Potential Neuroprotective Effects of Adiponectin in Alzheimer’s Disease
Abstract
:1. Introduction
1.1. Association between Alzheimer’s Disease and Insulin Signaling
Dysregulated Insulin Signaling in Clinical and Animal Studies
1.2. Enhancing Insulin Signaling as a Treatment for AD
2. Adiponectin
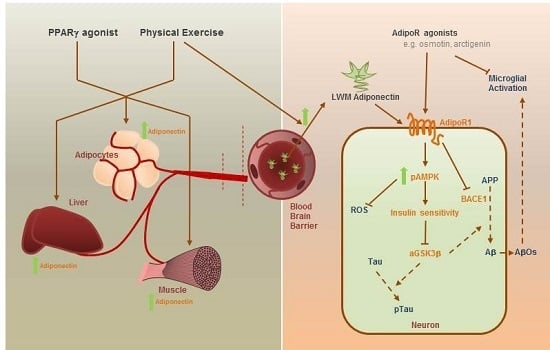
2.1. Adiponectin Receptors and Adiponectin Signaling
2.2. Adiponectin in the Brain
3. Pathophysiological Roles of Adiponectin in Alzheimer’s Disease
4. Therapeutic Potential of Adiponectin
5. Conclusions
Acknowledgments
Conflicts of Interest
Abbreviations
| AD | Alzheimer’s disease |
| Aβ | Amyloid-β |
| AdipoR | Adiponectin receptor |
| AMPK | 5′ adenosine monophosphate-activated protein kinase |
| APN | Adiponectin |
| APP | Amyloid precursor protein |
| APPL1 | Adaptor protein, phosphotyrosine interacting with PH domain and leucine zipper 1 |
| BACE1 | β-site APP cleaving enzyme 1 |
| BBB | Blood-brain barrier |
| CNS | Central nervous system |
| ERK1/2 | Extracellular signal-regulated kinase1/2 |
| FAD | Familial Alzheimer’s disease |
| GSK | Glycogen synthase kinase |
| HMW | High molecular weight |
| ICV | Intracerebroventricular |
| IV | Intravenous |
| IDE | Insulin degrading enzyme |
| IL1β | Interleukin 1β |
| IR | Insulin receptor |
| IRS-1 | Insulin receptor substrate 1 |
| JNK | c-Jun N-terminal kinase |
| LMW | Low molecular weight |
| LPS | Lipopolysaccharide |
| MAPK | Mitogen-activated protein kinases |
| MCI | Mild cognitive impairment |
| NFT | Neurofibrillary tangles |
| mTOR | mammalian target of rapamycin |
| NF-κB | Nuclear factor κ B |
| PPARα | Peroxisome proliferator-activated receptor α |
| PPARγ | Peroxisome proliferator-activated receptor γ |
| PS | Presenilin |
| SIRT-1 | NAD-dependent deacetylase sirtuin-1 |
| T2DM | Type 2 diabetes mellitus |
| TLR4 | Toll-like receptor 4 |
| TNFα | Tumor necrosis factor α |
References
- Stelzmann, R.A.; Norman Schnitzlein, H.; Reed Murtagh, F. Uber eine eigenartige Erkrankung der Hirnrinde. Allg. Z. Psychiatr. 1907, 64, 146–148. [Google Scholar]
- Glenner, G.G.; Wong, C.W. Alzheimer’s disease: Initial report of the purification and characterization of a novel cerebrovascular amyloid protein. Biochem. Biophys. Res. Commun. 1984, 120, 885–890. [Google Scholar] [CrossRef]
- Hardy, J.; Selkoe, D.J. The amyloid hypothesis of Alzheimer’s disease: Progress and problems on the road to therapeutics. Science 2002, 297, 353–356. [Google Scholar] [CrossRef] [PubMed]
- Oakley, H.; Cole, S.L.; Logan, S.; Maus, E.; Shao, P.; Craft, J.; Guillozet-Bongaarts, A.; Ohno, M.; Disterhoft, J.; VanEldik, L.; et al. Intraneuronal β-amyloid aggregates, neurodegeneration, and neuron loss in transgenic mice with five familial Alzheimer’s disease mutations: Potential factors in amyloid plaque formation. J. Neurosci. 2006, 26, 10129–10140. [Google Scholar] [CrossRef] [PubMed]
- Serrano-Pozo, A.; Frosch, M.P.; Masliah, E.; Hyman, B.T. Neuropathological alterations in Alzheimer disease. Cold Spring Harb. Perspect. Med. 2011, 1, a006189. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Ma, Q.; Zhang, Y.; Xu, H. Proteolytic processing of Alzheimer’s β-amyloid precursor protein. J. Neurochem. 2012, 120, 9–21. [Google Scholar] [CrossRef] [PubMed]
- Sun, X.; Bromley-Brits, K.; Song, W. Regulation of β-site APP-cleaving enzyme 1 gene expression and its role in Alzheimer’s Disease. J. Neurochem. 2012, 120 (Suppl. S1), 62–70. [Google Scholar] [CrossRef] [PubMed]
