(−)-β-Caryophyllene, a CB2 Receptor-Selective Phytocannabinoid, Suppresses Motor Paralysis and Neuroinflammation in a Murine Model of Multiple Sclerosis
Abstract
:1. Introduction
2. Results and Discussion
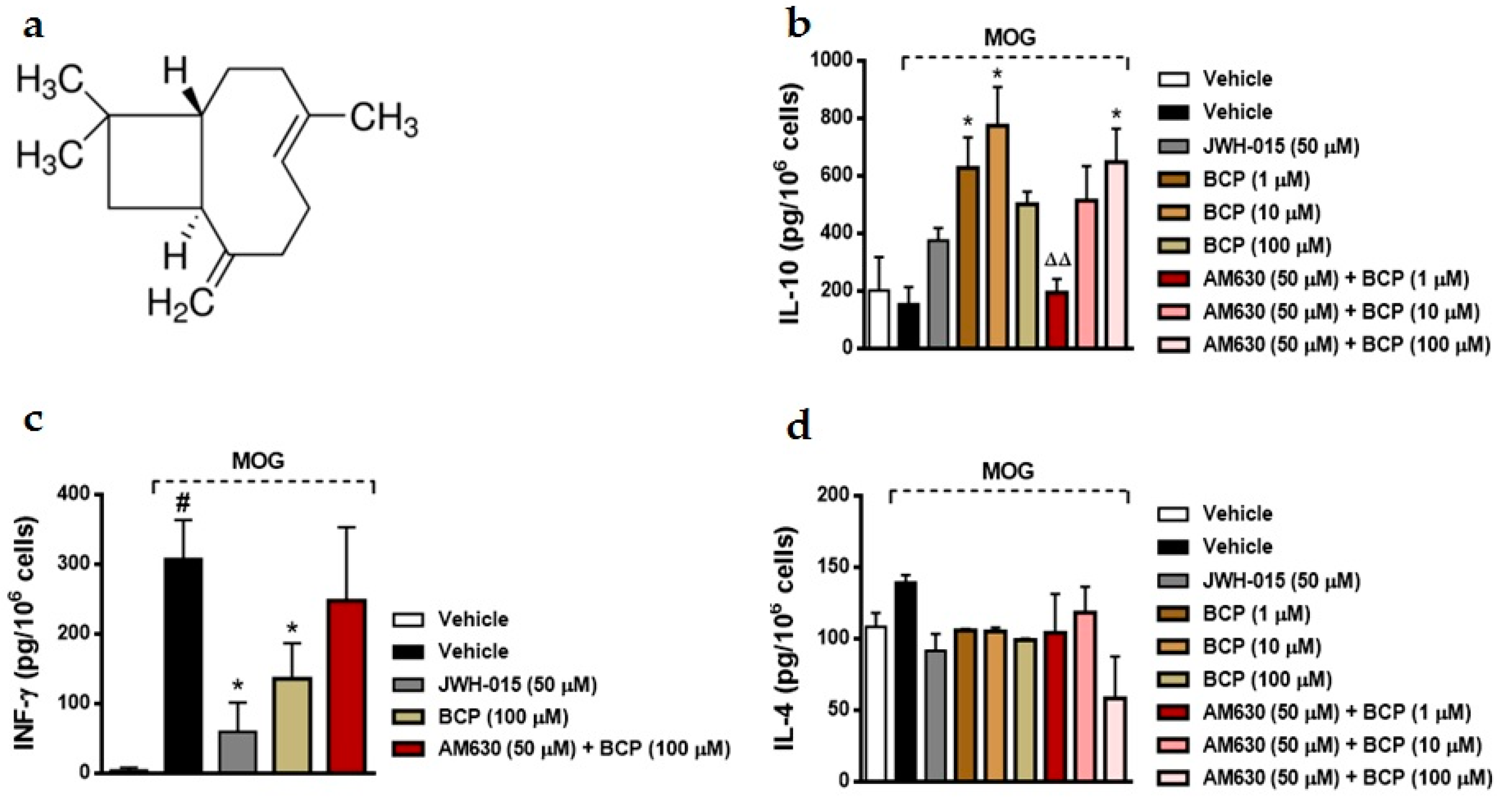
2.1. BCP Anti-Inflammatory Effects Are Mediated by Modulation of CB2 Receptor in MOG-Primed T Cells
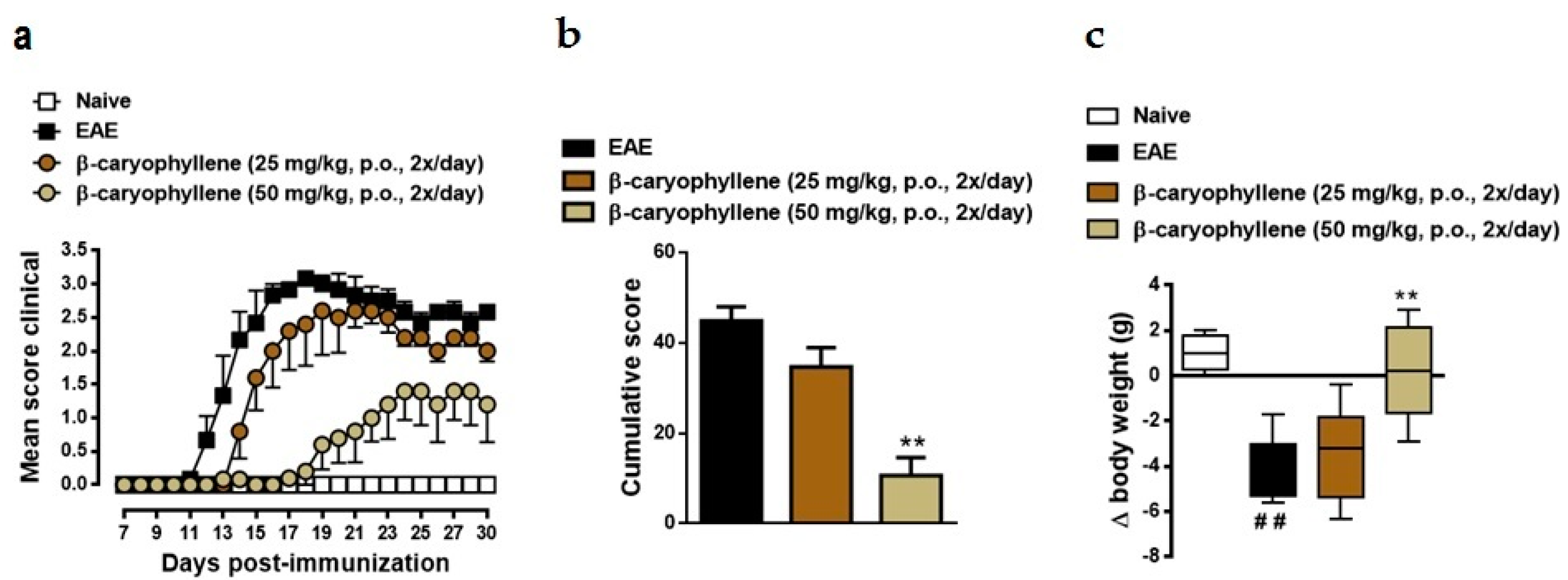
2.2. BCP Attenuates Disease Progression in Chronic EAE Mice Model
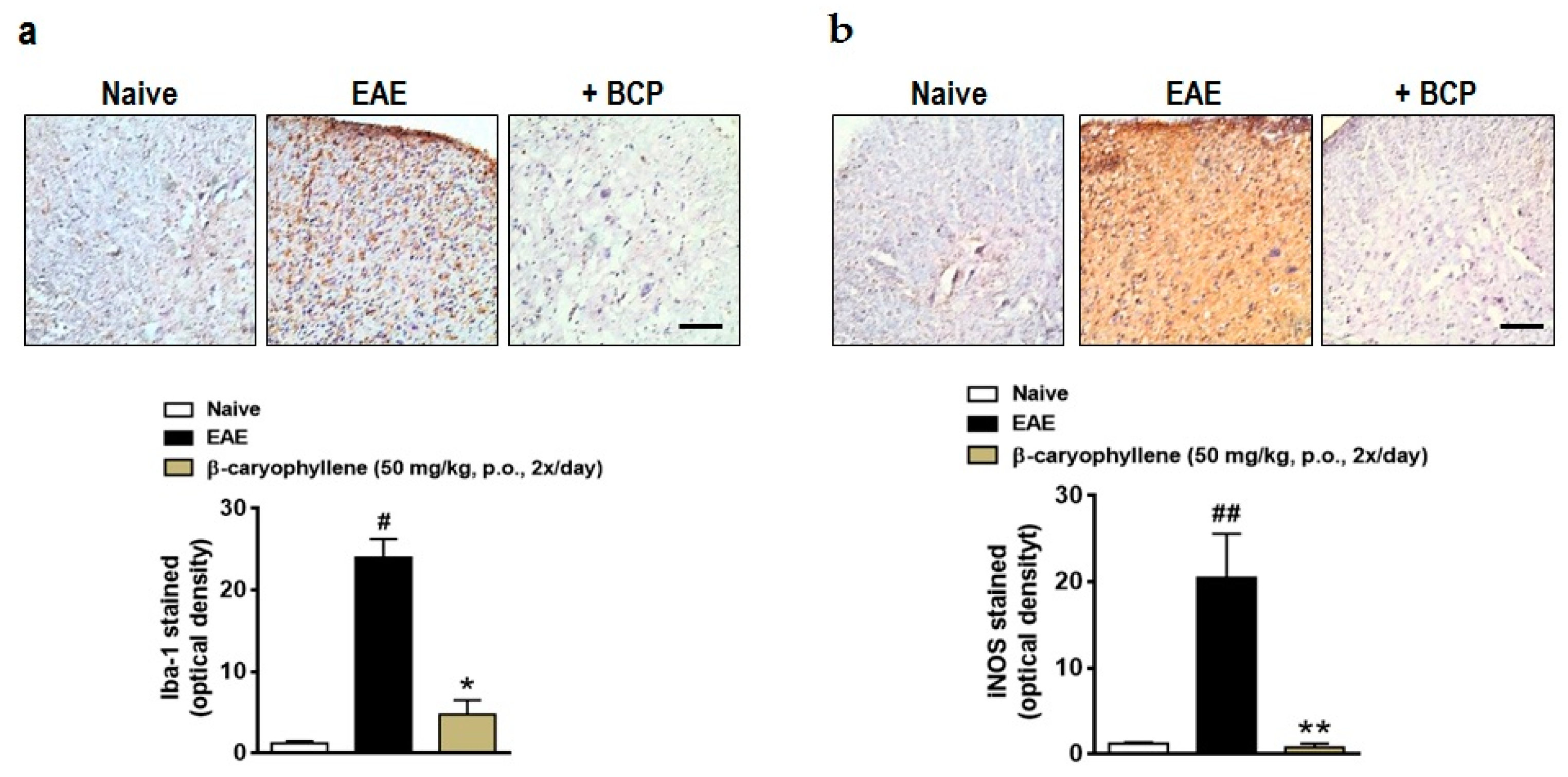
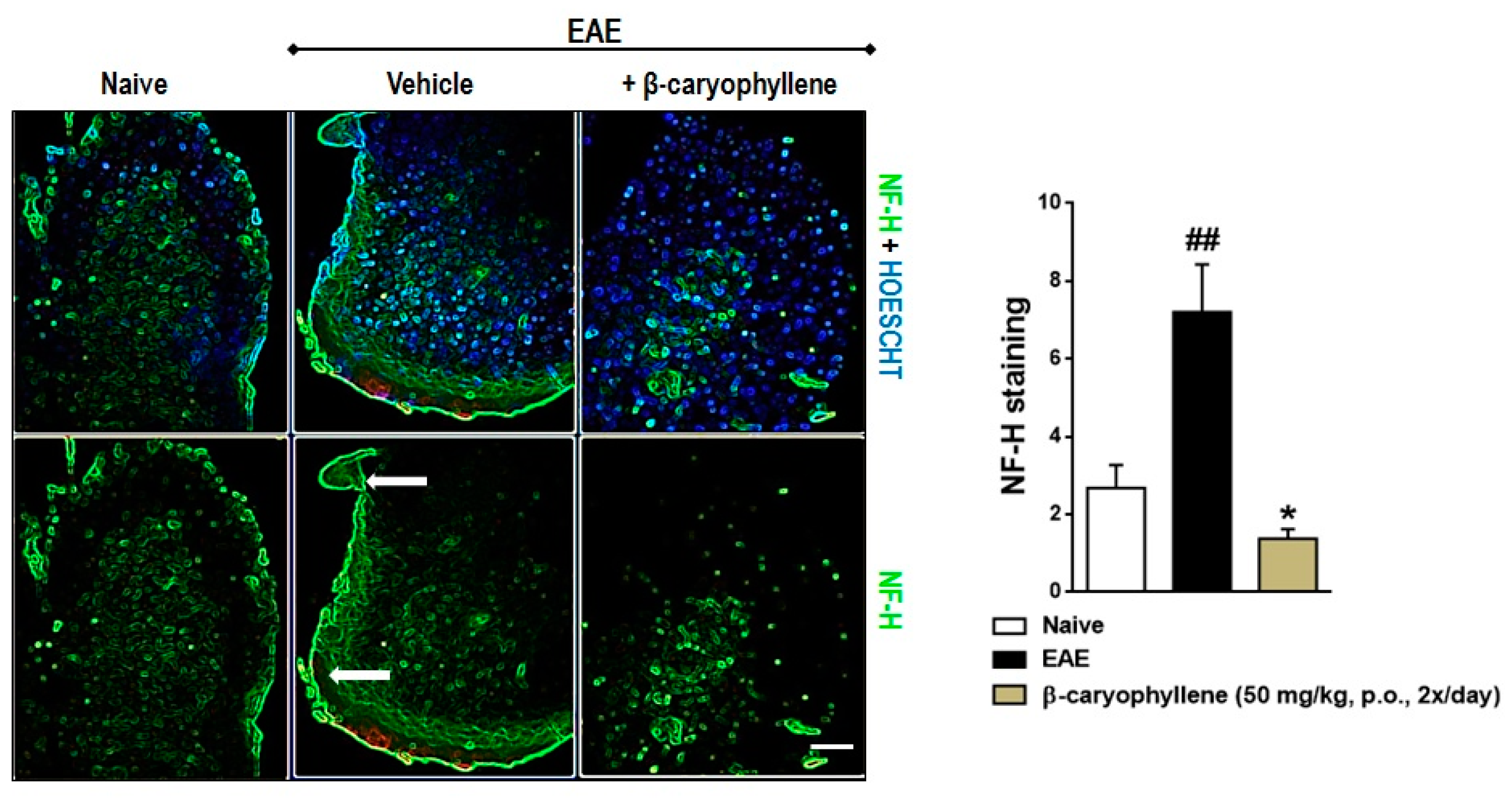
2.3. BCP Inhibits Glial Activation, Oxidative Damage, and Demyelination during EAE Development
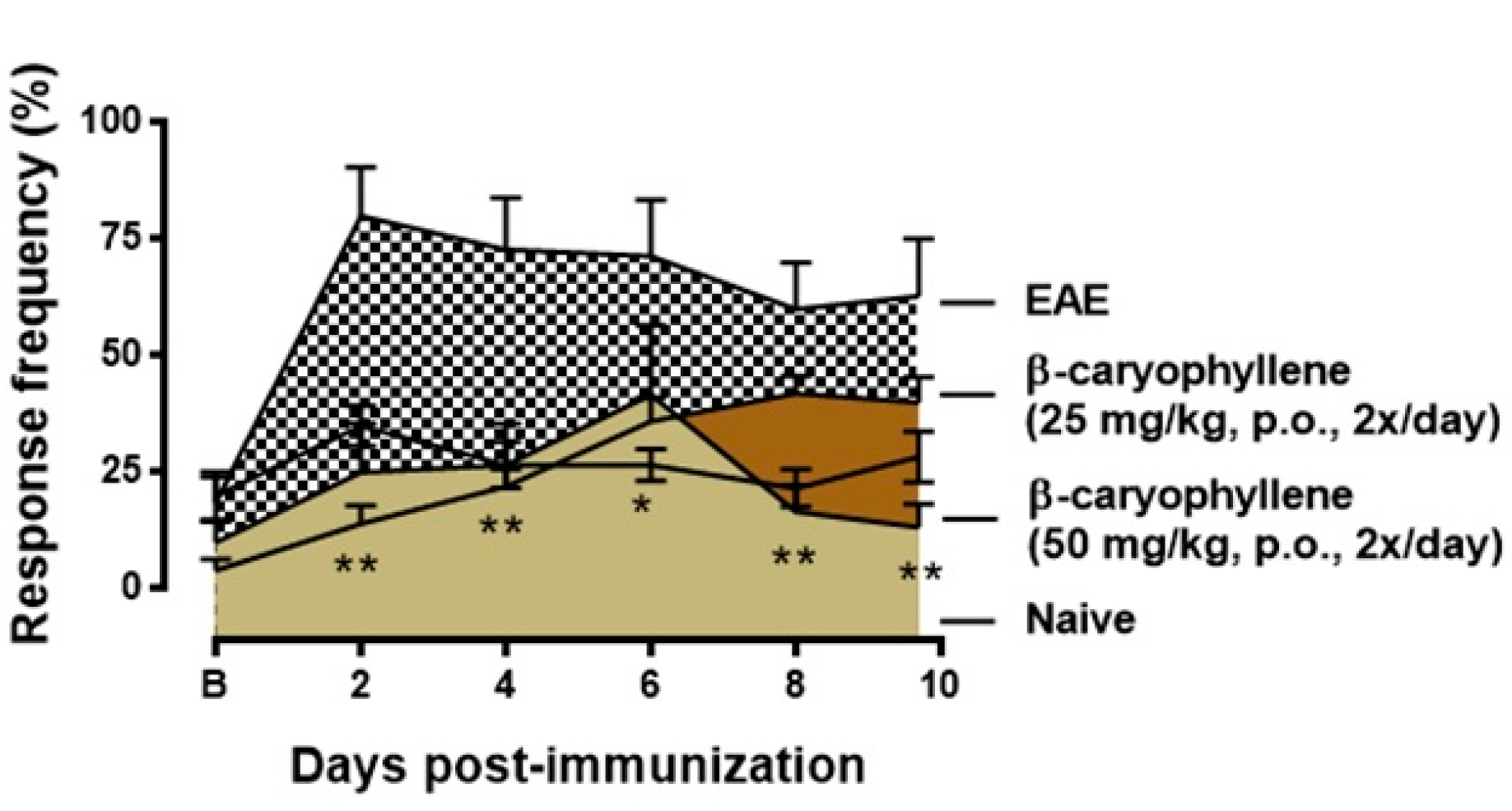
2.4. BCP Therapeutic Treatment Inhibits Progression the Clinical Signs of EAE
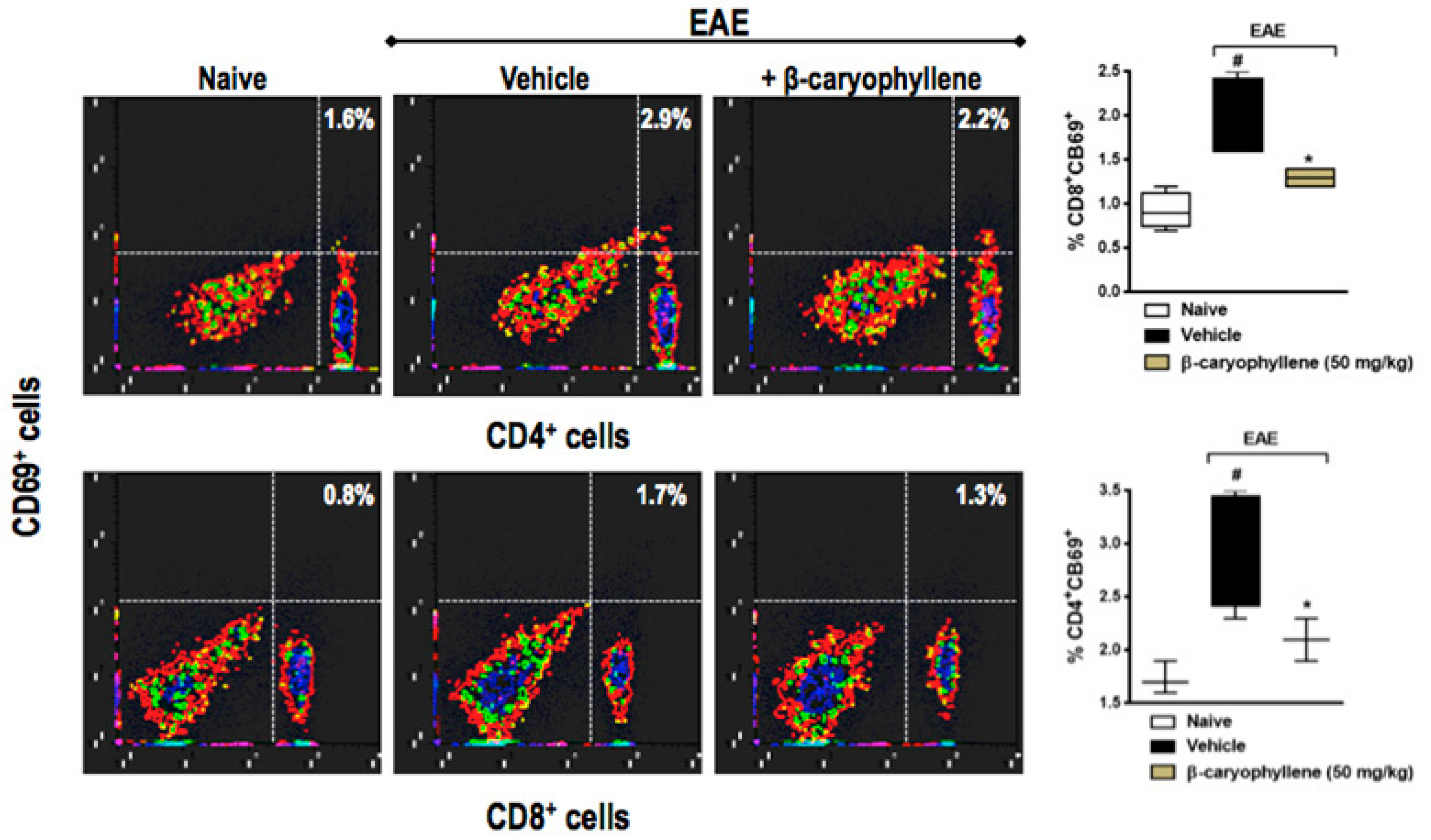
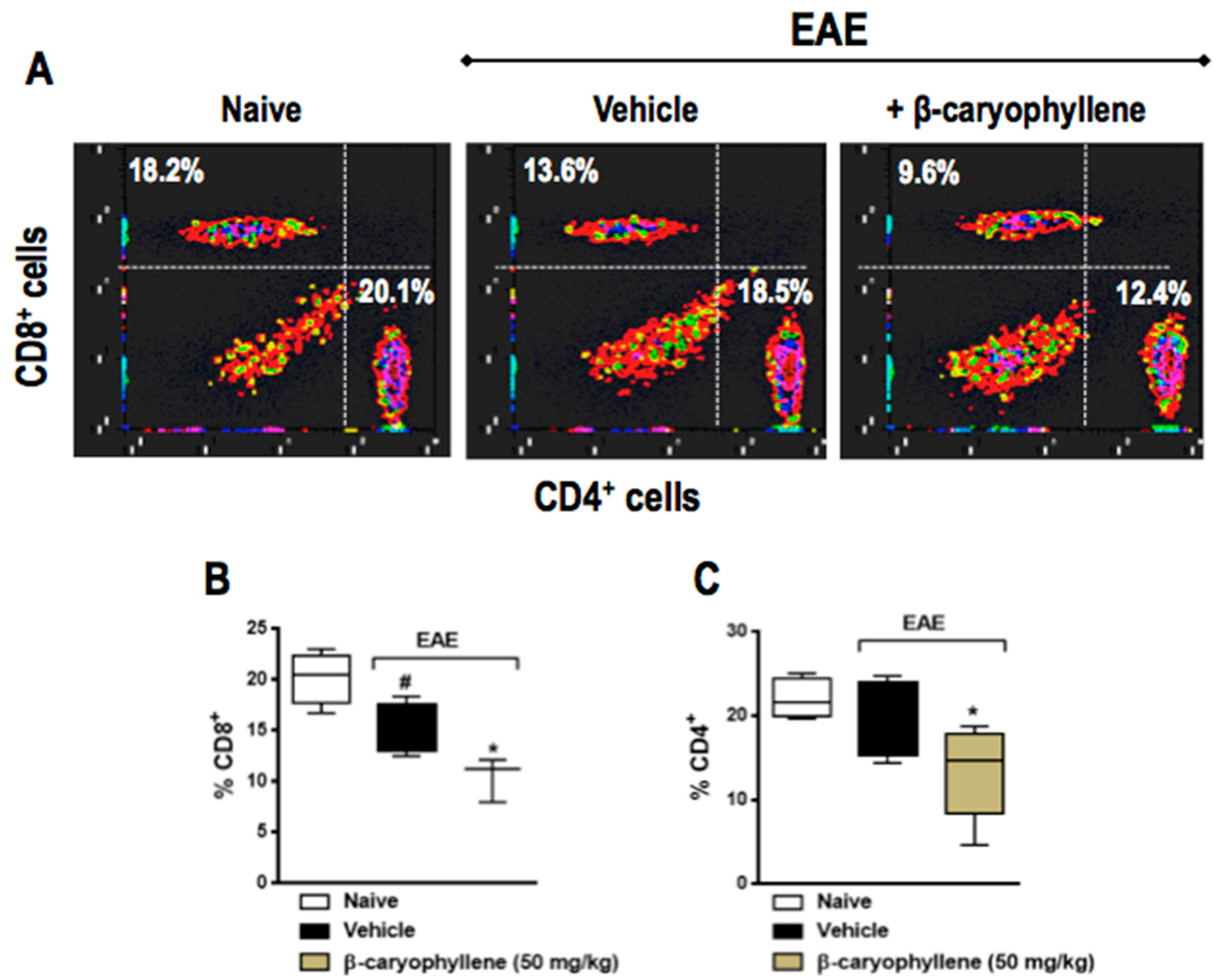
2.5. BCP Prophylactic Treatment Downregulated CD4+ and CD8+ T Lymphocytes
3. Materials and Methods
3.1. Chemicals and Reagents
3.2. Animals
3.3. Active EAE Induction in C57BL/6 Mice
3.4. Clinical Evaluation
3.5. Treatment Procedure
3.6. Isolation of Lymphocytes
3.7. Flow Cytometry Assay
3.8. Histology
3.9. Mechanical Hyperalgesia
3.10. Statistical Analysis
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Wagner, C.A.; Goverman, J.M. Novel insights and therapeutics in multiple sclerosis. F1000Res 2015, 4, 517. [Google Scholar] [CrossRef] [PubMed]
- Iglesias, A.; Bauer, J.; Litzenburger, T.; Schubart, A.; Linington, C. T- and B-cell responses to myelin oligodendrocyte glycoprotein in experimental autoimmune encephalomyelitis and multiple sclerosis. Glia 2001, 36, 220–234. [Google Scholar] [CrossRef] [PubMed]
- Cannella, B.; Raine, C.S. The adhesion molecule and cytokine profile of multiple sclerosis lesions. Ann. Neurol. 1995, 37, 424–435. [Google Scholar] [CrossRef] [PubMed]
- Muller, D.M.; Pender, M.P.; Greer, J.M. Blood–brain barrier disruption and lesion localisation in experimental autoimmune encephalomyelitis with predominant cerebellar and brainstem involvement. J. Neuroimmunol. 2005, 160, 162–169. [Google Scholar] [CrossRef] [PubMed]
