“Bligh and Dyer” and Folch Methods for Solid–Liquid–Liquid Extraction of Lipids from Microorganisms. Comprehension of Solvatation Mechanisms and towards Substitution with Alternative Solvents
Abstract
:1. Introduction
2. Results and Discussion
2.1. Bligh and Dyer: Principle
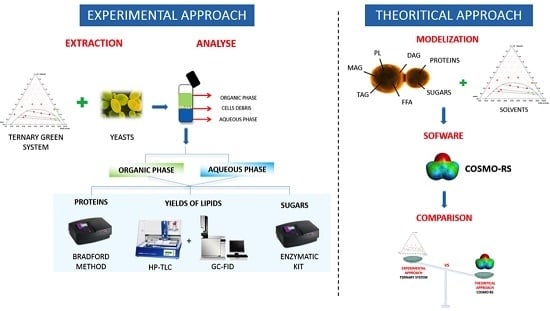
2.2. Strategy for Selection of Bio-Sourced Solvents: COSMO-RS Approach
2.3. Partition of Macro-Constituents from Y.L Yeast into Pure Solvents
2.4. Evaluation of Lipids Extraction from Y.L with B & D Solvent Pair versus the Alternative Solvent Pair
2.4.1. Evaluation of Total Lipid Contents
2.4.2. Lipid Classes of Y.L Yeast
2.4.3. Determination of Proteins and Sugars in Both Systems
2.5. COSMO-RS Calculations: Comparison of Experimental and Theoretical Approach
3. Material and Methods
3.1. Computational Method: Theoretical Prediction with COSMO-RS
3.2. Strain, Culture and Harvesting Conditions
3.3. Chemicals
3.4. Procedure for Construction of the Demixing Curve
3.5. Lipid Extractions: Application on Yeast
3.5.1. Bligh and Dyer Extractions: Classical and Alternative Ternary Systems
3.5.2. Classical Bligh and Dyer Procedure
3.6. Determination of Lipids, Proteins and Sugars
3.6.1. Qualitative and Quantitative Analysis of Total Lipids
Evaluation of Total Lipids Content in Tissue by Gravimetry
Analysis by Gas Chromatography Coupled with Flame Ionization Detector
Determination by High Performance Thin-Layer Chromatography
3.6.2. Quantitative Analysis of Proteins
3.6.3. Quantitative Analysis of Glucose
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
Abbreviations
| COSMO-RS | Conductor-like screening model for realistic solvation |
| HSP | Hansen solubility parameters |
| RED | Relative energy difference |
| HPTLC | High performance thin-layer chromatography |
| GC | Gas chromatography |
| FID | Flame ionization detector |
| CPME | Cyclopentyl methyl ether |
| MeTHF | 2-methyltetrahydrofuran |
| DMC | Dimethyl carbonate |
| IPA | Isopropanol |
| EtOAc | Ethyl acetate |
| TAGs | Triglycerides |
| DAGs | Diglycerides |
| MAGs | Monoglycerides |
| FFAs | Free fatty acids |
| PLs | Phospholipids |
| FAMEs | Fatty acid methyl esters |
References
- Meullemiestre, A.; Breil, C.; Vian, M.; Chemat, F. Modern Techniques and Solvents for the Extraction of Microbial oils; Springer Briefs in Molecular Science; Springer International Publishing AG: Dordrecht, The Netherlands, 2015. [Google Scholar]
- Saenge, C.; Cheirsilp, B.; Suksaroge, T.T.; Bourtoom, T. Potential use of oleaginous red yeast Rhodotorula glutinis for the bioconversion of crude glycerol from biodiesel plant to lipids and carotenoids. Process Biochem. 2011, 46, 210–218. [Google Scholar] [CrossRef]
- Spolaore, P.; Joannis-Cassan, C.; Duran, E.; Isambert, A. Commercial applications of microalgae. J. Biosci. Bioeng. 2006, 101, 87–96. [Google Scholar] [CrossRef] [PubMed]
