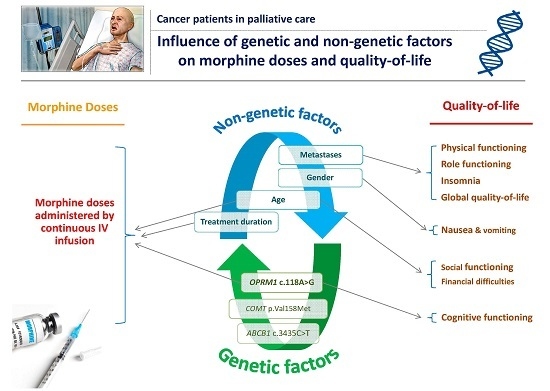
OPRM1 c.118A>G Polymorphism and Duration of Morphine Treatment Associated with Morphine Doses and Quality-of-Life in Palliative Cancer Pain Settings
Abstract
:1. Introduction
2. Results
2.1. Patient Population
2.2. Genotype and Allele Distribution
2.3. Variables Associated with Morphine Doses
2.4. Association to Pain
2.5. Variables Associated with Quality of Life and Morphine Side Effects
3. Discussion
Strengths and Limitations
4. Materials and Methods
4.1. Study Design and Patients
4.2. Genotyping
4.3. Data and Statistical Analysis
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
Abbreviations
| ABCB1 | ATP-binding cassette, subfamily B, member 1 gene |
| AP | Appetite loss |
| ASCO | American Society of Clinical Oncology |
| BMI | Body mass index |
| CF | Cognitive functioning |
| CI | Confidence Interval |
| CO | Constipation |
| COMT | Catechol-O-methyltransferase (enzyme and gene) |
| DI | Diarrhea |
| DNA | Deoxyribonucleic acid |
| DY | Dyspnea |
| EDTA | Ethylenediaminetetraacetic acid |
| EF | Emotional functioning |
| EORTC-QLQ-C30 | European Organisation for Research and Treatment of Cancer Core Quality of Life Questionnaire |
| EPOS | European Pharmacogenetic Opioid Study |
| FA | Fatigue |
| FI | Financial difficulties |
| GQOL | Global health status/QoL |
| MMSE | Mini-Mental State Examination |
| NV | Nausea and vomiting |
| PA | Pain |
| OPRM1 | Mu opioid receptor gene |
| PF | Physical functioning |
| P-gp | P-glycoprotein |
| RF | Role functioning |
| SD | Standard deviation |
| SF | Social functioning |
| SL | Insomnia |
| QOL | Quality-of-life |
| SNP | Single nucleotide polymorphism |
| SPSS | Statistical Package Software for Social Science (for Windows) |
| VAS | Visual analog scale |
| WHO | World Health Organization |
References
- Droney, J.; Riley, J. Recent advances in the use of opioids for cancer pain. J. Pain Res. 2009, 2, 135–155. [Google Scholar] [CrossRef] [PubMed]
- Van Hecke, O.; Torrance, N.; Smith, B.H. Chronic pain epidemiology and its clinical relevance. Br. J. Anaesth. 2013, 111, 13–18. [Google Scholar] [CrossRef] [PubMed]
- Hajj, A.; Khabbaz, L.; Laplanche, J.L.; Peoc’h, K. Pharmacogenetics of opiates in clinical practice: The visible tip of the iceberg. Pharmacogenomics 2013, 14, 575–585. [Google Scholar] [CrossRef] [PubMed]
- Gong, X.D.; Wang, J.Y.; Liu, F.; Yuan, H.H.; Zhang, W.Y.; Guo, Y.H.; Jiang, B. Gene polymorphisms of OPRM1 A118G and ABCB1 C3435T may influence opioid requirements in Chinese patients with cancer pain. Asian Pac. J. Cancer Prev. 2013, 14, 2937–2943. [Google Scholar] [CrossRef] [PubMed]
- Klepstad, P.; Rakvag, T.T.; Kaasa, S.; Holthe, M.; Dale, O.; Borchgrevink, P.C.; Baar, C.; Vikan, T.; Krokan, H.E.; Skorpen, F. The 118A>G polymorphism in the human μ-opioid receptor gene may increase morphine requirements in patients with pain caused by malignant disease. Acta Anaesthesiol. Scand. 2004, 48, 1232–1239. [Google Scholar] [CrossRef] [PubMed]
- Rakvag, T.T.; Klepstad, P.; Baar, C.; Kvam, T.M.; Dale, O.; Kaasa, S.; Krokan, H.E.; Skorpen, F. The Val158Met polymorphism of the human catechol-O-methyltransferase (COMT) gene may influence morphine requirements in cancer pain patients. Pain 2005, 116, 73–78. [Google Scholar] [CrossRef] [PubMed]
- Lachman, H.M.; Papolos, D.F.; Saito, T.; Yu, Y.M.; Szumlanski, C.L.; Weinshilboum, R.M. Human catechol-O-methyltransferase pharmacogenetics: Description of a functional polymorphism and its potential application to neuropsychiatric disorders. Pharmacogenetics 1996, 6, 243–250. [Google Scholar] [CrossRef] [PubMed]