- Steinman, L. Assessment of animal models for MS and demyelinating disease in the design of rational therapy. Neuron 1999, 24, 511–514. [Google Scholar] [CrossRef]
- Liblau, R.S.; Singer, S.M.; McDevitt, H.O. Th1 and Th2 CD4+ T cells in the pathogenesis of organ-specific autoimmune diseases. Immunol. Today 1995, 16, 34–38. [Google Scholar] [CrossRef]
- Harrington, L.E.; Hatton, R.D.; Mangan, P.R.; Turner, H.; Murphy, T.L.; Murphy, K.M.; Weaver, C.T. Interleukin 17-producing CD4+ effector T cells develop via a lineage distinct from the T helper type 1 and 2 lineages. Nat. Immunol. 2005, 6, 1123–1132. [Google Scholar] [CrossRef] [PubMed]
- Park, H.; Li, Z.; Yang, X.O.; Chang, S.H.; Nurieva, R.; Wang, Y.H.; Wang, Y.; Hood, L.; Zhu, Z.; Tian, Q.; et al. A distinct lineage of CD4 T cells regulates tissue inflammation by producing interleukin 17. Nat. Immunol. 2005, 6, 1133–1141. [Google Scholar] [CrossRef] [PubMed]
- Gajofatto, A.; Bacchetti, P.; Grimes, B.; High, A.; Waubant, E. Switching first-line disease-modifying therapy after failure: Impact on the course of relapsing-remitting multiple sclerosis. Mult. Scler. 2009, 15, 50–58. [Google Scholar] [CrossRef] [PubMed]
- Bermel, R.A.; You, X.; Foulds, P.; Hyde, R.; Simon, J.H.; Fisher, E.; Rudick, R.A. Predictors of long-term outcome in multiple sclerosis patients treated with interferon β. Ann. Neurol. 2013, 73, 95–103. [Google Scholar] [CrossRef] [PubMed]
- Compston, A.; Coles, A. Multiple sclerosis. Lancet 2008, 372, 1502–1517. [Google Scholar] [CrossRef]
- Sharma, C.; Al Kaabi, J.M.; Nurulain, S.M.; Goyal, S.N.; Kamal, M.A.; Ojha, S. Polypharmacological properties and therapeutic potential of β-caryophyllene: A dietary phytocannabinoid of pharmaceutical promise. Curr. Pharm. Des. 2016, 22, 3237–3264. [Google Scholar] [PubMed]