- Lubián, L.M.; Montero, O.; Moreno-Garrido, I.; Huertas, I.E.; Sobrino, C.; González-del Valle, M.; Parés, G. Nannochloropsis (Eustigmatophyceae) as source of commercially valuable pigments. J. Appl. Phycol. 2000, 12, 249–255. [Google Scholar] [CrossRef]
- Folch, J.; Ascoli, I.; Lees, M.; Meath, J.A.; LeBaron, F.N. Preparation of lipid extracts from brain tissue. J. Biol. Chem. 1951, 191, 833–841. [Google Scholar] [PubMed]
- Folch, J.; Lees, M.; Sloane-Stanley, G.H. A simple method for the isolation and purification of total lipids from animal tissues. J. Biol. Chem. 1957, 226, 497–509. [Google Scholar] [PubMed]
- Bligh, E.G.; Dyer, W.J. A rapid method of total lipid extraction and purification. J. Biochem. Physiol. 1959, 37, 911–917. [Google Scholar]
- Kunz, W.; Holmberg, K.; Zemb, T. Hydrotropes. Curr. Opin. Colloid Interface Sci. 2016, 22, 99–107. [Google Scholar] [CrossRef]
- Lipke, P.; Ovalle, R. Cell wall architecture in yeast: New structure and New challenges. J. Bacteriol. 1998, 180, 3735–3740. [Google Scholar] [PubMed]
- Vukovic, R.; Mrsa, V. Structure of the saccharomyces cerevisae cell wall. Croat. Chem. Acta 1995, 68, 597–605. [Google Scholar]
- Breil, C.; Meullemiestre, A.; Vian, M.; Chemat, F. Bio-Based Solvents for Green Extraction of Lipids from Oleaginous Yeast Biomass for Sustainable Aviation Biofuel. Molecules 2016, 21, 196. [Google Scholar] [CrossRef] [PubMed]
- Smedes, F.; Thomasen, T.K. Evaluation of the Bligh & Dyer lipid determination method. Mar. Pollut. Bull. 1996, 32, 681–688. [Google Scholar]
- Cot, M. Etude Physiologiques de L’adaptation et de la Résistance de la Levure Saccharomyces Cerevisiae au Cours de la Production Intensive D’éthanol; University of Toulouse, Institute of National Sciences: Toulouse, France, 2006. [Google Scholar]
- Hara, A.; Radin, N. Lipid Extraction of Tissues with Low-Toxicity Solvent. Anal. Biochem. 1978, 90, 420–426. [Google Scholar] [CrossRef]
- Grima, E.M.; Medina, A.R.; Giménez, A.G.; Pérez, J.S.; Camacho, F.G.; Sánchez, J.G. Comparison between extraction of lipids and fatty acids from microalgal biomass. J. Am. Oil Chem. Soc. 1994, 71, 955–959. [Google Scholar] [CrossRef]
- Lee, S.J.; Yoon, B.-D.; Oh, H.-M. Rapid method for the determination of lipid from the green alga Botryococcus braunii. Biotechnol. Tech. 1998, 12, 553–556. [Google Scholar] [CrossRef]
- Sheng, J.; Vannela, R.; Rittmann, B.E. Evaluation of methods to extract and quantify lipids from Synechocystis PCC 6803. Bioresour. Technol. 2011, 102, 1697–1703. [Google Scholar] [CrossRef] [PubMed]
- Caprioli, G.; Giusti, F.; Ballini, R.; Sagratini, G.; Vila-Donat, P.; Vittori, S.; Fiorini, D. Lipid nutritional value of legumes: Evaluation of different extraction methods and determination of fatty acid composition. Food Chem. 2016, 192, 965–971. [Google Scholar] [CrossRef] [PubMed]