- Lotta, T.; Vidgren, J.; Tilgmann, C.; Ulmanen, I.; Melen, K.; Julkunen, I.; Taskinen, J. Kinetics of human soluble and membrane-bound catechol-O-methyltransferase: A revised mechanism and description of the thermolabile variant of the enzyme. Biochemistry 1995, 34, 4202–4210. [Google Scholar] [CrossRef] [PubMed]
- Reyes-Gibby, C.C.; Shete, S.; Rakvag, T.; Bhat, S.V.; Skorpen, F.; Bruera, E.; Kaasa, S.; Klepstad, P. Exploring joint effects of genes and the clinical efficacy of morphine for cancer pain: OPRM1 and COMT gene. Pain 2007, 130, 25–30. [Google Scholar] [CrossRef] [PubMed]
- Hoffmeyer, S.; Burk, O.; von Richter, O.; Arnold, H.P.; Brockmoller, J.; Johne, A.; Cascorbi, I.; Gerloff, T.; Roots, I.; Eichelbaum, M.; et al. Functional polymorphisms of the human multidrug-resistance gene: Multiple sequence variations and correlation of one allele with P-glycoprotein expression and activity in vivo. Proc. Natl. Acad. Sci. USA 2000, 97, 3473–3478. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Johnson, A.D.; Papp, A.C.; Kroetz, D.L.; Sadee, W. Multidrug resistance polypeptide 1 (MDR1, ABCB1) variant 3435C>T affects mrna stability. Pharmacogenet. Genom. 2005, 15, 693–704. [Google Scholar] [CrossRef]
- Campa, D.; Gioia, A.; Tomei, A.; Poli, P.; Barale, R. Association of ABCB1/MDR1 and OPRM1 gene polymorphisms with morphine pain relief. Clin. Pharmacol. Ther. 2008, 83, 559–566. [Google Scholar] [CrossRef] [PubMed]
- Meineke, I.; Freudenthaler, S.; Hofmann, U.; Schaeffeler, E.; Mikus, G.; Schwab, M.; Prange, H.W.; Gleiter, C.H.; Brockmoller, J. Pharmacokinetic modelling of morphine, morphine-3-glucuronide and morphine-6-glucuronide in plasma and cerebrospinal fluid of neurosurgical patients after short-term infusion of morphine. Br. J. Clin. Pharmacol. 2002, 54, 592–603. [Google Scholar] [PubMed]
- Lotsch, J.; von Hentig, N.; Freynhagen, R.; Griessinger, N.; Zimmermann, M.; Doehring, A.; Rohrbacher, M.; Sittl, R.; Geisslinger, G. Cross-sectional analysis of the influence of currently known pharmacogenetic modulators on opioid therapy in outpatient pain centers. Pharmacogenet. Genom. 2009, 19, 429–436. [Google Scholar] [CrossRef]
- Hajj, A.; Peoc’h, K.; Laplanche, J.L.; Jabbour, H.; Naccache, N.; Abou Zeid, H.; Yazbeck, P.; Khabbaz, L.R. Genotyping test with clinical factors: Better management of acute postoperative pain? Int. J. Mol. Sci. 2015, 16, 6298–6311. [Google Scholar] [CrossRef] [PubMed]
- National Center for Biotechnology Information. Available online: https://www.ncbi.nlm.nih.gov/projects/snp/snp_ref.Cgi?Rs=4680 (accessed on 12 December 2016).
- National Center for Biotechnology Information. Available online: http://www.ncbi.nlm.nih.gov/snp/?Term=rs1799971 (accessed on 12 December 2016).
- National Center for Biotechnology Information. Available online: http://www.ncbi.nlm.nih.gov/snp/?Term=rs1045642 (accessed on 12 December 2016).
- Stevens, J. Applied Multivariate Statistics for the Social Sciences, 4th ed.; Routledge: Mahwah, NJ, USA, 2002. [Google Scholar]
- Aaronson, N.K.; Ahmedzai, S.; Bergman, B.; Bullinger, M.; Cull, A.; Duez, N.J.; Filiberti, A.; Flechtner, H.; Fleishman, S.B.; de Haes, J.C.; et al. The European Organization for Research and Treatment of Cancer QLQ-C30: A quality-of-life instrument for use in international clinical trials in oncology. J. Natl. Cancer Inst. 1993, 85, 365–376. [Google Scholar] [CrossRef] [PubMed]
- International Association for the Study of Pain (IASP), 2008–2009 Global Year against Cancer Pain 2008. Available online: https://www.iasp-pain.org/GlobalYear/Cancer (accessed on 10 February 2015).
