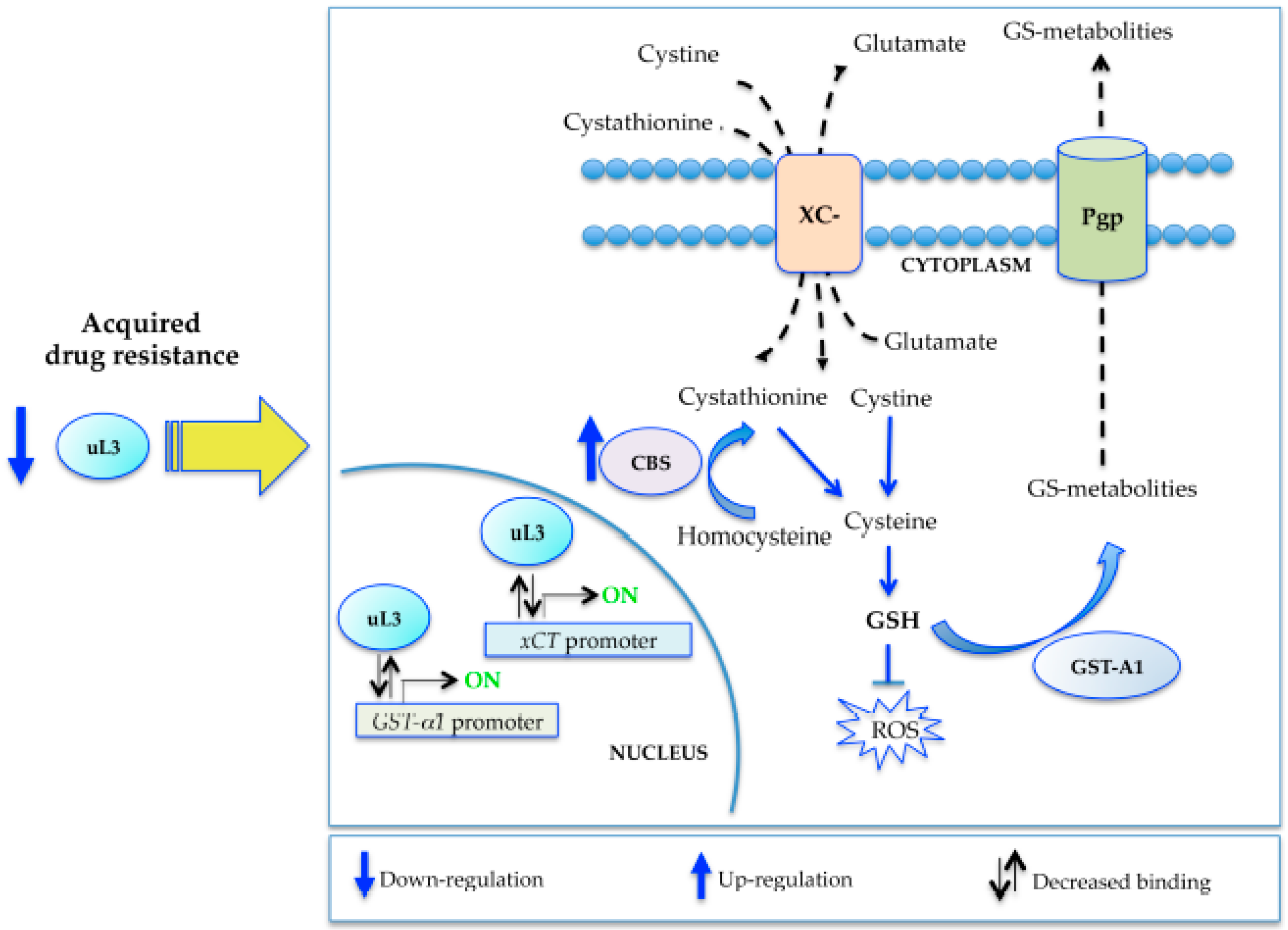
2.4. uL3 Mediates Anti-Oxidative Cell Response in rCalu-6 Cells
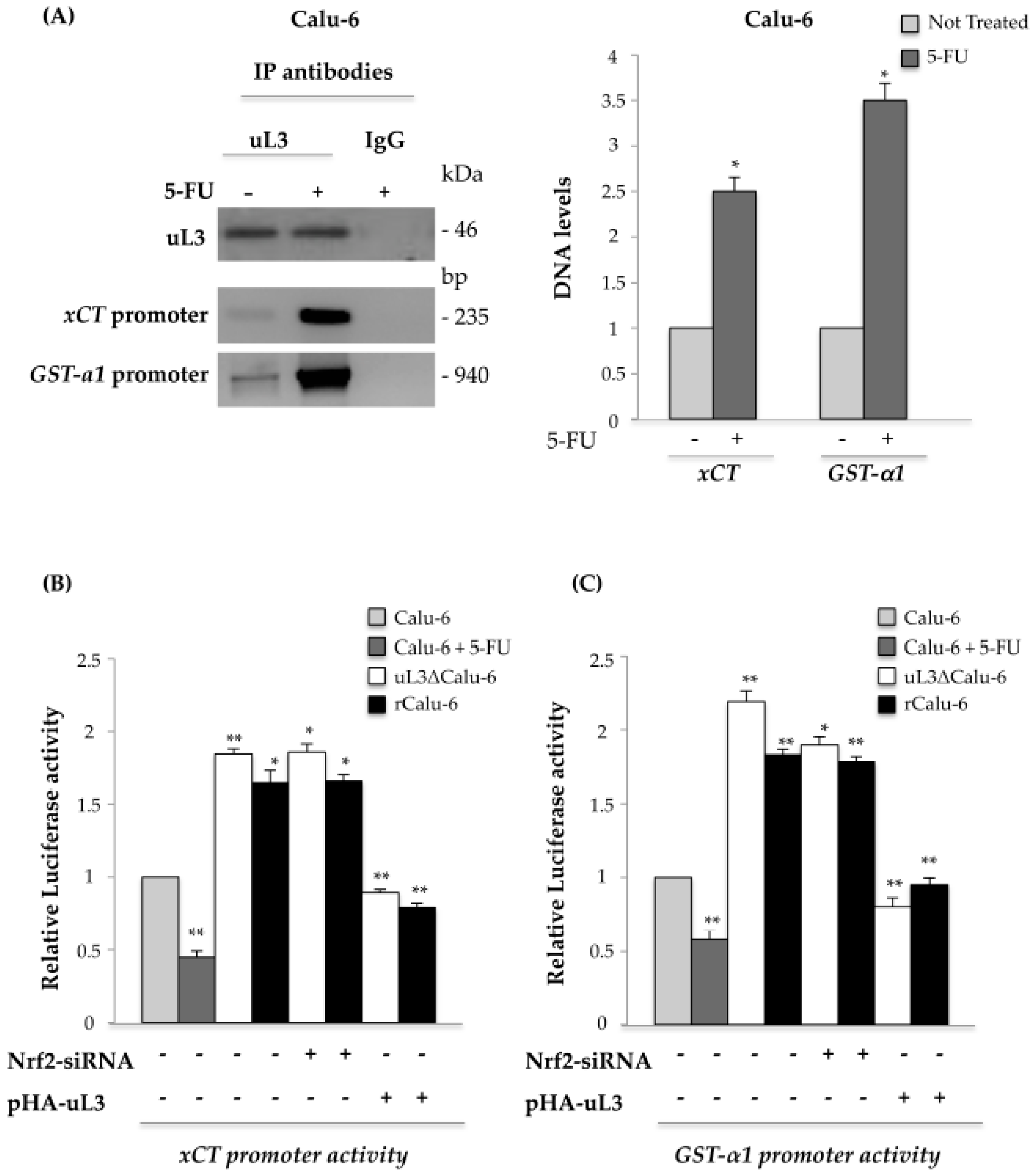
2.5. uL3 Reduces xCT and GST-α1 Expression Levels
2.6. uL3 Is a Transcriptional Repressor of xCT and GST-α1
3. Discussion
4. Materials and Methods
4.1. Cell Cultures, Drug Treatment, and Production of Multidrug Resistant Calu-6 Cells
4.2. Cell Viability Assay and Clonogenic Assay
4.3. Glutathione Measurement
4.4. Glutamate Analysis
4.5. Cystine Uptake
4.6. Reactive Oxygen Species (ROS) Assay
4.7. DNA Constructs, siRNA, and Transfection
4.8. Dual Luciferase Assays
4.9. Chromatin Immunoprecipitation
4.10. RNA Isolation, RT, and Quantitative Real-Time PCR
4.11. Western Blot Analysis
4.12. Immunoprecipitation Assay
4.13. Statistical Analysis
5. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- DeSantis, C.E.; Lin, C.C.; Mariotto, A.B.; Siegel, R.L.; Stein, K.D.; Kramer, J.L.; Alteri, R.; Robbins, A.S.; Jemal, A. Cancer treatment and survivorship statistics, 2014. CA Cancer J. Clin. 2014, 64, 252–271. [Google Scholar] [CrossRef] [PubMed]
- Maione, P.; Perrone, F.; Gallo, C.; Manzione, L.; Piantedosi, F.; Barbera, S.; Cigolari, S.; Rosetti, F.; Piazza, E.; Robbiati, S.F.; et al. Pretreatment quality of life and functional status assessment significantly predict survival of elderly patients with advanced non-small-cell lung cancer receiving chemotherapy: A prognostic analysis of the multicenter italian lung cancer in the elderly study. J. Clin. Oncol. 2005, 23, 6865–6872. [Google Scholar] [PubMed]
- Chang, A. Chemotherapy, chemoresistance and the changing treatment landscape for NSCLC. Lung Cancer 2011, 71, 3–10. [Google Scholar] [CrossRef] [PubMed]
- Chunga, Y.M.; Parka, S.; Parka, J.K.; Kimb, Y.; Kanga, Y.; Yooa, Y.D. Establishment and characterization of 5-fuorouracil-resistant gastric cancer cells. Cancer Lett. 2000, 159, 95–101. [Google Scholar] [CrossRef]