- Resa, J.M.; Goenaga, J.M.; Iglesias, M.; Gonzalez-Olmos, R.; Pozuelo, D. Liquid–liquid equilibrium diagrams of ethanol+ water+ (ethyl acetate or 1-pentanol) at several temperatures. J. Chem. Eng. Data 2006, 51, 1300–1305. [Google Scholar] [CrossRef]
- Lofgren, L.; Forsberg, G.-B.; Stahlman, M. The BUME method: A new rapid and simple chloroform-free method for TOTL lipid extraction of animal tissue. Sci. Rep. 2016, 6, 27688. [Google Scholar] [CrossRef] [PubMed]
- Iverson, S.J.; Lang, S.L.; Cooper, M.H. Comparison of the Bligh and Dyer and Folch methods for total lipid determination in a broad range of marine tissue. Lipids 2001, 36, 1283–1287. [Google Scholar] [CrossRef] [PubMed]
- Axelsson, M.; Gentili, F. A single-step method for rapid extraction of total lipids from green microalgae. PLoS ONE 2014, 9, e89643. [Google Scholar] [CrossRef] [PubMed]
- Berndmeyer, C.; Thiel, V.; Blumenberg, M. Test of microwave, ultrasound and Bligh & Dyer extraction for quantitative extraction of bacteriohopanepolyols (BHPs) from marine sediments. Org. Geochem. 2014, 68, 90–94. [Google Scholar]
- Ryckebosch, E.; Muylaert, K.; Foubert, I. Optimization of an Analytical Procedure for Extraction of Lipids from Microalgae. J. Am. Oil Chem. Soc. 2012, 89, 189–198. [Google Scholar] [CrossRef]
- Teo, C.L.; Idris, A. Enhancing the various solvent extraction method via microwave irradiation for extraction of lipids from marine microalgae in biodiesel production. Bioresour. Technol. 2014, 171, 477–481. [Google Scholar] [CrossRef] [PubMed]
- Medina, A.L.; da Silva, M.A.O.; de Sousa Barbosa, H.; Arruda, M.A.Z.; Marsaioli, A.; Bragagnolo, N. Rapid microwave assisted extraction of meat lipids. Food Res. Int. 2015, 78, 124–130. [Google Scholar] [CrossRef]
- Lee, J.-Y.; Yoo, C.; Jun, S.-Y.; Ahn, C.-Y.; Oh, H.-M. Comparison of several methods for effective lipid extraction from microalgae. Bioresour. Technol. 2010, 101, S75–S77. [Google Scholar] [CrossRef] [PubMed]
- Cescut, J.; Severac, E.; Molina-Jouve, C.; Uribelarrea, J.-L. Optimizing pressurized liquid extraction of microbial lipids using the response surface method. J. Chromatogr. A 2011, 1218, 373–379. [Google Scholar] [CrossRef] [PubMed]
- Meullemiestre, A.; Breil, C.; Abert-Vian, M.; Chemat, F. Microwave, ultrasound, thermal treatments, and bead milling as intensification techniques for extraction of lipids from oleaginous Yarrowia lipolytica yeast for a biojetfuel application. Bioresour. Technol. 2016, 211, 190–199. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Chen, L.; Rakesh, B.; Qin, Y.; Lv, R. Technologies for extracting lipids from oleaginous microorganisms for biodiesel production. Front. Energy 2012, 6, 266–274. [Google Scholar] [CrossRef]
- Chemat, F. Alternative Solvents for Natural Products Extraction; Dalian Institute of Chemical Physics, Chinese Academy of Sciences: Dalian, China, 2014. [Google Scholar]