- Bahi, A.; Al Mansouri, S.; Al Memari, E.; Al Ameri, M.; Nurulain, S.M.; Ojha, S. β-Caryophyllene, a CB2 receptor agonist produces multiple behavioral changes relevant to anxiety and depression in mice. Physiol. Behav. 2014, 135, 119–124. [Google Scholar] [CrossRef] [PubMed]
- Gertsch, J.; Leonti, M.; Raduner, S.; Racz, I.; Chen, J.Z.; Xie, X.Q.; Altmann, K.H.; Karsak, M.; Zimmer, A. β-caryophyllene is a dietary cannabinoid. Proc. Natl. Acad. Sci. USA 2008, 105, 9099–9104. [Google Scholar] [CrossRef] [PubMed]
- Andrade-Silva, M.; Correa, L.B.; Candea, A.L.; Cavalher-Machado, S.C.; Barbosa, H.S.; Rosas, E.C.; Henriques, M.G. The cannabinoid 2 receptor agonist β-caryophyllene modulates the inflammatory reaction induced by Mycobacterium bovis BCG by inhibiting neutrophil migration. Inflamm. Res. 2016, 65, 869–879. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.J.; Guo, S.S.; You, C.X.; Geng, Z.F.; Liang, J.Y.; Deng, Z.W.; Wang, C.F.; Du, S.S.; Wang, Y.Y. Chemical composition of essential oils from zanthoxylum bungeanum maxim. and their bioactivities against Lasioderma serricorne. J. Oleo Sci. 2016, 65, 871–879. [Google Scholar] [CrossRef] [PubMed]
- Neves, R.C.; Camara, C.A. Chemical composition and acaricidal activity of the essential oils from Vitex agnus-castus L. (Verbenaceae) and selected monoterpenes. An. Acad. Bras. Cienc. 2016, 88, 1221–1233. [Google Scholar] [CrossRef] [PubMed]
- McNeil, M.; Facey, P.; Porter, R. Essential oils from the Hyptis genus—A review (1909–2009). Nat. Prod. Commun. 2011, 6, 1775–1796. [Google Scholar] [PubMed]
- Maggio, A.; Riccobono, L.; Spadaro, V.; Campisi, P.; Bruno, M.; Senatore, F. Volatile constituents of the aerial parts of Pulicaria sicula (L.) Moris growing wild in Sicily: Chemotaxonomic volatile markers of the genus Pulicaria Gaertn. Chem. Biodivers. 2015, 12, 781–799. [Google Scholar] [CrossRef] [PubMed]