- Gu, W.; Fang, F.-F.; Li, B.; Cheng, B.-B.; Ling, C.-Q. Characterization and resistance mechanisms of a 5-fluorouracil-resistant hepatocellular carcinoma cell line. Asian Pac. J. Cancer Prev. 2012, 13, 4807–4814. [Google Scholar] [CrossRef] [PubMed]
- Longley, D.B.; Harkin, D.P.; Johnston, P.G. 5-Fluorouracil: Mechanisms of action and clinical strategies. Nat. Rev. Cancer 2003, 3, 330–338. [Google Scholar] [CrossRef] [PubMed]
- Boulon, S.; Westman, B.J.; Hutten, S.; Boisvert, F.M.; Lamond, A.I. The nucleolus under stress. Mol. Cell 2010, 40, 216–227. [Google Scholar] [CrossRef] [PubMed]
- Lewinska, A.; Wnuk, M.; Grzelak, A.; Bartosz, G. Nucleolus as an oxidative stress sensor in the yeast saccharomyces cerevisiae. Redox Rep. 2010, 15, 87–96. [Google Scholar] [CrossRef] [PubMed]
- Challagundla, K.B.; Sun, X.X.; Zhang, X.; de Vine, T.; Zhang, Q.; Sears, R.C.; Dai, M.S. Ribosomal protein L11 recruits miR-24/miRISC to repress c-Myc expression in response to ribosomal stress. Mol. Cell. Biol. 2011, 31, 4007–4021. [Google Scholar] [CrossRef] [PubMed]
- De Marval, P.L.M.; Zhang, Y. The RP-Mdm2-p53 pathway and tumorigenesis. Oncotarget 2011, 234–238. [Google Scholar] [CrossRef] [PubMed]
- Cuccurese, M.; Russo, G.; Russo, A.; Pietropaolo, C. Alternative splicing and nonsense-mediated mRNA decay regulate mammalian ribosomal gene expression. Nucleic Acids Res. 2005, 33, 5965–5977. [Google Scholar] [CrossRef] [PubMed]
- Russo, G.; Cuccurese, M.; Monti, G.; Russo, A.; Amoresano, A.; Pucci, P.; Pietropaolo, C. Ribosomal protein L7a binds RNA through two distinct RNA-binding domains. Biochem. J. 2005, 385, 289–299. [Google Scholar] [CrossRef] [PubMed]
- Russo, A.; Russo, G.; Cuccurese, M.; Garbi, C.; Pietropaolo, C. The 3′-untranslated region directs ribosomal protein-encoding mRNAs to specific cytoplasmic regions. Biochim. Biophys. Acta 2006, 1763, 833–843. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Russo, A.; Cirulli, C.; Amoresano, A.; Pucci, P.; Pietropaolo, C.; Russo, G. Cis-acting sequences and trans-acting factors in the localization of mRNA for mitochondrial ribosomal proteins. Biochim. Biophys. Acta 2008, 1779, 820–829. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Russo, A.; Russo, G. Ribosomal proteins control or bypass p53 during nucleolar stress. Int. J. Mol. Sci. 2017, 18. [Google Scholar] [CrossRef] [PubMed]
- Ban, N.; Beckmann, R.; Cate, J.H.; Dinman, J.D.; Dragon, F.; Ellis, S.R.; Lafontaine, D.L.; Lindahl, L.; Liljas, A.; Lipton, J.M.; et al. A new system for naming ribosomal proteins. Curr. Opin. Struct. Biol. 2014, 24, 165–169. [Google Scholar] [CrossRef] [PubMed]
- Russo, A.; Siciliano, G.; Catillo, M.; Giangrande, C.; Amoresano, A.; Pucci, P.; Pietropaolo, C.; Russo, G. hnRNP H1 and intronic G runs in the splicing control of the human rpL3 gene. Biochim. Biophys. Acta 2010, 1799, 419–428. [Google Scholar] [CrossRef] [PubMed]
- Russo, A.; Catillo, M.; Esposito, D.; Briata, P.; Pietropaolo, C.; Russo, G. Autoregulatory circuit of human rpL3 expression requires hnRNP H1, NPM and KHSRP. Nucleic Acids Res. 2011, 39, 7576–7585. [Google Scholar] [CrossRef] [PubMed]
- Russo, A.; Esposito, D.; Catillo, M.; Pietropaolo, C.; Crescenzi, E.; Russo, G. Human rpL3 induces G1/S arrest or apoptosis by modulating p21waf1/cip1 levels in a p53-independent manner. Cell Cycle 2013, 12, 76–87. [Google Scholar] [CrossRef] [PubMed]
- Esposito, D.; Crescenzi, E.; Sagar, V.; Loreni, F.; Russo, A.; Russo, G. Human rpL3 plays a crucial role in cell response to nucleolar stress induced by 5-FU and L-OHP. Oncotarget 2014, 5, 11737–11751. [Google Scholar] [CrossRef] [PubMed]
- Russo, A.; Pagliara, V.; Albano, F.; Esposito, D.; Sagar, V.; Loreni, F.; Irace, C.; Santamaria, R.; Russo, G. Regulatory role of rpL3 in cell response to nucleolar stress induced by act D in tumor cells lacking functional p53. Cell Cycle 2016, 15, 41–51. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Russo, A.; Saide, A.; Cagliani, R.; Cantile, M.; Botti, G.; Russo, G. rpL3 promotes the apoptosis of p53 mutated lung cancer cells by down-regulating CBS and NFκB upon 5-FU treatment. Sci. Rep. 2016, 6, 38369. [Google Scholar] [CrossRef] [PubMed]