- Strittmatter, W.J.; Saunders, A.M.; Schmechel, D.; Pericak-Vance, M.; Enghild, J.; Salvesen, G.S.; Roses, A.D. Proteolytic processing of Alzheimer’s β-amyloid precursor protein. Proc. Natl. Acad. Sci. USA 1993, 90, 1977–1981. [Google Scholar] [CrossRef] [PubMed]
- Sharp, D.J.; Brooks, D.J.; Matthews, P.M.; Heckemann, R.A.; Gentleman, S.M.; Turkheimer, F.E.; Greenwood, R.J.; Leech, R.; Veronese, M.; Cole, J.; et al. Amyloid pathology and axonal injury after brain trauma. Neurology 2016, 86, 821–828. [Google Scholar]
- Kanekiyo, T.; Xu, H.; Bu, G. ApoE and Aβ in Alzheimer’s disease: Accidental encounters or partners? Neuron 2014, 81, 740–754. [Google Scholar] [CrossRef] [PubMed]
- Annus, T.; Wilson, L.R.; Hong, Y.T.; Acosta-Cabronero, J.; Fryer, T.D.; Cardenas-Blanco, A.; Smith, R.; Boros, I.; Coles, J.P.; Aigbirhio, F.I.; et al. The pattern of amyloid accumulation in the brains of adults with Down syndrome. Alzheimer’s Dement. 2016, 12, 538–545. [Google Scholar] [CrossRef] [PubMed]
- Association, A. 2013 Alzheimer’s disease facts and figures. Alzheimer’s Dement. 2013, 9, 208–245. [Google Scholar]
- García-Casares, N.; Jorge, R.E.; García-Arnés, J.A.; Acion, L.; Berthier, M.L.; Gonzalez-Alegre, P.; Nabrozidis, A.; Gutiérrez, A.; Ariza, M.J.; Rioja, J.; et al. Cognitive dysfunctions in middle-aged type 2 diabetic patients and neuroimaging correlations: A cross-sectional study. J. Alzheimer’s Dis. 2014, 42, 1337–1346. [Google Scholar]
- Talbot, K.; Wang, H.; Kazi, H.; Han, L.; Bakshi, K.P.; Stucky, A.; Fuino, R.L.; Kawaguchi, K.R.; Samoyedny, A.J.; Wilson, R.S.; et al. Demonstrated brain insulin resistance in Alzheimer’s disease patients is assocaited with IGF-1 resisitance, IRS-1 dysregulation, and cogntive decline. J. Clin. Investig. 2012, 122, 1316–1338. [Google Scholar] [CrossRef] [PubMed]
- Razay, G.; Wilcock, G.K. Hyperinsulinaemia and Alzheimer s Disease. Age Ageing 1994, 23, 396–399. [Google Scholar] [CrossRef] [PubMed]
- Craft, S.; Peskind, E.; Schwartz, M.W.; Schellenberg, G.D.; Raskind, M.; Porte, D. Cerebrospinal fluid and plasma insulin levels in Alzheimer’s disease Relationship to severity of dementia and apolipoprotein E genotype. Neurology 1998, 50, 164–168. [Google Scholar] [CrossRef] [PubMed]
- Yarchoan, M.; Toledo, J.B.; Lee, E.B.; Arvanitakis, Z.; Kazi, H.; Han, L.Y.; Louneva, N.; Lee, V.M.Y.; Kim, S.F.; Trojanowski, J.Q.; et al. Abnormal serine phosphorylation of insulin receptor substrate 1 is associated with tau pathology in Alzheimer’s disease and tauopathies. Acta Neuropathol. 2014, 128, 679–689. [Google Scholar] [CrossRef] [PubMed]
- Steen, E.; Terry, B.M.; Rivera, E.J.; Cannon, J.L.; Neely, T.R.; Tavares, R.; Xu, X.J.; Wands, J.R.; dela Monte, S.M. Impaired insulin and insulin-like growth factor expression and signaling mechanisms in Alzheimer’s disease—Is this type 3 diabetes? J. Alzheimer’s Dis. 2005, 7, 63–80. [Google Scholar]
- Moloney, A.M.; Griffin, R.J.; Timmons, S.; O’Connor, R.; Ravid, R.; O’Neill, C. Defects in IGF-1 receptor, insulin receptor and IRS-1/2 in Alzheimer’s disease indicate possible resistance to IGF-1 and insulin signalling. Neurobiol. Aging 2010, 31, 224–243. [Google Scholar] [CrossRef] [PubMed]
- Pei, J.-J.; Sabiha, K.; An, W.-L.; Maria, N.; Toshihisa, T.; Heiko, B.; Ichiro, T.; Masatoshi, T.; Irina, A.; Bengt, W.; et al. Role of protein kinase B in Alzheimer’s neurofibrillary pathology. Acta Neuropathol. 2003, 105, 381–392. [Google Scholar] [PubMed]