- Ulukanli, Z.; Karaborklu, S.; Bozok, F.; Ates, B.; Erdogan, S.; Cenet, M.; Karaaslan, M.G. Chemical composition, antimicrobial, insecticidal, phytotoxic and antioxidant activities of Mediterranean Pinus brutia and Pinus pinea resin essential oils. Chin. J. Nat. Med. 2014, 12, 901–910. [Google Scholar] [CrossRef]
- Thang, T.D.; Dai, D.N.; Luong, N.X.; Ogunwande, I.A. Constituents of essential oils from the leaves, stem barks and resins of Canarium parvum Leen., and Canarium tramdenanum Dai et Yakovl. (Burseracea) grown in Vietnam. Nat. Prod. Res. 2014, 28, 461–466. [Google Scholar] [CrossRef] [PubMed]
- Veiga Junior, V.F.; Rosas, E.C.; Carvalho, M.V.; Henriques, M.G.; Pinto, A.C. Chemical composition and anti-inflammatory activity of copaiba oils from Copaifera cearensis Huber ex Ducke, Copaifera reticulata Ducke and Copaifera multijuga Hayne—A comparative study. J. Ethnopharmacol. 2007, 112, 248–254. [Google Scholar] [CrossRef] [PubMed]
- Messer, A.; McCormick, K.; Sunjaya; Hagedorn, H.H.; Tumbel, F.; Meinwald, J. Defensive role of tropical tree resins: Antitermitic sesquiterpenes from Southeast Asian Dipterocarpaceae. J. Chem. Ecol. 1990, 16, 3333–3352. [Google Scholar] [CrossRef] [PubMed]
- Geron, C.D.; Daly, R.W.; Arnts, R.R.; Guenther, A.B.; Mowry, F.L. Canopy level emissions of 2-methyl-3-buten-2-ol, monoterpenes, and sesquiterpenes from an experimental Pinus taeda plantation. Sci. Total Environ. 2016, 565, 730–741. [Google Scholar] [CrossRef] [PubMed]
- Sadraei, H.; Asghari, G.; Alipour, M. Anti-spasmodic assessment of hydroalcoholic extract and essential oil of aerial part of Pycnocycla caespitosa Boiss.&Hausskn on rat ileum contractions. Res. Pharm. Sci. 2016, 11, 33–42. [Google Scholar] [PubMed]
- Kamikubo, R.; Kai, K.; Tsuji-Naito, K.; Akagawa, M. β-Caryophyllene attenuates palmitate-induced lipid accumulation through AMPK signaling by activating CB2 receptor in human HepG2 hepatocytes. Mol. Nutr. Food Res. 2016, 60, 2228–2242. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Y.; Dong, Z.; Liu, S. β-Caryophyllene ameliorates the Alzheimer-like phenotype in APP/PS1 Mice through CB2 receptor activation and the PPARγ pathway. Pharmacology 2014, 94, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Guo, K.; Mou, X.; Huang, J.; Xiong, N.; Li, H. Trans-caryophyllene suppresses hypoxia-induced neuroinflammatory responses by inhibiting NF-κB activation in microglia. J. Mol. Neurosci. 2014, 54, 41–48. [Google Scholar] [CrossRef] [PubMed]
- Klauke, A.L.; Racz, I.; Pradier, B.; Markert, A.; Zimmer, A.M.; Gertsch, J.; Zimmer, A. The cannabinoid CB2 receptor-selective phytocannabinoid β-caryophyllene exerts analgesic effects in mouse models of inflammatory and neuropathic pain. Eur. Neuropsychopharmacol. 2014, 24, 608–620. [Google Scholar] [CrossRef] [PubMed]
- Choi, I.Y.; Ju, C.; Anthony Jalin, A.M.; Lee, D.I.; Prather, P.L.; Kim, W.K. Activation of cannabinoid CB2 receptor-mediated AMPK/CREB pathway reduces cerebral ischemic injury. Am. J. Pathol. 2013, 182, 928–939. [Google Scholar] [CrossRef] [PubMed]
- Horvath, B.; Mukhopadhyay, P.; Kechrid, M.; Patel, V.; Tanchian, G.; Wink, D.A.; Gertsch, J.; Pacher, P. β-Caryophyllene ameliorates cisplatin-induced nephrotoxicity in a cannabinoid 2 receptor-dependent manner. Free Radic. Biol. Med. 2012, 52, 1325–1333. [Google Scholar] [CrossRef] [PubMed]
- Bifulco, M.; Laezza, C.; Malfitano, A.M. Endocannabionoid system in neurological disorders. Recent Pat. CNS Drug Discov. 2015, 10, 90–112. [Google Scholar]
- Kaur, R.; Ambwani, S.R.; Singh, S. Endocannabinoid system: A multi-facet therapeutic target. Curr. Clin. Pharmacol. 2016, 11, 110–117. [Google Scholar] [CrossRef] [PubMed]
- Katchan, V.; David, P.; Shoenfeld, Y. Cannabinoids and autoimmune diseases: A systematic review. Autoimmun. Rev. 2016, 15, 513–528. [Google Scholar] [CrossRef] [PubMed]
- Haugh, O.; Penman, J.; Irving, A.J.; Campbell, V.A. The emerging role of the cannabinoid receptor family in peripheral and neuro-immune interactions. Curr. Drug Targets 2016, 17, 1834–1840. [Google Scholar] [CrossRef] [PubMed]