- Van den Beuken-van, M.H.; Hochstenbach, L.M.; Joosten, E.A.; Tjan-Heijnen, V.C.; Janssen, D.J. Update on prevalence of pain in patients with cancer: Systematic review and meta-analysis. J. Pain Symptom Manag. 2016, 51, 1070–1090. [Google Scholar] [CrossRef]
- Porter, L.S.; Keefe, F.J. Psychosocial issues in cancer pain. Curr. Pain Headache Rep. 2011, 15, 263–270. [Google Scholar] [CrossRef] [PubMed]
- Kroenke, K.; Theobald, D.; Wu, J.; Loza, J.K.; Carpenter, J.S.; Tu, W. The association of depression and pain with health-related quality of life, disability, and health care use in cancer patients. J. Pain Symptom Manag. 2010, 40, 327–341. [Google Scholar] [CrossRef] [PubMed]
- Abbasi, J. Getting pharmacogenomics into the clinic. JAMA 2016, 316, 1533–1535. [Google Scholar] [CrossRef] [PubMed]
- Paice, J.A.; Portenoy, R.; Lacchetti, C.; Campbell, T.; Cheville, A.; Citron, M.; Constine, L.S.; Cooper, A.; Glare, P.; Keefe, F.; et al. Management of chronic pain in survivors of adult cancers: American society of clinical oncology clinical practice guideline. J. Clin. Oncol. 2016, 34, 3325–3345. [Google Scholar] [CrossRef] [PubMed]
- Bentley, J.N.; Viswanathan, A.; Rosenberg, W.S.; Patil, P.G. Treatment of medically refractory cancer pain with a combination of intrathecal neuromodulation and neurosurgical ablation: Case series and literature review. Pain Med. 2014, 15, 1488–1495. [Google Scholar] [CrossRef] [PubMed]
- Cargnin, S.; Magnani, F.; Viana, M.; Tassorelli, C.; Mittino, D.; Cantello, R.; Sances, G.; Nappi, G.; Canonico, P.L.; Genazzani, A.A.; et al. An opposite-direction modulation of the COMT Val158Met polymorphism on the clinical response to intrathecal morphine and triptans. J. Pain 2013, 14, 1097–1106. [Google Scholar] [CrossRef] [PubMed]
- Caraceni, A.; Hanks, G.; Kaasa, S.; Bennett, M.I.; Brunelli, C.; Cherny, N.; Dale, O.; de Conno, F.; Fallon, M.; Hanna, M.; et al. Use of opioid analgesics in the treatment of cancer pain: Evidence-based recommendations from the eapc. Lancet Oncol. 2012, 13, 58–68. [Google Scholar] [CrossRef]
- Zalloua, P.A.; Xue, Y.; Khalife, J.; Makhoul, N.; Debiane, L.; Platt, D.E.; Royyuru, A.K.; Herrera, R.J.; Hernanz, D.F.; Blue-Smith, J.; et al. Y-chromosomal diversity in lebanon is structured by recent historical events. Am. J. Hum. Genet. 2008, 82, 873–882. [Google Scholar] [CrossRef] [PubMed]
- Coulbault, L.; Beaussier, M.; Verstuyft, C.; Weickmans, H.; Dubert, L.; Tregouet, D.; Descot, C.; Parc, Y.; Lienhart, A.; Jaillon, P.; et al. Environmental and genetic factors associated with morphine response in the postoperative period. Clin. Pharmacol. Ther. 2006, 79, 316–324. [Google Scholar] [CrossRef] [PubMed]
- Macintyre, P.E.; Jarvis, D.A. Age is the best predictor of postoperative morphine requirements. Pain 1996, 64, 357–364. [Google Scholar] [CrossRef]
- Sia, A.T.; Lim, Y.; Lim, E.C.; Goh, R.W.; Law, H.Y.; Landau, R.; Teo, Y.Y.; Tan, E.C. A118G single nucleotide polymorphism of human μ-opioid receptor gene influences pain perception and patient-controlled intravenous morphine consumption after intrathecal morphine for postcesarean analgesia. Anesthesiology 2008, 109, 520–526. [Google Scholar] [CrossRef] [PubMed]
- King, T.; Ossipov, M.H.; Vanderah, T.W.; Porreca, F.; Lai, J. Is paradoxical pain induced by sustained opioid exposure an underlying mechanism of opioid antinociceptive tolerance? Neurosignals 2005, 14, 194–205. [Google Scholar] [CrossRef] [PubMed]
- Nestler, E.J. Molecular mechanisms of drug addiction. J. Neurosci. 1992, 12, 2439–2450. [Google Scholar] [CrossRef] [PubMed]