- Hoscheidt, S.M.; Starks, E.J.; Oh, J.M.; Zetterberg, H.; Blennow, K.; Krause, R.A.; Gleason, C.E.; Puglielli, L.; Atwood, C.S.; Carlsson, C.M.; et al. Insulin Resistance is Associated with Increased Levels of Cerebrospinal Fluid Biomarkers of Alzheimer’s Disease and Reduced Memory Function in At-Risk Healthy Middle-Aged Adults. J. Alzheimer’s Dis. 2016, 52, 1374–1483. [Google Scholar] [CrossRef] [PubMed]
- Anderson, N.J.; King, M.R.; Delbruck, L.; Jolivalt, C.G. Role of insulin signaling impairment, adiponectin and dyslipidemia in peripheral and central neuropathy in mice. Dis. Model. Mech. 2014, 7, 625–633. [Google Scholar] [CrossRef] [PubMed]
- Abbondante, S.; Baglietto-Vargas, D.; Rodriguez-Ortiz, C.J.; Estrada-Hernandez, T.; Medeiros, R.; LaFerla, F.M. Genetic ablation of tau mitigates cognitive impairment induced by type 1 diabetes. Am. J. Pathol. 2014, 184, 819–826. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Zhao, Y.; Dai, C.-L.; Liang, Z.; Run, X.; Iqbal, K.; Liu, F.; Gong, C.X. Intranasal insulin restores insulin signaling, increases synaptic proteins, and reduces Aβ level and microglia activation in the brains of 3xTg-AD mice. Exp. Neurol. 2014, 261, 610–619. [Google Scholar] [CrossRef] [PubMed]
- Vandal, M.; White, P.J.; Tremblay, C.; St-Amour, I.; Chevrier, G.; Emond, V.; Lefrancois, D.; Virgili, J.; Planel, E.; Giguere, Y.; et al. Insulin reverses the high-fat diet-induced increase in brain Aβ and improves memory in an animal model of Alzheimer disease. Diabetes 2014, 63, 4291–4301. [Google Scholar] [CrossRef] [PubMed]
- Rajasekar, N.; Nath, C.; Hanif, K.; Shukla, R. Intranasal Insulin Administration Ameliorates Streptozotocin (ICV)-Induced Insulin Receptor Dysfunction, Neuroinflammation, Amyloidogenesis, and Memory Impairment in Rats. Mol. Neurobiol. 2016. [Google Scholar] [CrossRef] [PubMed]
- Park, C.R.; Seeley, R.J.; Craft, S.; Woods, S.C. Intracerebroventricular insulin enhances memory in a passive-avoidance task. Physiol. Behav. 2000, 68, 509–514. [Google Scholar] [CrossRef]
- Reger, M.A.; Watson, G.S.; Green, P.S.; Wilkinson, C.W.; Baker, L.D.; Cholerton, B.; Fishel, M.A.; Plymate, S.R.; Breitner, J.C.; DeGroodt, W.; et al. Intranasal insulin improves cognition and modulates β-amyloid in early AD. Neurology 2008, 70, 440–448. [Google Scholar] [CrossRef] [PubMed]
- Watson, G.S.; Claxton, A.; Arbuckle, M.; Leverenz, J.; Cross, D.; Gerton, B. Intranasal Insulin Therapy for Alzheimer Disease and Amnestic Mild Cognitive Impairment. Arch. Neurol. 2012, 69, 29–38. [Google Scholar]
- Scherer, P.E.; Williams, S.; Fogliano, M.; Baldini, G.; Lodish, H.F. A novel serum protein similar to C1q, produced exclusively in adipocytes. J. Biol. Chem. 1995, 270, 26746–26749. [Google Scholar] [CrossRef] [PubMed]
- Combs, T.P.; Berg, A.H.; Obici, S.; Scherer, P.E.; Rossetti, L. Endogenous glucose production is inhibited by the adipose-derived protein Acrp30. J. Clin. Investig. 2001, 108, 1875–1881. [Google Scholar] [CrossRef] [PubMed]
- Waki, H.; Yamauchi, T.; Kamon, J.; Ito, Y.; Uchida, S.; Kita, S.; Hara, K.; Hada, Y.; Vasseur, F.; Froguel, P.; et al. Impaired multimerization of human adiponectin mutants associated with diabetes. Molecular structure and multimer formation of adiponectin. J. Biol. Chem. 2003, 278, 40352–40363. [Google Scholar] [CrossRef] [PubMed]
- Tsao, T.S.; Murrey, H.E.; Hug, C.; Lee, D.H.; Lodish, H.F. Oligomerization state-dependent activation of NF-κB signaling pathway by adipocyte complement-related protein of 30 kDa (Acrp30). J. Biol. Chem. 2002, 277, 29359–29362. [Google Scholar] [CrossRef] [PubMed]