- Pagliara, V.; Saide, A.; Mitidieri, E.; di Villa Bianca, D.R.; Sorrentino, R.; Russo, G.; Russo, A. 5-Fu targets rpL3 to induce mitochondrial apoptosis via cystathionine-β-synthase in colon cancer cells lacking p53. Oncotarget 2016, 7, 50333–50348. [Google Scholar] [CrossRef] [PubMed]
- Russo, A.; Maiolino, S.; Pagliara, V.; Ungaro, F.; Tatangelo, F.; Leone, A.; Scalia, G.; Budillon, A.; Quaglia, F.; Russo, G. Enhancement of 5-FU sensitivity by the proapoptotic rpL3 gene in p53 null colon cancer cells through combined polymer nanoparticles. Oncotarget 2016, 7, 79670–79687. [Google Scholar] [CrossRef] [PubMed]
- Russo, A.; Pellosi, D.S.; Pagliara, V.; Milone, M.R.; Pucci, B.; Caetano, W.; Hioka, N.; Budillon, A.; Ungaro, F.; Russo, G.; et al. Biotin-targeted pluronic® P123/F127 mixed micelles delivering niclosamide: A repositioning strategy to treat drug-resistant lung cancer cells. Int. J. Pharm. 2016, 511, 127–139. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shen, D.W.; Liang, X.J.; Suzuki, T.; Gottesman, M.M. Identification by functional cloning from a retroviral cDNA library of cDNAs for ribosomal protein l36 and the 10-kDa heat shock protein that confer cisplatin resistance. Mol. Pharmacol. 2006, 69, 1383–1388. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.J.; Youn, H.; Seong, K.M.; Jin, Y.W.; Kim, J.; Youn, B. Phosphorylation of ribosomal protein S3 and antiapoptotic TRAF2 protein mediates radioresistance in non-small cell lung cancer cells. J. Biol. Chem. 2013, 288, 2965–2975. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.-L.; Kong, Q.-S.; Liu, H.-S.; Tan, W.-B. Drug resistance effects of ribosomal protein L24 overexpression in hepatocellular carcinoma HepG2 cells. Asian Pac. J. Cancer Prev. 2014, 15, 9853–9857. [Google Scholar] [CrossRef] [PubMed]