- Allouche, S.; Noble, F.; Marie, N. Opioid receptor desensitization: Mechanisms and its link to tolerance. Front. Pharmacol. 2014, 5, 280. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Wang, D.; Johnson, A.D.; Papp, A.C.; Sadee, W. Allelic expression imbalance of human μ opioid receptor (OPRM1) caused by variant A118G. J. Biol. Chem. 2005, 280, 32618–32624. [Google Scholar] [CrossRef] [PubMed]
- Bond, C.; LaForge, K.S.; Tian, M.; Melia, D.; Zhang, S.; Borg, L.; Gong, J.; Schluger, J.; Strong, J.A.; Leal, S.M.; et al. Single-nucleotide polymorphism in the human μ opioid receptor gene alters β-endorphin binding and activity: Possible implications for opiate addiction. Proc. Natl. Acad. Sci. USA 1998, 95, 9608–9613. [Google Scholar] [CrossRef] [PubMed]
- Lotsch, J.; Skarke, C.; Grosch, S.; Darimont, J.; Schmidt, H.; Geisslinger, G. The polymorphism A118G of the human μ-opioid receptor gene decreases the pupil constrictory effect of morphine-6-glucuronide but not that of morphine. Pharmacogenetics 2002, 12, 3–9. [Google Scholar] [CrossRef] [PubMed]
- Klepstad, P.; Fladvad, T.; Skorpen, F.; Bjordal, K.; Caraceni, A.; Dale, O.; Davies, A.; Kloke, M.; Lundstrom, S.; Maltoni, M.; et al. Influence from genetic variability on opioid use for cancer pain: A european genetic association study of 2294 cancer pain patients. Pain 2011, 152, 1139–1145. [Google Scholar] [CrossRef] [PubMed]
- Droney, J.M.; Gretton, S.K.; Sato, H.; Ross, J.R.; Branford, R.; Welsh, K.I.; Cookson, W.; Riley, J. Analgesia and central side-effects: Two separate dimensions of morphine response. Br. J. Clin. Pharmacol. 2013, 75, 1340–1350. [Google Scholar] [CrossRef] [PubMed]
- Buffart, L.M.; Kalter, J.; Sweegers, M.G.; Courneya, K.S.; Newton, R.U.; Aaronson, N.K.; Jacobsen, P.B.; May, A.M.; Galvao, D.A.; Chinapaw, M.J.; et al. Effects and moderators of exercise on quality of life and physical function in patients with cancer: An individual patient data meta-analysis of 34 RCTs. Cancer Treat. Rev. 2016, 52, 91–104. [Google Scholar] [CrossRef] [PubMed]
- Berhili, S.; Kadiri, S.; Bouziane, A.; Aissa, A.; Marnouche, E.; Ogandaga, E.; Echchikhi, Y.; Touil, A.; Loughlimi, H.; Lahdiri, I.; et al. Associated factors with psychological distress in moroccan breast cancer patients: A cross-sectional study. Breast 2016, 31, 26–33. [Google Scholar] [CrossRef] [PubMed]
- Teke, F.; Bucaktepe, P.; Kibrisli, E.; Demir, M.; Ibiloglu, A.; Inal, A. Quality of life, psychological burden, and sleep quality in patients with brain metastasis undergoing whole brain radiation therapy. Clin. J. Oncol. Nurs. 2016, 20, AE-02. [Google Scholar] [CrossRef] [PubMed]
- Jansson-Frojmark, M.; Lindblom, K. A bidirectional relationship between anxiety and depression, and insomnia? A prospective study in the general population. J. Psychosom. Res. 2008, 64, 443–449. [Google Scholar] [CrossRef] [PubMed]
- Johnson, E.O.; Roth, T.; Breslau, N. The association of insomnia with anxiety disorders and depression: Exploration of the direction of risk. J. Psychiatr. Res. 2006, 40, 700–708. [Google Scholar] [CrossRef] [PubMed]
- Banks, S.; Dinges, D.F. Behavioral and physiological consequences of sleep restriction. J. Clin. Sleep Med. 2007, 3, 519–528. [Google Scholar] [PubMed]
- Choueiry, N.; Salamoun, T.; Jabbour, H.; El Osta, N.; Hajj, A.; Rabbaa Khabbaz, L. Insomnia and relationship with anxiety in university students: A cross-sectional designed study. PLoS ONE 2016, 11, e0149643. [Google Scholar] [CrossRef]