- Tsao, T.S.; Tomas, E.; Murrey, H.E.; Hug, C.; Lee, D.H.; Ruderman, N.B.; Heuser, J.E.; Lodish, H.F. Role of Disulfide Bonds in Acrp30/Adiponectin Structure and Signaling Specificity: Different oligomers activate different signal transduction pathways. J. Biol. Chem. 2003, 278, 50810–50817. [Google Scholar] [CrossRef] [PubMed]
- Fruebis, J.; Tsao, T.-S.; Javorschi, S.; Ebbets-Reed, D.; Erickson, M.R.S.; Yen, F.T.; Bihain, B.E.; Lodish, H.F. Proteolytic cleavage product of 30-kDa adipocyte complement-related protein increases fatty acid oxidation in muscle and causes weight loss in mice. Proc. Natl. Acad. Sci. USA 2001, 98, 2005–2010. [Google Scholar] [CrossRef] [PubMed]
- Yukio, A.; Shinji, K.; Noriyuki, O.; Masahiko, T.; Kazuhisa, M.; Jun-ichiro, M.; Kikuko, H.; Lichiro, S.; Tadashi, N.; Koji, M.; et al. Paradoxical Decrease of an Adipose-Specific Protein, Adiponectin, in Obesity. Biochem. Biophys. Res. Commun. 1999, 257, 79–83. [Google Scholar]
- Thundyil, J.; Pavlovski, D.; Sobey, C.G.; Arumugam, T.V. Adiponectin receptor signalling in the brain. Br. J. Pharm. 2012, 165, 313–327. [Google Scholar] [CrossRef] [PubMed]
- Protein, A.A.; Yang, W.; Lee, W.; Funahashi, T.; Tanaka, S.; Matsuzawa, Y.; Chao, C.; Chen, C.; Tai, T.; Chuang, L. Weight Reduction Increases Plasma Levels of an adipose-derived anti-inflammatory protein, adiponectin. J. Clin. Endocrinol. Metab. 2001, 86, 3815–3819. [Google Scholar]
- Hotta, K.; Funahashi, T.; Bodkin, N.L.; Ortmeyer, H.K.; Arita, Y.; Hansen, B.C.; Matsuzawa, Y. Circulating concentrations of the adipocyte protein adponectin are decreased in parallel with reduced insulin sensitivity during the progression to type 2 diabetes in Rhesys Monkeys. Diabetes 2001, 50, 1126–1133. [Google Scholar] [CrossRef] [PubMed]
- Kondo, H.; Shimomura, L.; Matsukawa, Y.; Kumada, M.; Takahashi, M.; Matsuda, M.; Ouchi, N.; Kihara, S.; Kawamoto, T.; Sumitsuji, S.; et al. Association of adiponectin mutation with type 2 diabetes: A candidate gene for the insulin resistance syndrome. Diabetes 2002, 51, 2325–2328. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Mao, X.; Wang, L.; Liu, M.; Wetzel, M.D.; Guan, K.L.; Dong, L.Q.; Liu, F. Adiponectin sensitizes insulin signaling by reducing p70 S6 kinase-mediated serine phosphorylation of IRS-1. J. Biol. Chem. 2007, 282, 7991–7996. [Google Scholar] [CrossRef] [PubMed]
- Yamauchi, T.; Kamon, J.; Minokoshi, Y.; Ito, Y.; Waki, H.; Uchida, S.; Yamashita, S.; Noda, M.; Kita, S.; Ueki, K.; et al. Adiponectin stimulates glucose utilization and fatty-acid oxidation by activating AMP-activated protein kinase. Nat. Med. 2002, 8, 1288–1295. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.; Motoshima, H.; Mahadev, K.; Stalker, T.J.; Scalia, R.; Goldstein, B.J. Involvement of AMP-activated protein kinase in glucose uptake stimulated by the globular domain of adiponectin in primary rat adipocytes. Diabetes 2003, 52, 1355–1363. [Google Scholar] [CrossRef] [PubMed]
- Ryu, J.; Galan, A.K.; Xin, X.; Dong, F.; Abdul-Ghani, M.A.; Zhou, L.; Wang, C.; Li, C.; Holmes, B.M.; Sloane, L.B.; et al. APPL1 potentiates insulin sensitivity by facilitating the binding of IRS1/2 to the insulin receptor. Cell Rep. 2014, 7, 1227–1238. [Google Scholar] [CrossRef] [PubMed]
- 88Patel, S.A.; Hoehn, K.L.; Lawrence, R.T.; Sawbridge, L.; Talbot, N.A.; Tomsig, J.L.; Turner, N.; Cooney, G.J.; Whitehead, J.P.; Kraegen, E.W.; et al. Overexpression of the adiponectin receptor AdipoR1 in rat skeletal muscle amplifies local insulin sensitivity. Endocrinology 2012, 153, 5231–5246. [Google Scholar]