- Klamt, A.; Eckert, F.; Arlt, W. COSMO-RS: An alternative to simulation for simulation for calculating thermodynamic properties of liquid mixtures. Annu. Rev. Chem. Biomol. Eng. 2010, 1, 101–122. [Google Scholar] [CrossRef] [PubMed]
- Aguilar-Uscanga, B.; Francois, J.M. A study of the yeast cell wall composition and structure in response to growth conditions and mode of cultivation. Lett. Appl. Microbiol. 2003, 37, 268–274. [Google Scholar] [CrossRef] [PubMed]
- Klis, F.M.; Boorsma, A.; de Groot, P.W.J. Cell wall construction inSaccharomyces cerevisiae. Yeast 2006, 23, 185–202. [Google Scholar] [CrossRef] [PubMed]
- Inokuma, K.; Liao, J.C.; Okamoto, M.; Hanai, T. Improvement of isopropanol production by metabolically engineered Escherichia coli using gas stripping. J. Biosci. Bioeng. 2010, 110, 696–701. [Google Scholar] [CrossRef] [PubMed]
- Gonçalves, F.A.; Ruiz, H.A.; Silvino dos Santos, E.; Teixeira, J.A.; de Macedo, G.R. Bioethanol production by Saccharomyces cerevisiae, Pichia stipitis and Zymomonas mobilis from delignified coconut fibre mature and lignin extraction according to biorefinery concept. Renew. Energy 2016, 94, 353–365. [Google Scholar] [CrossRef]
- Song, Y.; Wi, S.G.; Kim, H.M.; Bae, H.-J. Cellulosic bioethanol production from Jerusalem artichoke (Helianthus tuberosus L.) using hydrogen peroxide-acetic acid (HPAC) pretreatment. Bioresour. Technol. 2016, 214, 30–36. [Google Scholar] [CrossRef] [PubMed]
- Shuai, W.; Chen, N.; Li, B.; Zhou, D.; Gao, J. Life cycle assessment of common reed (Phragmites australis (Cav) Trin. ex Steud) cellulosic bioethanol in Jiangsu Province, China. Biomass Bioenergy 2016, 92, 40–47. [Google Scholar] [CrossRef]
- Sicaire, A.-G.; Vian, M.; Fine, F.; Joffre, F.; Carré, P.; Tostain, S.; Chemat, F. Alternative Bio-Based Solvents for Extraction of Fat and Oils: Solubility Prediction, Global Yield, Extraction Kinetics, Chemical Composition and Cost of Manufacturing. Int. J. Mol. Sci. 2015, 16, 8430–8453. [Google Scholar] [CrossRef] [PubMed]
- Anastas, P.; Eghbali, N. Green Chemistry: Principles and Practice. Chem. Soc. Rev. 2010, 39, 301–312. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, K.; Yamagiwa, N.; Torisawa, Y. Cyclopentyl Methyl Ether as a New and Alternative Process Solvent. Org. Process Res. Dev. 2007, 11, 251–258. [Google Scholar] [CrossRef]
- Mehler, C.; Klamt, A.; Peukert, W. Use of COSMO-RS for the prediction of adsorption equilibria. AIChE J. 2002, 48, 1093–1099. [Google Scholar] [CrossRef]
- Filly, A.; Fabiano-Tixier, A.S.; Fernandez, X.; Chemat, F. Alternative solvents for extraction of food aromas. Experimental and COSMO-RS study. Food Sci. Technol. 2015, 61, 33–40. [Google Scholar] [CrossRef]
- Morrison, W.R.; Smith, L.M. Preparation of fatty acid methyl esters and dimethylacetals from lipids with boron fluoride–methanol. J. Lipid Res. 1964, 5, 600–608. [Google Scholar] [PubMed]