- Notcutt, W.G. Clinical use of cannabinoids for symptom control in multiple sclerosis. Neurotherapeutics 2015, 12, 769–777. [Google Scholar] [CrossRef] [PubMed]
- Kasper, L.H.; Reder, A.T. Immunomodulatory activity of interferon-β. Ann. Clin. Transl. Neurol. 2014, 1, 622–631. [Google Scholar] [CrossRef] [PubMed]
- Tanasescu, R.; Constantinescu, C.S. Cannabinoids and the immune system: An overview. Immunobiology 2010, 215, 588–597. [Google Scholar] [CrossRef] [PubMed]
- Pertwee, R.G. Cannabinoids and multiple sclerosis. Pharmacol. Ther. 2002, 95, 165–174. [Google Scholar] [CrossRef]
- Rog, D.J. Cannabis-based medicines in multiple sclerosis—A review of clinical studies. Immunobiology 2010, 215, 658–672. [Google Scholar] [CrossRef] [PubMed]
- Kavia, R.B.; de Ridder, D.; Constantinescu, C.S.; Stott, C.G.; Fowler, C.J. Randomized controlled trial of Sativex to treat detrusor overactivity in multiple sclerosis. Mult. Scler. 2010, 16, 1349–1359. [Google Scholar] [CrossRef] [PubMed]
- Pryce, G.; Ahmed, Z.; Hankey, D.J.; Jackson, S.J.; Croxford, J.L.; Pocock, J.M.; Ledent, C.; Petzold, A.; Thompson, A.J.; Giovannoni, G.; et al. Cannabinoids inhibit neurodegeneration in models of multiple sclerosis. Brain 2003, 126, 2191–2202. [Google Scholar] [CrossRef]
- Varga, Z.V.; Matyas, C.; Erdelyi, K.; Cinar, R.; Nieri, D.; Chicca, A.; Nemeth, B.T.; Paloczi, J.; Lajtos, T.; Corey, L.; et al. β-Caryophyllene protects against alcoholic steatohepatitis by attenuating inflammation and metabolic dysregulation in mice. Br. J. Pharmacol. 2017. [CrossRef] [PubMed]
- Quintans-Junior, L.J.; Araujo, A.A.; Brito, R.G.; Santos, P.L.; Quintans, J.S.; Menezes, P.P.; Serafini, M.R.; Silva, G.F.; Carvalho, F.M.; Brogden, N.K.; et al. β-Caryophyllene, a dietary cannabinoid, complexed with β-cyclodextrin produced anti-hyperalgesic effect involving the inhibition of Fos expression in superficial dorsal horn. Life Sci. 2016, 149, 34–41. [Google Scholar] [CrossRef] [PubMed]