- Kurita, G.P.; Ekholm, O.; Kaasa, S.; Klepstad, P.; Skorpen, F.; Sjogren, P. Genetic variation and cognitive dysfunction in opioid-treated patients with cancer. Brain Behav. 2016, 6, e00471. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kurita, G.P.; Sjogren, P.; Ekholm, O.; Kaasa, S.; Loge, J.H.; Poviloniene, I.; Klepstad, P. Prevalence and predictors of cognitive dysfunction in opioid-treated patients with cancer: A multinational study. J. Clin. Oncol. 2011, 29, 1297–1303. [Google Scholar] [CrossRef] [PubMed]
- Bilkei-Gorzo, A.; Mauer, D.; Michel, K.; Zimmer, A. Dynorphins regulate the strength of social memory. Neuropharmacology 2014, 77, 406–413. [Google Scholar] [CrossRef] [PubMed]
- Negrete, R.; Garcia Gutierrez, M.S.; Manzanares, J.; Maldonado, R. Involvement of the dynorphin/KOR system on the nociceptive, emotional and cognitive manifestations of joint pain in mice. Neuropharmacology 2016, 24, 315–327. [Google Scholar] [CrossRef] [PubMed]
- Carey, A.N.; Lyons, A.M.; Shay, C.F.; Dunton, O.; McLaughlin, J.P. Endogenous kappa opioid activation mediates stress-induced deficits in learning and memory. J. Neurosci. 2009, 29, 4293–4300. [Google Scholar] [CrossRef] [PubMed]
- Laugsand, E.A.; Fladvad, T.; Skorpen, F.; Maltoni, M.; Kaasa, S.; Fayers, P.; Klepstad, P. Clinical and genetic factors associated with nausea and vomiting in cancer patients receiving opioids. Eur. J. Cancer 2011, 47, 1682–1691. [Google Scholar] [CrossRef] [PubMed]
- De Gregori, M.; Garbin, G.; de Gregori, S.; Minella, C.E.; Bugada, D.; Lisa, A.; Govoni, S.; Regazzi, M.; Allegri, M.; Ranzani, G.N. Genetic variability at COMT but not at OPRM1 and UGT2b7 loci modulates morphine analgesic response in acute postoperative pain. Eur. J. Clin. Pharmacol. 2013, 69, 1651–1658. [Google Scholar] [CrossRef] [PubMed]
- Henker, R.A.; Lewis, A.; Dai, F.; Lariviere, W.R.; Meng, L.; Gruen, G.S.; Sereika, S.M.; Pape, H.; Tarkin, I.S.; Gowda, I.; et al. The associations between OPRM 1 and COMT genotypes and postoperative pain, opioid use, and opioid-induced sedation. Biol. Res. Nurs. 2013, 15, 309–317. [Google Scholar] [CrossRef] [PubMed]
- Kolesnikov, Y.; Gabovits, B.; Levin, A.; Veske, A.; Qin, L.; Dai, F.; Belfer, I. Chronic pain after lower abdominal surgery: Do catechol-O-methyl transferase/opioid receptor μ-1 polymorphisms contribute? Mol. Pain 2013, 9, 19. [Google Scholar] [CrossRef] [PubMed]
- Facit Measurement System. Available online: http://www.facit.org/facitorg/overview/relatedliterature. (accessed on 18 January 2017).
- Eortc Quality of Life. Available online: http://groups.eortc.be/qol/why-do-we-need-modules (accessed on 18 January 2017).
- Center, E.D. EORTC QLQ-C30 Scoring Manual; Publisher: Brussels, Belgium, 2011. [Google Scholar]
- World Health Organization, Cancer, Who’s Cancer Pain Ladder for Adults. Available online: http://www.who.int/cancer/palliative/painladder/en/ (accessed on 26 Januray 2016).
- Grosch, S.; Niederberger, E.; Lotsch, J.; Skarke, C.; Geisslinger, G. A rapid screening method for a single nucleotide polymorphism (SNP) in the human MOR gene. Br. J. Clin. Pharmacol. 2001, 52, 711–714. [Google Scholar] [CrossRef] [PubMed]
- Nauck, M.; Stein, U.; von Karger, S.; Marz, W.; Wieland, H. Rapid detection of the C3435T polymorphism of multidrug resistance gene 1 using fluorogenic hybridization probes. Clin. Chem. 2000, 46, 1995–1997. [Google Scholar] [PubMed]
- Primer3. Available online: http://fokker.wi.mit.edu/primer3/input.htm (accessed on 22 February 2011).