- Mandal, P.; Pratt, B.T.; Barnes, M.; McMullen, M.R.; Nagy, L.E. Molecular mechanism for adiponectin-dependent m2 macrophage polarization link between the metabolic and innate immune activity of full-length adiponectin. J. Biol. Chem. 2011, 286, 13460–13469. [Google Scholar] [CrossRef] [PubMed]
- Folco, E.J.; Rocha, V.Z.; López-Ilasaca, M.; Libby, P. Adiponectin inhibits pro-inflammatory signaling in human macrophages independent of interleukin-10. J. Biol. Chem. 2009, 284, 25569–25575. [Google Scholar] [CrossRef] [PubMed]
- Huang, H.; Park, P.H.; McMullen, M.R.; Nagy, L.E. Mechanisms for the anti-inflammatory effects of adiponectin in macrophages. J. Gastroenterol. Hepatol. 2008, 23 (Suppl. S1), S50–S53. [Google Scholar] [CrossRef] [PubMed]
- Nigro, E.; Scudiero, O.; Sarnataro, D.; Mazzarella, G.; Sofia, M.; Bianco, A.; Daniele, A. Adiponectin affects lung epithelial A549 cell viability counteracting TNFa and IL-1β toxicity through AdipoR1. Int J. Biochem. Cell Biol. 2013, 45, 1145–1153. [Google Scholar] [CrossRef] [PubMed]
- Wan, Z.; Mah, D.; Simtchouk, S.; Klegeris, A.; Little, J.P. Globular adiponectin induces a pro-inflammatory response in human astrocytic cells. Biochem. Biophys. Res. Commun. 2014, 446, 37–42. [Google Scholar] [CrossRef] [PubMed]
- Shrestha, A.; Park, P.H. Globular adiponectin attenuates LPS-induced reactive oxygen species production in HepG2 cells via FoxO3A and HO-1 signaling. Life Sci. 2016, 148, 71–79. [Google Scholar] [CrossRef] [PubMed]
- Park, M.; Youn, B.; Zheng, X.L.; Wu, D.; Xu, A.; Sweeney, G. Globular adiponectin, acting via AdipoR1/APPL1, protects H9c2 cells from hypoxia/reoxygenation-induced apoptosis. PLoS ONE 2011, 6, e19143. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Qi, Y.; Takahashi, N.; Hileman, S.M.; Patel, H.R.; Berg, A.H.; Pajvani, U.B.; Scherer, P.E.; Ahima, R.S. Adiponectin acts in the brain to decrease body weight. Nat. Med. 2004, 10, 524–529. [Google Scholar] [CrossRef] [PubMed]
- Spranger, J.; Verma, S.; Göhring, I.; Bobbert, T.; Seifert, J.; Sindler, A.L.; Pfeiffer, A.; Hileman, S.M.; Tschöp, M.; Banks, W.A. Adiponectin Does Not Cross the Blood-Brain Barrier but Modifies Cytokine Expression of Brain Endothelial Cells. Diabetes 2005, 55, 141–147. [Google Scholar] [CrossRef]
- Pan, W.; Tu, H.; Kastin, A.J. Differential BBB interactions of three ingestive peptides: Obestatin, ghrelin, and adiponectin. Peptides 2006, 27, 911–916. [Google Scholar] [CrossRef] [PubMed]
- Neumeier, M.; Weigert, J.; Buettner, R.; Wanninger, J.; Schäffler, A.; Mü, A.M.; Killian, S.; Sauerbruch, S.; Schlachetzki, F.; Steinbrecher, A.; et al. Detection of adiponectin in cerebrospinal fluid in humans. Am. J. Physiol. Endocrinol. Metab. 2007, 293, E965–E969. [Google Scholar] [CrossRef] [PubMed]
- Kusminski, C.M.; McTernan, P.G.; Schraw, T.; Kos, K.; O’Hare, J.P.; Ahima, R.; Kumar, S.; Scherer, P.E. Adiponectin complexes in human cerebrospinal fluid: Distinct complex distribution from serum. Diabetologia 2007, 50, 634–642. [Google Scholar] [CrossRef] [PubMed]
- Kos, K.; Harte, A.L.; DaSilva, N.F.; Tonchev, A.; Chaldakov, G.; James, S.; Snead, D.R.; Hoggart, B.; O’Hare, J.P.; McTernan, P.G.; et al. Adiponectin and resistin in human cerebrospinal fluid and expression of adiponectin receptors in the human hypothalamus. J. Clin. Endocrinol. Metab. 2007, 92, 1129–1136. [Google Scholar] [CrossRef] [PubMed]