- Baguley, B.C. Multiple drug resistance mechanisms in cancer. Mol. Biotechnol. 2010, 46, 308–316. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, K.; Tanaka, M.; Inagaki, A.; Wanibuchi, H.; Izumi, Y.; Miura, K.; Nagayama, K.; Shiota, M.; Iwao, H. Establishment of a 5-fluorouracil-resistant triple-negative breast cancer cell line. Int. J. Oncol. 2013, 43, 1985–1991. [Google Scholar] [PubMed]
- Hayes, J.D.; McLellan, L.I. Glutathione and glutathione-dependent enzymes represent a co-ordinately regulated defence against oxidative stress. Free Radic. Res. 1999, 31, 273–300. [Google Scholar] [CrossRef] [PubMed]
- Traverso, N.; Ricciarelli, R.; Nitti, M.; Marengo, B.; Furfaro, A.L.; Pronzato, M.A.; Marinari, U.M.; Domenicotti, C. Role of glutathione in cancer progression and chemoresistance. Oxid. Med. Cell. Longev. 2013, 2013, 972913. [Google Scholar] [CrossRef] [PubMed]
- Homma, S.; Ishii, Y.; Morishima, Y.; Yamadori, T.; Matsuno, Y.; Haraguchi, N.; Kikuchi, N.; Satoh, H.; Sakamoto, T.; Hizawa, N.; et al. Nrf2 enhances cell proliferation and resistance to anticancer drugs in human lung cancer. Clin. Cancer Res. 2009, 15, 3423–3432. [Google Scholar] [CrossRef] [PubMed]
- Faraonio, R.; Vergara, P.; di Marzo, D.; Napolitano, M.; Russo, T.; Cimino, F. Transcription regulation in NIH3T3 cell clones resistant to diethylmaleate-induced oxidative stress and apoptosis. Antioxid. Redox Signal. 2006, 8, 365–374. [Google Scholar] [CrossRef] [PubMed]
- Sparaneo, A.; Fabrizio, F.P.; Muscarella, L.A. Nrf2 and notch signaling in lung cancer: Near the crossroad. Oxid. Med. Cell. Longev. 2016, 2016, 7316492. [Google Scholar] [CrossRef] [PubMed]
- Sharma, M.K.; Seidlitz, E.P.; Singh, G. Cancer cells release glutamate via the cystine/glutamate antiporter. Biochem. Biophys. Res. Commun. 2010, 391, 91–95. [Google Scholar] [CrossRef] [PubMed]