- Tabachnick, B.; Fidell, L. Using Multivariate Statistics, 4th ed.; Allyn and Bacon: Needham Heights, MA, USA, 2001. [Google Scholar]

| Characteristics of the Subjects (n = 89) | n (%) |
|---|---|
| Men | 46 (51.7) |
| Women | 43 (48.3) |
| Ethnicity | |
| Lebanese | 84 (94.4) |
| Non-Lebanese 1 | 5 (5.6) |
| Diagnosis | |
| Gastrointestinal tract | 20 (22.5) |
| Breast | 16 (18.0) |
| Lung | 15 (16.9) |
| Hematologic | 9 (10.1) |
| Urogenital | 6 (6.7) |
| Gynecologic | 6 (6.7) |
| Prostate | 4 (4.5) |
| Pancreas | 4 (4.5) |
| Head and neck | 2 (2.25) |
| Sarcoma | 2 (2.25) |
| Others | 5 (5.6) |
| Metastases | 62 (69.7) |
| Concomitant use of opioids 2 | 6 (6.7) |
| Concomitant use of other drugs for neuropathic pain 3 | 64 (71.9) |
| Variables | Mean ± SD |
| Age (years) | 56.94 ± 13.88 |
| Weight (kg) | 64.41 ± 14.26 |
| Creatinine clearance (mL/min) | 121.01 ± 46.68 |
| Visual analog scale (VAS) score at rest at 24 h | 3.4 ± 2.93 |
| Dose of morphine at 24 h (mean ± SD—mg) Pump infusion | 34.78 ± 33.26 |
| Duration of morphine treatment (mean ± SD—days) | 121.96 ± 383.87 |
| Duration since cancer diagnosis (mean ± SD—months) | 37.08 ± 50.65 |
| Gene dbSNP | Genotype Frequencies 1 | Allelic Frequencies | p 2 | |||
|---|---|---|---|---|---|---|
| OPRM1 rs1799971 | AA | AG | GG | A | G | |
| Lebanese patients n = 84 3 (Current study, cancer) | 67 (79.8) | 17 (20.2) | 0 (0.0) | 0.90 | 0.10 | – |
| Lebanese patients n = 96 [15] | 76 (79.2) | 18 (18.8) | 2 (2.1) | 0.89 | 0.11 | 0.41 |
| European HapMap n = 113 | 80 (70.8) | 31 (27.4) | 2 (1.8) | 0.84 | 0.16 | 0.22 |
| Japanese HapMap n = 86 | 29 (33.7) | 34 (39.5) | 23 (26.7) | 0.53 | 0.47 | <0.0001 * |
| Chinese HapMap n = 43 | 18 (41.9) | 19 (44.2) | 6 (14) | 0.64 | 0.36 | <0.0001 * |
| Sub-Saharan African HapMap n = 60 | 60 (100) | 0 (0) | 0 (0) | 1 | 0 | 0.001 * |
| COMT rs4680 | Val/Val | Val/Met | Met/Met | Val | Met | |
| Lebanese patients n = 84 3 (Current study, cancer) | 22 (26.2) | 42 (50) | 20 (23.8) | 0.51 | 0.49 | – |
| Lebanese patients n = 96 [15] | 23 (24) | 48 (50) | 25 (26) | 0.49 | 0.51 | 0.92 |
| European HapMap n = 113 | 28 (24.8) | 52 (46) | 33 (29.2) | 0.48 | 0.52 | 0.67 |
| Japanese HapMap n = 86 | 6 (7) | 37 (43) | 43 (50) | 0.28 | 0.72 | <0.0001 * |
| Chinese HapMap n = 43 | 3 (7) | 14 (32.5) | 26 (60.5) | 0.23 | 0.77 | 0.0001 * |
| Sub-Saharan African HapMap n = 113 | 10 (8.8) | 51 (45.2) | 52 (46) | 0.31 | 0.69 | 0.0004 * |
| ABCB1 rs1045642 | CC | CT | TT | C | T | |
| Lebanese patients n = 84 3 (Current study, cancer) | 29 (34.5) | 32 (38.1) | 23 (27.4) | 0.54 | 0.46 | – |
| Lebanese patients n = 96 [15] | 34 (35.4) | 38 (39.6) | 24 (25) | 0.55 | 0.44 | 0.94 |
| European HapMap n = 113 | 17 (15) | 63 (55.8) | 33 (29.2) | 0.43 | 0.57 | 0.0041 * |
| Japanese HapMap n = 86 | 22 (25.6) | 49 (57) | 15 (17.4) | 0.54 | 0.46 | 0.045 * |
| Chinese HapMap n = 42 | 16 (38.1) | 17 (40.5) | 9 (21.4) | 0.58 | 0.42 | 0.76 |
| Sub-Saharan African HapMap n = 113 | 89 (78.8) | 23 (20.4) | 1 (0.8) | 0.89 | 0.11 | <0.0001 * |