- Ebinuma, H.; Yamauchi, T.; Hada, Y.; Hara, K. Improved ELISA for Selective Measurement of Adiponectin Multimers and Identification of Adiponectin in Human Cerebrospinal Fluid. Clin. Chem. 2007, 53, 1541–1548. [Google Scholar] [CrossRef] [PubMed]
- Yau, S.-Y.; Li, A.; Hoo, R.L.C.; Ching, Y.P.; Christie, B.R.; Lee, T.M.C.; Xu, A.; So, K.F. Physical exercise-induced hippocampal neurogenesis and antidepressant effects are mediated by the adipocyte hormone adiponectin. Proc. Natl. Acad. Sci. USA 2014, 111, 15810–15815. [Google Scholar] [CrossRef] [PubMed]
- Fry, M.; Smith, P.M.; Hoyda, T.D.; Duncan, M.; Ahima, R.S.; Sharkey, K.A.; Ferguson, A.V. Area postrema neurons are modulated by the adipocyte hormone adiponectin. J. Neurosci. 2006, 26, 9695–9702. [Google Scholar] [CrossRef] [PubMed]
- Kubota, N.; Yano, W.; Kubota, T.; Yamauchi, T.; Itoh, S.; Kumagai, H.; Kozono, H.; Takamoto, I.; Okamoto, S.; Shiuchi, T.; et al. Adiponectin Stimulates AMP-Activated Protein Kinase in the Hypothalamus and Increases Food Intake. Cell Metab. 2007, 6, 55–68. [Google Scholar] [CrossRef] [PubMed]
- Wen, J.P.; Liu, C.E.; Hu, Y.T.; Chen, G.; Lin, L.X. Globular adiponectin regulates energy homeostasis through AMP-activated protein kinase-acetyl-CoA carboxylase (AMPK/ACC) pathway in the hypothalamus. Mol. Cell. Biochem. 2010, 344, 109–115. [Google Scholar] [CrossRef] [PubMed]
- Park, S.; Kim, D.S.; Kwon, D.Y.; Yang, H.J. Long-term central infusion of adiponectin improves energy and glucose homeostasis by decreasing fat storage and suppressing hepatic gluconeogenesis without changing food intake. J. Neuroendocrinol. 2011, 23, 687–698. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.; Guo, M.; Zhang, W. LUX:Adiponectin stimulates proliferation of adult hippocampal neural stem/progenitor cells through activation of p38 mitogen-activated protein kinase (p38MAPK)/glycogen synthase kinase 3β (GSK-3β)/β-catenin signaling cascade. J. Biol. Chem. 2011, 12, 44913–44920. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.; Wang, X.; Lu, X.Y. Adiponectin exerts neurotrophic effects on dendritic arborization, spinogenesis, and neurogenesis of the dentate gyrus of male mice. Endocrinology 2016, 157, 2853–2869. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Guo, M.; Zhang, D.; Cheng, S.Y.; Liu, M.; Ding, J.; Scherer, P.E.; Liu, F.; Lu, X.Y. Adiponectin is critical in determining susceptibility to depressive behaviors and has antidepressant-like activity. Proc. Natl. Acad. Sci. USA 2012, 109, 12248–12253. [Google Scholar] [CrossRef] [PubMed]
- Chabry, J.; Nicolas, S.; Cazareth, J.; Murris, E.; Guyon, A.; Glaichenhaus, N.; Heurteaux, C.; Petit-Paitel, A. Enriched environment decreases microglia and brain macrophages inflammatory phenotypes through adiponectin-dependent mechanisms: Relevance to depressive-like behavior. Brain Behav. Immun. 2015, 50, 275–287. [Google Scholar] [CrossRef] [PubMed]
- Nicolas, S.; Veyssière, J.; Gandin, C.; Zsürger, N.; Pietri, M.; Heurteaux, C.; Glaichenhaus, N.; Petit-Paitel, A.; Chabry, J. Neurogenesis-independent antidepressant-like effects of enriched environment is dependent on adiponectin. Psychoneuroendocrinology 2015, 57, 72–83. [Google Scholar] [CrossRef] [PubMed]
- Mikesell, R.; Ryu, J.; Hsieh, C.; Cremasco, V. Lack of adiponectin leads to increased lymphocyte activation and worse severity of a mouse model of multiple sclerosis. Eur. J. Immunol. 2013, 43, 2089–2100. [Google Scholar]