- Miniaci, M.C.; Irace, C.; Capuozzo, A.; Piccolo, M.; di Pascale, A.; Russo, A.; Lippiello, P.; Lepre, F.; Russo, G.; Santamaria, R. Cysteine prevents the reduction in keratin synthesis induced by iron deficiency in human keratinocytes. J. Cell. Biochem. 2016, 117, 402–412. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Maiolino, S.; Russo, A.; Pagliara, V.; Conte, C.; Ungaro, F.; Russo, G.; Quaglia, F. Biodegradable nanoparticles sequentially decorated with polyethyleneimine and hyaluronan for the targeted delivery of docetaxel to airway cancer cells. J. Nanobiotechnol. 2015, 13, 29. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kobayashi, S.; Sato, M.; Kasakoshi, T.; Tsutsui, T.; Sugimoto, M.; Osaki, M.; Okada, F.; Igarashi, K.; Hiratake, J.; Homma, T.; et al. Cystathionine is a novel substrate of cystine/glutamate transporter: Implications for immune function. J. Biol. Chem. 2015, 290, 8778–8788. [Google Scholar] [CrossRef] [PubMed]
- Di Villa Bianca, D.R.; Mitidieri, E.; Esposito, D.; Donnarumma, E.; Russo, A.; Fusco, F.; Ianaro, A.; Mirone, V.; Cirino, G.; Russo, G.; et al. Human cystathionine-β-synthase phosphorylation on serine227 modulates hydrogen sulfide production in human urothelium. PLoS ONE 2015, 10, e0136859. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Di Villa Bianca, D.R.; Mitidieri, E.; Fusco, F.; Russo, A.; Pagliara, V.; Tramontano, T.; Donnarumma, E.; Mirone, V.; Cirino, G.; Russo, G.; et al. Urothelium muscarinic activation phosphorylates CBSSer227 via cGMP/PKG pathway causing human bladder relaxation through H2S production. Sci. Rep. 2016, 6, 31491. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bhattacharyya, S.; Saha, S.; Giri, K.; Lanza, I.R.; Nair, K.S.; Jennings, N.B.; Rodriguez-Aguayo, C.; Lopez-Berestein, G.; Basal, E.; Weaver, A.L.; et al. Cystathionine β-synthase (CBS) contributes to advanced ovarian cancer progression and drug resistance. PLoS ONE 2013, 8, e79167. [Google Scholar] [CrossRef] [PubMed]