- Cheng, Y.; Wei, H.; Sun, R.; Tian, Z.; Zheng, X. Rapid method for protein quantitation by Bradford assay after elimination of the interference of polysorbate 80. Anal. Biochem. 2016, 494, 37–39. [Google Scholar] [CrossRef] [PubMed]
- Chemat, F. Eco-Extraction du Vegetal. Procédés Innovants et Solvants Alternatifs; Technique et Ingénierue; Dunod: Paris, France, 2015. [Google Scholar]







| Solvents/Molecules | TAG LLL | TAG LOO | TAG OOO | DAG LGL | DAG LGO | DAG OGO | FFA18 1n9 | FFA18 2n6 | FFA 16 | PC LL | PC OL | PE LL | PE OL | Lanosterol | Ergosterol | 1.6 bd Glucan | 1.4 bd Glucan | Chitine | Glycerol | Histidine | Arginine | Glucose |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Water | −23.96 | −24.57 | −24.55 | −15.09 | −17.26 | −16.23 | −8.18 | −7.99 | −7.67 | −7.86 | −5.92 | −14.56 | −14.13 | −10.05 | −9.19 | 0.00 | −1.01 | −0.21 | 0.00 | 0.00 | 0.00 | 0.00 |
| Methanol | −3.97 | −4.36 | −4.39 | 0.00 | −2.73 | −1.98 | −0.13 | −0.26 | −0.30 | 0.00 | 0.00 | −0.11 | −0.08 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 |
| Ethanol | −2.11 | −2.41 | −2.40 | 0.00 | −0.99 | −0.11 | −0.10 | −0.13 | 0.12 | 0.00 | 0.00 | −0.27 | −0.16 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 |
| Isopropanol | 0.00 | 0.00 | 0.00 | −0.19 | −0.07 | −0.20 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 |
| Chloroform | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | −0.39 | −0.17 | −0.29 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | −0.22 | 0.00 | −3.65 | 0.00 | 0.00 | 0.00 | 0.00 | −4.66 |
| Ethyl acetate | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | −7.86 | −5.92 | −14.56 | −14.13 | −17.48 | −20.22 | 0.00 |
| MeTHF | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 |
| CPME | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | −0.95 | −0.21 | 0.00 | 0.00 | 0.00 | 0.00 | −2.25 | 0.00 | 0.00 | 0.00 | 0.00 | −3.08 | 0.00 |
| DMC | −0.17 | −0.10 | −0.10 | −0.10 | −0.24 | −0.19 | −0.10 | 0.00 | −0.20 | 0.00 | 0.00 | −0.65 | −0.21 | −0.09 | −0.06 | −0.17 | 0.00 | 0.00 | 0.00 | −0.28 | −0.20 | 0.00 |
| Ethyl lactate | −2.71 | −3.24 | −3.24 | 0.00 | −1.60 | −0.09 | −0.15 | −0.04 | −0.14 | 0.00 | 0.00 | −0.07 | −0.04 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 |
| α−Pinene | 0.00 | 0.00 | 0.00 | 0.00 | −0.19 | −0.17 | 0.00 | 0.00 | 0.00 | −3.52 | −4.56 | 0.00 | 0.00 | 0.00 | 0.00 | −9.94 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 |
| d−Limonene | 0.00 | 0.00 | 0.00 | −0.20 | −0.10 | −0.37 | 0.00 | 0.00 | 0.00 | −2.93 | −3.97 | 0.00 | 0.00 | −0.07 | 0.00 | −9.29 | −8.71 | −7.64 | −5.11 | −4.85 | −7.25 | −7.55 |
| p−Cymene | 0.00 | 0.00 | 0.00 | −0.17 | −0.14 | −0.14 | 0.00 | 0.00 | 0.00 | −2.21 | −3.40 | 0.00 | 0.00 | −0.04 | 0.00 | −8.92 | −8.42 | −7.31 | −4.96 | −4.56 | −6.96 | −7.37 |