- Shen, D.; Xing, S.; Chen, C. Adiponectin gene polymorphisms contributes to ischemic stroke risk: A meta-analysis. J. Renin Angiotensin Aldosterone Syst. 2015, 16, 178–184. [Google Scholar] [CrossRef] [PubMed]
- Liu, F.; He, Z.; Deng, S.; Zhang, H.; Li, N.; Xu, J. Association of adiponectin gene polymorphisms with the risk of ischemic stroke in a Chinese Han population. Mol. Biol. Rep. 2011, 38, 1983–1988. [Google Scholar] [CrossRef] [PubMed]
- Shen, L.; Miao, J.; Zhao, Y.; Zhao, Y.; Liang, W. Expression of brain adiponectin in a murine model of transient cerebral ischemia. Int. J. Clin. Exp. Med. 2014, 7, 4590–4596. [Google Scholar] [PubMed]
- Miao, J.; Shen, L.H.; Tang, Y.H.; Wang, Y.T.; Tao, M.X.; Jin, K.L.; Zhao, Y.J.; Yang, G.Y. Overexpression of adiponectin improves neurobehavioral outcomes after focal cerebral ischemia in aged mice. CNS Neurosci. Ther. 2013, 19, 969–977. [Google Scholar] [CrossRef] [PubMed]
- Une, K.; Takei, Y.A.; Tomita, N.; Asamura, T.; Ohrui, T.; Furukawa, K.; Arai, H. Adiponectin in plasma and cerebrospinal fluid in MCI and Alzheimer’s disease. Eur. J. Neurol. 2011, 18, 1006–1009. [Google Scholar] [CrossRef] [PubMed]
- Himbergen, T.M.V.; Alexa, S.B.; Ai, M.; Seshadri, S.; Otokozawa, S.; Au, R.; Thongtang, N.; Wolf, P.A.; Schaefer, E.J. Biomarkers for Insulin Resistance and Inflammation and the Risk for All-Cause Dementia and Alzheimer Disease Results From the Framingham Heart Study. Arch. Neurol. 2012, 69, 564–600. [Google Scholar]
- Teixeira, A.L.; Diniz, B.S.; Campos, A.C.; Miranda, A.S.; Rocha, N.P.; Talib, L.L.; Gattaz, W.F.; Forlenza, O.V. Decreased levels of circulating adiponectin in mild cognitive impairment and alzheimer’s disease. Neuromol. Med. 2013, 15, 115–121. [Google Scholar] [CrossRef] [PubMed]
- Wennberg, A.M.V.; Gustafson, D.; Hagen, C.E.; Roberts, R.O.; Knopman, D.; Jack, C.; Petersen, R.C.; Mielke, M.M. Serum Adiponectin Levels, Neuroimaging, and Cognition in the Mayo Clinic Study of Aging. J. Alzheimer’s Dis. 2016, 53, 573–581. [Google Scholar] [CrossRef] [PubMed]
- Waragai, M.; Adame, A.; Trinh, I.; Sekiyama, K.; Takamatsu, Y.; Une, K.; Masliah, E.; Hashimoto, M. Possible Involvement of Adiponectin, the Anti-Diabetes Molecule, in the Pathogenesis of Alzheimer’s Disease. J. Alzheimer’s Dis. 2016, 52, 1453–1459. [Google Scholar] [CrossRef] [PubMed]
- Dukic, L.; Simundic, A.M.; Martinic-Popovic, I.; Kackov, S.; Diamandis, A.; Begcevic, I.; Diamandis, E.P. The role of human kallikrein 6, clusterin and adiponectin as potential blood biomarkers of dementia. Clin. Biochem. 2016, 49, 213–218. [Google Scholar] [CrossRef] [PubMed]
- Yu, Z.; Li, W.; Hou, D.; Zhou, L.; Deng, Y.; Tian, M.; Feng, X. Relationship between Adiponectin Gene Polymorphisms and Late-Onset Alzheimer’s Disease. PLoS ONE 2015, 10, e0125186. [Google Scholar] [CrossRef] [PubMed]
- García-Casares, N.; García-Arnés, J.A.; Rioja, J.; Ariza, M.J.; Gutiérrez, A.; Alfaro, F.; Nabrozidis, A.; González-Alegre, P.; González-Santos, P. Alzheimer’s like brain changes correlate with low adiponectin plasma levels in type 2 diabetic patients. J. Diabetes Complicat. 2016, 30, 281–286. [Google Scholar] [CrossRef] [PubMed]
- Gorska-Ciebiada, M.; Saryusz-Wolska, M.; Borkowska, A.; Ciebiada, M.; Loba, J. Adiponectin, leptin and IL-1 β in elderly diabetic patients with mild cognitive impairment. Metab. Brain Dis. 2016, 31, 257–266. [Google Scholar] [CrossRef] [PubMed]
- Kurata, T.; Miyazaki, K.; Morimoto, N.; Kawai, H.; Ohta, Y.; Ikeda, Y.; Abe, K. Atorvastatin and pitavastatin reduce oxidative stress and improve IR/LDL-R signals in Alzheimer’s disease. Neurol. Res. 2013, 35, 193–205. [Google Scholar] [CrossRef] [PubMed]