| Characteristics of the Subjects | n | Dose of Morphine at 24 h (Mean—mg) | Standard Deviation | p |
|---|---|---|---|---|
| OPRM1 c.118A>G | ||||
| AA | 69 | 29.97 | 26.96 | 0.010 2 |
| AG 1 | 20 | 51.37 | 46.35 | |
| COMT p.Val158Met | ||||
| Val/Val | 23 | 34.85 | 26.96 | 0.307 |
| Val/Met | 43 | 30.52 | 33.06 | |
| Met/Met | 23 | 42.68 | 38.87 | |
| Val/Val | 23 | 34.85 | 26.96 | 0.701 |
| ValMet/MetMet | 66 | 34.76 | 35.38 | |
| ABCB1 c.3435C>T | ||||
| CC | 29 | 32.87 | 22.75 | 0.857 |
| CT | 35 | 41.38 | 46.41 | |
| CC | 25 | 27.76 | 16.75 | |
| CC | 29 | 32.87 | 22.75 | 0.750 |
| CT/TT | 60 | 35.71 | 37.44 | |
| Metastasis | ||||
| Yes | 62 | 32.54 | 28.92 | 0.476 |
| No | 27 | 39.94 | 41.73 | |
| Concomitant use of opioids 3 | ||||
| Yes | 6 | 22.50 | 8.22 | 0.015 |
| No | 83 | 35.67 | 34.22 | |
| Concomitant use of other drugs 4 | ||||
| Yes | 64 | 39.63 | 37.37 | 0.002 |
| No | 25 | 22.36 | 12.94 |
| Doses de Morphine | Unstandardized Coefficients | Standardized Coefficients | t 4 | Sig. 5 | 95% Confidence Interval (CI) for B | ||
|---|---|---|---|---|---|---|---|
| B 1 | S.E. 2 | β 3 | Lower Bound | Upper Bound | |||
| Age 6 | −0.342 | 0.167 | −0.143 | −2.053 | 0.043 | −0.675 | −0.010 |
| Treatment duration | 0.061 | 0.006 | 0.700 | 10.112 | <0.001 | 0.049 | 0.073 |
| OPRM1 c.118A>G | 12.838 | 5.756 | 0.162 | 2.230 | 0.029 | 1.381 | 24.295 |
| Concomitant use of other drugs | −10.525 | 6.567 | −0.119 | −1.603 | 0.113 | −23.596 | 2.547 |
| Concomitant opioids use | 2.440 | 9.096 | 0.018 | 0.268 | 0.789 | −15.665 | 20.545 |
| Items of the QOL 1 | n | Mean | Std. Deviation | Univariate Analyses | p 2 | Multivariate Analyses | Std. Error 4 | Standardized Coefficients | 95% CI for B 6 | Upper Bound | p 7 |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Unstandardized Coefficients B 3 | β 5 | Lower Bound | |||||||||
| Physical functioning (PF) | 89 | 27.42 | 32.67 | Metastases 8 | 0.048 | - | - | - | - | - | - |
| Role functioning (RF) | 89 | 21.91 | 37.66 | ABCB1 SNP | 0.159 | −9.185 | 8.898 | −0.115 | −26.883 | 8.512 | 0.305 |
| OPRM1 SNP | 0.119 | 10.335 | 9.676 | 0.115 | −8.910 | 29.579 | 0.289 | ||||
| Metastases | 0.059 | −8.745 | 7.888 | −0.117 | 0.126 | 35.609 | 0.048 | ||||
| Gender | 0.189 | 17.867 | 8.92 | 0.219 | -0.513 | 0.709 | 0.751 | ||||
| Social functioning (SF) | 89 | 52.62 | 38.43 | OPRM1 SNP | 0.097 | 15.961 | 9.444 | 0.174 | −2.812 | 34.734 | 0.095 |
| Age | 0.034 | 0.646 | 0.286 | 0.233 | 0.078 | 1.213 | 0.026 | ||||
| Emotional functioning (EF) | 89 | 44.8 | 34.36 | ABCB1 SNP | 0.191 | −9.805 | 7.741 | −0.123 | −25.193 | 5.583 | 0.209 |
| Gender | 0.18 | −7.695 | 7.26 | −0.113 | −22.128 | 6.738 | 0.292 | ||||
| Cognitive functioning (CF) | 89 | 65.73 | 32.31 | OPRM1 SNP | 0.014 | 19.448 | 7.985 | 0.253 | 3.571 | 35.325 | 0.017 |
| COMT SNP | 0.195 | −9.019 | 7.706 | −0.123 | −24.340 | 6.303 | 0.245 | ||||
| Metastases | 0.209 | 8.109 | 7.343 | 0.116 | −6.491 | 22.709 | 0.273 | ||||