| Compositions | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13 | 14 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Lipids yieds by GC | 14.55 ± 0.12 | 11.94 ± 0.52 | 6.25 ± 0.12 | 4.32 ± 0.15 | 5.31 ± 0.23 | 11.08 ± 0.24 | 14.12 ± 0.89 | 13.77 ± 0.56 | 13.45 ± 0.26 | 13.62 ± 0.27 | 13.39 ± 0.85 | 6.51± 0.07 | 9.51 ± 0.23 | 18.50 ± 0.75 |
| Lipids yields by gravimetry | 14.85 ± 0.10 | 9.12 ± 0.35 | 3.5 ± 0.20 | 4.85 ± 0.12 | 5.42 ± 0.17 | 12.25 ± 0.18 | 14.83 ± 0.42 | 14.25 ± 0.21 | 13.89 ± 0.14 | 14.63 ± 0.17 | 13.95 ± 0.15 | 7.02 ± 0.16 | 10.52 ± 0.24 | 9.25 ± 0.36 |
| Lipid classes composition (%) | ||||||||||||||
| FFA: Free fatty acid | 58.69 ± 0.98 | 57.89 ± 2.75 | 57.07 ± 0.40 | 58.96 ± 0.49 | 58.69 ± 2.13 | 57.23 ± 1.07 | 58.93 ± 2.11 | 58.45 ± 1.04 | 57.36 ± 1.21 | 61.23 ± 1.27 | 58.23 ± 1.40 | 58.52 ± 1.25 | 57.85 ± 1.74 | 58.63 ± 2.36 |
| TAG: Triacylglycerol | 20.03 ± 0.52 | 21.23 ± 1.02 | 20.96 ± 2.3 | 22.36 ± 1.02 | 23.78 ± 1.58 | 22.45 ± 1.82 | 21.36 ± 1.02 | 19.87 ± 0.95 | 18.65 ± 1.24 | 16.20 ± 1.52 | 18.25 ± 1.75 | 22.52 ± 1.36 | 20.60 ± 1.95 | 17.81 ± 1.52 |
| DAG: Diacylglycerol | 15.45 ± 0.23 | 15.26 ± 0.82 | 15.36 ± 0.47 | 14.56 ± 1.18 | 12.58 ± 0.40 | 14.25 ± 1.11 | 13.69 ± 1.36 | 14.25 ± 1.12 | 14.74 ± 1.23 | 14.10 ± 1.45 | 15.24 ± 1.91 | 13.63 ± 1.14 | 15.25 ± 1.02 | 14.63 ± 1.41 |
| PE: Phosphatdylethanolamine | 2.58 ± 0.25 | 2.58 ± 0.80 | 2.85 ± 0.40 | 2.47 ± 0.18 | 4.16 ± 0.42 | 3.81 ± 0.05 | 4.76 ± 0.05 | 3.69 ± 0.12 | 4.43 ± 0.14 | 3.69 ± 0.31 | 3.62 ± 0.25 | 3.56 ± 0.32 | 3.58 ± 012 | 4.52 ± 0.36 |
| PC: Phosphatydylcholine | 0.15 ± 0.003 | 0.23 ± 0.008 | 0.24 ± 0.003 | 0.54 ± 0.006 | 0.24 ± 0.008 | 0.21 ± 0.007 | 0.29 ± 0.002 | 0.17 ± 0.007 | 0.01 ± 0.001 | 0.2 ± 0.02 | 0.14 ± 0.01 | 0.25 ± 0.01 | 0.14 ± 0.10 | 0.96 ± 0.01 |
| PI: Phosphatydylinositol | 3.10 ± 0.01 | 2.81 ± 0.94 | 3.52 ± 0.05 | 1.11 ± 0.08 | 0.55 ± 0.001 | 2.05 ± 0.008 | 0.97 ± 0.004 | 3.57 ± 0.004 | 4.81 ± 0.20 | 4.58 ± 0.041 | 4.52 ± 0.43 | 1.52 ± 0.04 | 2.58 ± 0.21 | 3.45 ± 0.02 |
| Fatty acids composition (%) | ||||||||||||||
| C16 | 10.26 ± 0.02 | 10.20 ± 0.02 | 10.49 ± 0.08 | 11.18 ± 0.07 | 10.00 ± 0.06 | 9.89 ± 0.05 | 10.84 ± 0.04 | 9.69 ± 0.12 | 10.62 ± 0.19 | 11.79 ± 0.03 | 9.60 ± 0.03 | 10.03 ± 0.01 | 9.37 ± 0.06 | 10.50 ± 0.06 |
| C18 | 3.25 ± 0.085 | 3.49 ± 0.005 | 3.52 ± 0.027 | 3.76 ± 0.023 | 3.59 ± 0.018 | 3.53 ± 0.02 | 3.91 ± 0.021 | 3.55 ± 0.05 | 3.33 ± 0.21 | 4.11 ± 0.013 | 3.36 ± 0.014 | 2.94 ± 0.004 | 3.55 ± 0.023 | 3.33 ± 0.019 |
| C16:1n9 | 4.28 ± 0.005 | 5.84 ± 0.009 | 3.98 ± 0.11 | 5.47 ± 0.009 | 5.36 ± 0.008 | 5.42 ± 0.03 | 5.48 ± 0.096 | 5.35 ± 0.07 | 5.57 ± 0.050 | 4.11 ± 0.041 | 5.32 ± 0.013 | 6.20 ± 0.007 | 5.40 ± 0.036 | 5.63 ± 0.023 |
| C18:1n9 | 50.36 ± 0.07 | 49.81 ± 0.07 | 50.92 ± 0.39 | 50.78 ± 0.81 | 51.39 ± 0.11 | 51.20 ± 0.30 | 52.91 ± 0.72 | 51.26 ± 0.65 | 51.23 ± 0.10 | 52.67 ± 0.25 | 48.37 ± 0.17 | 49.16 ± 0.062 | 51.61 ± 0.32 | 49.79 ± 0.29 |