- Pancani, T.; Anderson, K.L.; Brewer, L.D.; Kadish, I.; DeMoll, C.; Landfield, P.W.; Blalock, E.M.; Porter, N.M.; Thibault, O. Effect of high-fat diet on metabolic indices, cognition, and neuronal physiology in aging F344 rats. Neurobiol. Aging 2013, 34, 1977–1987. [Google Scholar] [CrossRef] [PubMed]
- Ng, R.C.L.; Cheng, O.Y.; Kwan, J.S.C.; Ho, P.W.L.; Cheng, K.K.Y.; Yeung, P.K.K.; Zhou, L.L.; Hoo, R.L.C.; Chung, S.K.; Xu, A.; et al. Chronic adiponectin deficiency leads to Alzheimer’s disease-like cognitive impairments through AMPK inactivation and cerebral insulin resistance in aged mice. Mol. Neurodegener. 2016, 11, 71. [Google Scholar] [CrossRef] [PubMed]
- Chan, K.H.; Lam, K.S.L.; Cheng, O.Y.; Kwan, J.S.C.; Ho, P.W.L.; Cheng, K.K.Y.; Chung, S.K.; Ho, J.W.M.; Guo, V.Y.; Xu, A. Adiponectin is Protective against Oxidative Stress Induced Cytotoxicity in Amyloid-β Neurotoxicity. PLoS ONE 2012, 7, e52354. [Google Scholar] [CrossRef] [PubMed]
- Ly, P.T.T.; Wu, Y.; Zou, H.; Wang, R.; Zhou, W.; Kinoshita, A.; Zhang, M.; Yang, Y.; Cai, F.; Woodgett, J.; et al. Inhibition of GSK3β-mediated BACE1 expression reduces Alzheimer-associated phenotypes. J. Clin. Investig. 2013, 123, 224–235. [Google Scholar] [CrossRef] [PubMed]
- Yang, D.S.; Stavrides, P.; Mohan, P.S.; Kaushik, S.; Kumar, A.; Ohno, M.; Schmidt, S.D.; Wesson, D.; Bandyopadhyay, U.; Jiang, Y.; et al. Reversal of autophagy dysfunction in the TgCRND8 mouse model of Alzheimer’s disease ameliorates amyloid pathologies and memory deficits. Brain 2011, 134, 258–277. [Google Scholar] [CrossRef] [PubMed]
- Reed, M.N.; Hofmeister, J.J.; Jungbauer, L.; Welzel, A.T.; Yu, C.; Sherman, M.A.; Lesné, S.; LaDu, M.J.; Walsh, D.M.; Ashe, K.H.; et al. Cognitive effects of cell-derived and synthetically derived Aβ oligomers. Neurobiol. Aging 2011, 32, 1784–1794. [Google Scholar] [CrossRef] [PubMed]
- Pákáski, M.; Fehér, Á.; Juhász, A.; Drótos, G.; Fazekas, Ö.C.; Kovács, J.; Janka, Z.; Kálmán, J. Serum adipokine levels modified by donepezil treatment in Alzheimer’s disease. J. Alzheimer’s Dis. 2014, 38, 371–377. [Google Scholar]
- Sun, Y.; Zang, Z.; Zhong, L.; Wu, M.; Su, Q.; Gao, X.; Zan, W.; Lin, D.; Zhao, Y.; Zhang, Z. Identification of Adiponectin Receptor Agonist Utilizing a Fluorescence Polarization Based High Throughput Assay. PLoS ONE 2013, 8, e63354. [Google Scholar] [CrossRef] [PubMed]
- Badshah, H.; Ali, T.; Kim, M.O. Osmotin attenuates LPS-induced neuroinflammation and memory impairments via the TLR4/NFκB signaling pathway. Sci Rep. 2016, 6, 24493. [Google Scholar] [CrossRef] [PubMed]


© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ng, R.C.-L.; Chan, K.-H. Potential Neuroprotective Effects of Adiponectin in Alzheimer’s Disease. Int. J. Mol. Sci. 2017, 18, 592. https://doi.org/10.3390/ijms18030592
Ng RC-L, Chan K-H. Potential Neuroprotective Effects of Adiponectin in Alzheimer’s Disease. International Journal of Molecular Sciences. 2017; 18(3):592. https://doi.org/10.3390/ijms18030592
Chicago/Turabian StyleNg, Roy Chun-Laam, and Koon-Ho Chan. 2017. "Potential Neuroprotective Effects of Adiponectin in Alzheimer’s Disease" International Journal of Molecular Sciences 18, no. 3: 592. https://doi.org/10.3390/ijms18030592
APA StyleNg, R. C. -L., & Chan, K. -H. (2017). Potential Neuroprotective Effects of Adiponectin in Alzheimer’s Disease. International Journal of Molecular Sciences, 18(3), 592. https://doi.org/10.3390/ijms18030592