| Fatigue (FA) | 89 | 74.28 | 26.04 | COMT SNP | 0.156 | −10.142 | 6.197 | −0.171 | −22.461 | 2.176 | 0.105 |
| Gender | 0.055 | 10.624 | 5.429 | 0.205 | -0.167 | 21.416 | 0.054 | ||||
| Pain (PA) (Normalized Pain Scores) | 89 | 70.32 | 32.86 | Age | 0.189 | −0.286 | 0.259 | −0.121 | −0.801 | 0.23 | 0.273 |
| Gender | 0.189 | 8.85 | 6.888 | 0.135 | −4.845 | 22.545 | 0.202 | ||||
| Metastases | 0.104 | 8.824 | 7.781 | 0.124 | −6.647 | 24.295 | 0.26 | ||||
| Nausea and vomiting (NV) | 89 | 35.11 | 38.89 | OPRM1 SNP | 0.087 | −9.604 | 9.964 | −0.104 | −29.419 | 10.21 | 0.338 |
| Gender | 0.038 | 19.578 | 7.969 | 0.253 | 3.731 | 35.426 | 0.016 | ||||
| Morphine dose | 0.132 | −0.177 | 0.125 | −0.151 | −0.425 | 0.071 | 0.16 | ||||
| VAS scores 9 | 1.481 | 1.37 | 0.111 | −1.242 | 4.205 | 0.282 | |||||
| Global health status/QoL (GQOL) | 89 | 39.05 | 28.58 | COMT SNP | 0.193 | −8.297 | 6.75 | −0.128 | −21.715 | 5.122 | 0.222 |
| Metastases | 0.007 | 16.185 | 6.428 | 0.262 | 3.406 | 28.963 | 0.014 | ||||
| Dyspnea (DY) | 89 | 32.4 | 33.93 | - | |||||||
| Insomnia (SL) | 89 | 48.69 | 42.66 | ABCB1 SNP | 0.098 | −11.636 | 9.66 | −0.129 | −30.842 | 7.571 | 0.232 |
| Metastases | 0.095 | 22.028 | 9.898 | 0.239 | 2.348 | 41.708 | 0.029 | ||||
| Age | 0.216 | 0.604 | 0.341 | 0.196 | −0.075 | 1.282 | 0.08 | ||||
| Appetite loss (AP) | 89 | 68.91 | 39.5 | - | |||||||
| Constipation (CO) | 89 | 60.67 | 42.51 | - | |||||||
| Diarrhea (DI) | 89 | 12.73 | 26.83 | - | |||||||
| Financial difficulties (FI) | 89 | 35.21 | 33.84 | ABCB1 SNP | 0.234 | 3.887 | 7.628 | 0.054 | −11.277 | 19.051 | 0.612 |
| Age | 0.004 | −0.699 | 0.259 | −0.287 | −1.214 | -0.184 | 0.008 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hajj, A.; Halepian, L.; Osta, N.E.; Chahine, G.; Kattan, J.; Rabbaa Khabbaz, L. OPRM1 c.118A>G Polymorphism and Duration of Morphine Treatment Associated with Morphine Doses and Quality-of-Life in Palliative Cancer Pain Settings. Int. J. Mol. Sci. 2017, 18, 669. https://doi.org/10.3390/ijms18040669
Hajj A, Halepian L, Osta NE, Chahine G, Kattan J, Rabbaa Khabbaz L. OPRM1 c.118A>G Polymorphism and Duration of Morphine Treatment Associated with Morphine Doses and Quality-of-Life in Palliative Cancer Pain Settings. International Journal of Molecular Sciences. 2017; 18(4):669. https://doi.org/10.3390/ijms18040669
Chicago/Turabian StyleHajj, Aline, Lucine Halepian, Nada El Osta, Georges Chahine, Joseph Kattan, and Lydia Rabbaa Khabbaz. 2017. "OPRM1 c.118A>G Polymorphism and Duration of Morphine Treatment Associated with Morphine Doses and Quality-of-Life in Palliative Cancer Pain Settings" International Journal of Molecular Sciences 18, no. 4: 669. https://doi.org/10.3390/ijms18040669
APA StyleHajj, A., Halepian, L., Osta, N. E., Chahine, G., Kattan, J., & Rabbaa Khabbaz, L. (2017). OPRM1 c.118A>G Polymorphism and Duration of Morphine Treatment Associated with Morphine Doses and Quality-of-Life in Palliative Cancer Pain Settings. International Journal of Molecular Sciences, 18(4), 669. https://doi.org/10.3390/ijms18040669