- Esposito, V.; Russo, A.; Amato, T.; Varra, M.; Vellecco, V.; Bucci, M.; Russo, G.; Virgilio, A.; Galeone, A. Backbone modified TBA analogues endowed with antiproliferative activity. Biochim. Biophys. Acta 2016. [Google Scholar] [CrossRef] [PubMed]
- Murphy, T.H.; Miyamoto, M.; Sastre, A.; Schnaar, R.L.; Coyle, J.T. Glutamate toxicity in a neuronal cell line involves inhibition of cystine transport leading to oxidative stress. Cell Press 1989, 2, 1547–1558. [Google Scholar] [CrossRef]
- Lavecchia, A.; di Giovanni, C.; Cerchia, C.; Russo, A.; Russo, G.; Novellino, E. Discovery of a novel small molecule inhibitor targeting the frataxin/ubiquitin interaction via structure-based virtual screening and bioassays. J. Med. Chem. 2013, 56, 2861–2873. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- De Filippis, D.; Russo, A.; D’Amico, A.; Esposito, G.; Pietropaolo, C.; Cinelli, M.; Russo, G.; Iuvone, T. Cannabinoids reduce granuloma-associated angiogenesis in rats by controlling transcription and expression of mast cell protease-5. Br. J. Pharmacol. 2008, 154, 1672–1679. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Faraonio, R.; Vergara, P.; di Marzo, D.; Pierantoni, M.G.; Napolitano, M.; Russo, T.; Cimino, F. p53 suppresses the Nrf2-dependent transcription of antioxidant response genes. J. Biol. Chem. 2006, 281, 39776–39784. [Google Scholar] [CrossRef] [PubMed]
- Russo, A.; Russo, G.; Peticca, M.; Pietropaolo, C.; di Rosa, M.; Iuvone, T. Inhibition of granuloma-associated angiogenesis by controlling mast cell mediator release: Role of mast cell protease-5. Br. J. Pharmacol. 2005, 145, 24–33. [Google Scholar] [CrossRef] [PubMed]
- De Nigris, F.; Mancini, F.P.; Schiano, C.; Infante, T.; Zullo, A.; Minucci, P.B.; Al-Omran, M.; Giordano, A.; Napoli, C. Osteosarcoma cells induce endothelial cell proliferation during neo-angiogenesis. J. Cell. Physiol. 2013, 228, 846–852. [Google Scholar] [CrossRef] [PubMed]