- De Oliveira, C.C.; de Oliveira, C.V.; Grigoletto, J.; Ribeiro, L.R.; Funck, V.R.; Grauncke, A.C.; de Souza, T.L.; Souto, N.S.; Furian, A.F.; Menezes, I.R.; et al. Anticonvulsant activity of β-caryophyllene against pentylenetetrazol-induced seizures. Epilepsy Behav. 2016, 56, 26–31. [Google Scholar] [CrossRef] [PubMed]
- Bento, A.F.; Marcon, R.; Dutra, R.C.; Claudino, R.F.; Cola, M.; Leite, D.F.; Calixto, J.B. β-Caryophyllene inhibits dextran sulfate sodium-induced colitis in mice through CB2 receptor activation and PPARγ pathway. Am. J. Pathol. 2011, 178, 1153–1166. [Google Scholar] [CrossRef] [PubMed]
- Ehrhart, J.; Obregon, D.; Mori, T.; Hou, H.; Sun, N.; Bai, Y.; Klein, T.; Fernandez, F.; Tan, J.; Shytle, R.D. Stimulation of cannabinoid receptor 2 (CB2) suppresses microglial activation. J. Neuroinflamm. 2005, 2, 29. [Google Scholar] [CrossRef] [PubMed]
- Buckley, N.E.; McCoy, K.L.; Mezey, E.; Bonner, T.; Zimmer, A.; Felder, C.C.; Glass, M.; Zimmer, A. Immunomodulation by cannabinoids is absent in mice deficient for the cannabinoid CB2 receptor. Eur. J. Pharmacol. 2000, 396, 141–149. [Google Scholar] [CrossRef]
- Galiegue, S.; Mary, S.; Marchand, J.; Dussossoy, D.; Carriere, D.; Carayon, P.; Bouaboula, M.; Shire, D.; Le Fur, G.; Casellas, P. Expression of central and peripheral cannabinoid receptors in human immune tissues and leukocyte subpopulations. Eur. J. Biochem. 1995, 232, 54–61. [Google Scholar] [CrossRef] [PubMed]
- Bleul, C.C.; Fuhlbrigge, R.C.; Casasnovas, J.M.; Aiuti, A.; Springer, T.A. A highly efficacious lymphocyte chemoattractant, stromal cell-derived factor 1 (SDF-1). J. Exp. Med. 1996, 184, 1101–1109. [Google Scholar] [CrossRef] [PubMed]
- Dutra, R.C. Kinin receptors: Key regulators of autoimmunity. Autoimmun. Rev. 2017, 16, 192–207. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Song, Z.; Liao, D.; Zhang, T.; Liu, F.; Zhuang, K.; Luo, K.; Yang, L. Neuroprotective effects of trans-caryophyllene against kainic acid induced seizure activity and oxidative stress in mice. Neurochem. Res. 2015, 40, 118–123. [Google Scholar] [CrossRef] [PubMed]