- De Filippis, D.; Russo, A.; de Stefano, D.; Cipriano, M.; Esposito, D.; Grassia, G.; Carnuccio, R.; Russo, G.; Iuvone, T. Palmitoylethanolamide inhibits rMCP-5 expression by regulating MITF activation in rat chronic granulomatous inflammation. Eur. J. Pharmacol. 2014, 725, 64–69. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- De Filippis, D.; Russo, A.; de Stefano, D.; Maiuri, M.C.; Esposito, G.; Cinelli, M.P.; Pietropaolo, C.; Carnuccio, R.; Russo, G.; Iuvone, T. Local administration of win 55,21-2 reduces chronic granuloma-associated angiogenesis in rat by inhibiting NF-κB activation. J. Mol. Med. 2007, 85, 635–645. [Google Scholar] [CrossRef] [PubMed]
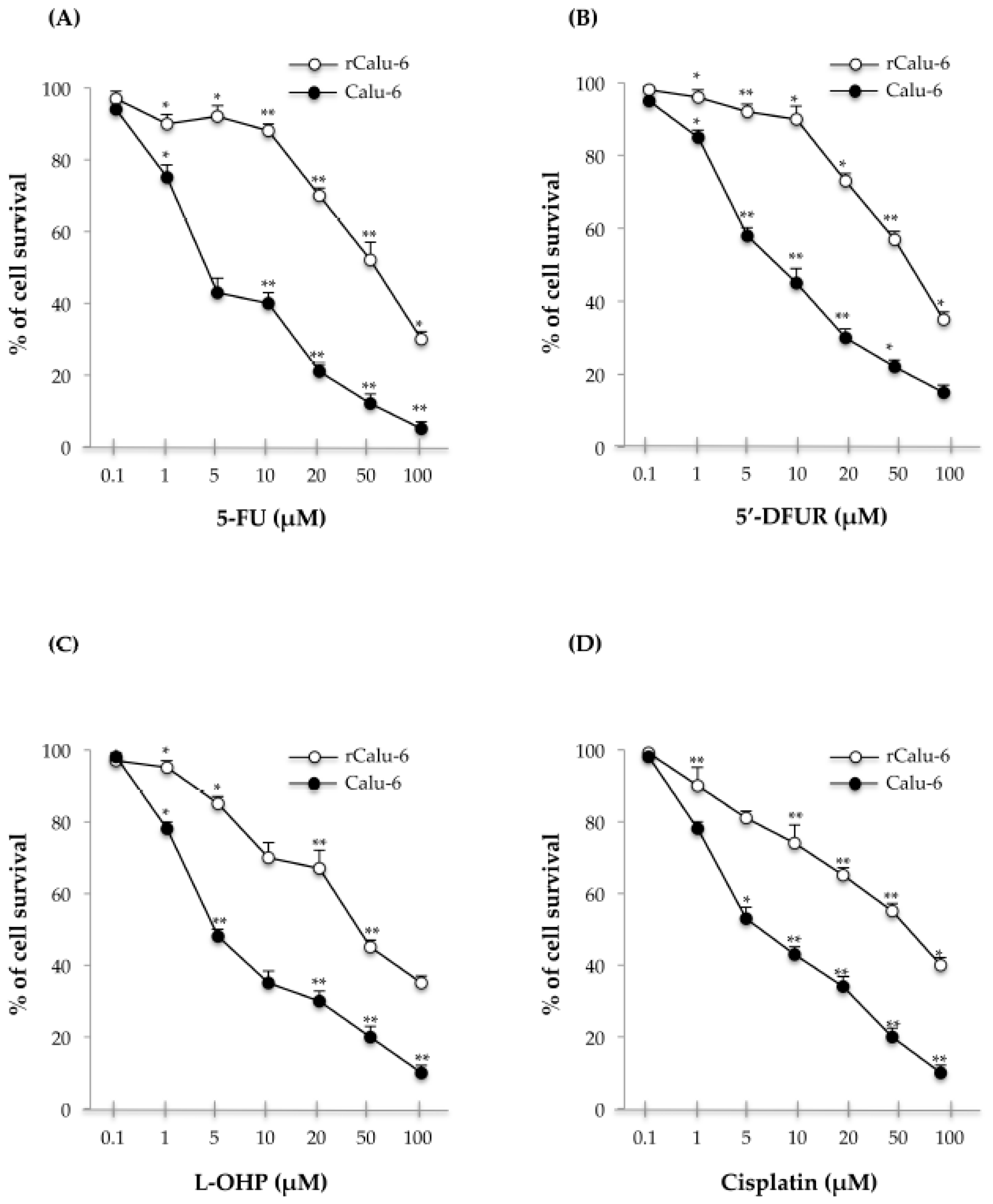


| Drug | IC50 (μM) | r/Calu-6 | p-Value (Calu-6 vs. rCalu-6) |
|---|---|---|---|
| Calu-6 | |||
| 5-FU | 4.5 ± 0.08 | 52.3 ± 0.02 (RI = 11.7X) | <0.01 |
| 5′-DFUR | 8.35 ± 0.06 | 56.7 ± 0.03 (RI = 7X) | <0.05 |
| L-OHP | 5 ± 0.07 | 50 ± 0.03 (RI = 10X) | <0.01 |
| Cisplatin | 10 ± 0.02 | 60 ± 0.05 (RI = 6X) | <0.01 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Russo, A.; Saide, A.; Smaldone, S.; Faraonio, R.; Russo, G. Role of uL3 in Multidrug Resistance in p53-Mutated Lung Cancer Cells. Int. J. Mol. Sci. 2017, 18, 547. https://doi.org/10.3390/ijms18030547
Russo A, Saide A, Smaldone S, Faraonio R, Russo G. Role of uL3 in Multidrug Resistance in p53-Mutated Lung Cancer Cells. International Journal of Molecular Sciences. 2017; 18(3):547. https://doi.org/10.3390/ijms18030547
Chicago/Turabian StyleRusso, Annapina, Assunta Saide, Silvia Smaldone, Raffaella Faraonio, and Giulia Russo. 2017. "Role of uL3 in Multidrug Resistance in p53-Mutated Lung Cancer Cells" International Journal of Molecular Sciences 18, no. 3: 547. https://doi.org/10.3390/ijms18030547
APA StyleRusso, A., Saide, A., Smaldone, S., Faraonio, R., & Russo, G. (2017). Role of uL3 in Multidrug Resistance in p53-Mutated Lung Cancer Cells. International Journal of Molecular Sciences, 18(3), 547. https://doi.org/10.3390/ijms18030547