- McCarthy, R.C.; Lu, D.Y.; Alkhateeb, A.; Gardeck, A.M.; Lee, C.H.; Wessling-Resnick, M. Characterization of a novel adult murine immortalized microglial cell line and its activation by amyloid-β. J. Neuroinflamm. 2016, 13, 21. [Google Scholar] [CrossRef] [PubMed]
- Assis, L.C.; Straliotto, M.R.; Engel, D.; Hort, M.A.; Dutra, R.C.; de Bem, A.F. β-Caryophyllene protects the C6 glioma cells against glutamate-induced excitotoxicity through the Nrf2 pathway. Neuroscience 2014, 279, 220–231. [Google Scholar] [CrossRef] [PubMed]
- Paszcuk, A.F.; Dutra, R.C.; da Silva, K.A.; Quintao, N.L.; Campos, M.M.; Calixto, J.B. Cannabinoid agonists inhibit neuropathic pain induced by brachial plexus avulsion in mice by affecting glial cells and MAP kinases. PLoS ONE 2011, 6, e24034. [Google Scholar] [CrossRef] [PubMed]

© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alberti, T.B.; Barbosa, W.L.R.; Vieira, J.L.F.; Raposo, N.R.B.; Dutra, R.C. (−)-β-Caryophyllene, a CB2 Receptor-Selective Phytocannabinoid, Suppresses Motor Paralysis and Neuroinflammation in a Murine Model of Multiple Sclerosis. Int. J. Mol. Sci. 2017, 18, 691. https://doi.org/10.3390/ijms18040691
Alberti TB, Barbosa WLR, Vieira JLF, Raposo NRB, Dutra RC. (−)-β-Caryophyllene, a CB2 Receptor-Selective Phytocannabinoid, Suppresses Motor Paralysis and Neuroinflammation in a Murine Model of Multiple Sclerosis. International Journal of Molecular Sciences. 2017; 18(4):691. https://doi.org/10.3390/ijms18040691
Chicago/Turabian StyleAlberti, Thaís Barbosa, Wagner Luiz Ramos Barbosa, José Luiz Fernandes Vieira, Nádia Rezende Barbosa Raposo, and Rafael Cypriano Dutra. 2017. "(−)-β-Caryophyllene, a CB2 Receptor-Selective Phytocannabinoid, Suppresses Motor Paralysis and Neuroinflammation in a Murine Model of Multiple Sclerosis" International Journal of Molecular Sciences 18, no. 4: 691. https://doi.org/10.3390/ijms18040691