| C18:2n6 | 28.39 ± 0.036 | 29.29 ± 0.04 | 29.80 ± 0.22 | 27.44 ± 0.76 | 28.44 ± 0.07 | 28.71 ± 0.18 | 25.76 ± 1.16 | 28.82 ± 0.35 | 27.52 ± 0.40 | 24.85 ± 0.57 | 28.12 ± 0.093 | 30.18 ± 0.0038 | 28.80 ± 0.17 | 29.38 ± 0.14 |
| C18:3n6 | 3.46 ± 0.036 | 1.35 ± 0.002 | 1.25 ± 0.008 | 1.34 ± 0.01 | 1.19 ± 0.007 | 1.21 ± 0.007 | 1.07 ± 0.054 | 1.31 ± 0.027 | 1.52 ± 0.014 | 1.00 ± 0.028 | 5.20 ± 0.029 | 1.47 ± 0.001 | 1.24 ± 0.008 | 1.33 ± 0.013 |
| Proteins yields (%) | ||||||||||||||
| Aqueous phases | 0.038 ± 0.001 | 0.328 ± 0.020 | 0.327 ± 0.030 | 0.158 ± 0.002 | 0.175 ± 0.015 | 0.25 ± 0.023 | 0.296 ± 0.021 | 0.314 ± 0.020 | 0.294 ± 0.025 | 0.290 ± 0.021 | 0.284 ± 0.021 | 0.0195 ± 0.001 | 0.012 ± 0.001 | 0.041 ± 0.001 |
| Organic phases | 0.0036 ± 1.10 × 10−4 | 0.0058 ± 1.10 × 10−4 | 0.0048 ± 1.10 × 10−4 | 0.0087 ± 1.10 × 10−4 | 0.0084 ± 1.10 × 10−4 | 0.0053 ± 1.10 × 10−4 | 0.0039 ± 1.10 × 10−4 | 0.0021 ± 1.10 × 10−4 | 0.015 ± 1.10 × 10−4 | 0.021 ± 1.10 × 10−4 | 0.0007 ± 1.10 × 10−4 | 0.0002 ± 1.10 × 10−4 | 0.00036 ± 1.10 × 10−4 | 0.00025 ± 1.10 × 10−4 |
| Sugars yields (%) | ||||||||||||||
| Aqueous phases | 0.17 ± 0.010 | 2.12 ± 0.17 | 2.23 ± 0.15 | 2.02 ± 0.18 | 2.06 ± 0.14 | 1.97 ± 0.12 | 1.85 ± 0.17 | 2.06 ± 0.10 | 2.04 ± 0.11 | 2.15 ± 0.14 | 2.08 ± 0.20 | 1.33 ± 0.04 | 0.74 ± 0.01 | 0.32 ± 0.02 |
| Organic phases | 0.13 ± 0.01 | 0.03 ± 0.001 | 0.025 ± 0.002 | 0.023 ± 0.002 | 0.13 ± 0.001 | 0.23 ± 0.012 | 0.12 ± 0.014 | 0.14 ± 0.017 | 0.19 ± 0.012 | 0.02 ± 0.001 | 0.13 ± 0.02 | 0.28 ± 0.014 | 0.08 ± 0.001 | 0.14 ± 0.001 |
| Compositions | A | B | C | D | E | F | G | H | I | J | K | L | M | N |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Lipids yieds by GC | 13.51 ± 0.09 | 11.44 ± 0.13 | 4.52 ± 0.17 | 6.78 ± 0.52 | 11.45 ± 0.12 | 13.53 ± 0.36 | 14.35 ± 0.63 | 14.38 ± 12 | 14.15 ± 0.15 | 14.24 ± 0.19 | 14.18 ± 0.13 | 5.63 ± 0.25 | 7.58 ± 0.36 | 8.69 ± 0.50 |
| Lipids yields by gravimetry | 14.03 ± 0.15 | 9.53 ± 0.48 | 5.03 ± 0.38 | 7.25 ± 0.12 | 11.97 ± 0.21 | 14.25 ± 0.46 | 14.75 ± 0.13 | 14.65 ± 0.23 | 14.28 ± 0.60 | 14.86 ± 0.64 | 14.52 ± 0.42 | 6.96 ± 0.31 | 7.86 ± 0.52 | 9.19 ± 0.07 |
| Lipid classes composition (%) | ||||||||||||||
| FFA: Free fatty acid | 59.84 ± 1.52 | 57.23 ± 2.8 | 58.62 ± 1.58 | 58.69 ± 2.53 | 57.85 ± 1.69 | 57.37 ± 1.52 | 58.06 ± 2.12 | 59.36 ± 1.58 | 57.38 ± 3.69 | 57.89 ± 1.88 | 57.43 ± 1.76 | 59.56 ± 1.97 | 59.63 ± 1.45 | 58.67 ± 1.47 |
| TAG: Triacylglycerol | 18.26 ± 0.85 | 19.20 ± 1.96 | 20.52 ± 1.02 | 20.41 ± 1.32 | 20.33 ± 2.47 | 19.78 ± 1.75 | 19.43 ± 1.19 | 19.85 ± 0.74 | 21.03 ± 2.12 | 18.55 ± 1.23 | 21.81 ± 2.15 | 18.56+ ± 1.41 | 16.57 ± 1.41 | 17.85 ± 1.20 |
| DAG: Diacylglycerol | 12.30 ± 1.07 | 14.85 ± 0.89 | 15.69 ± 0.47 | 12.64 ± 1.21 | 13.67 ± 0.21 | 14.16 ± 1.03 | 16.20 ± 1.24 | 13.60 ± 1.23 | 12.76 ± 1.12 | 15.23 ± 1.45 | 12.87 ± 1.17 | 13.65 ± 0.55 | 14.78 ± 0.51 | 14.52 ± 0.57 |
| PE: Phosphatdylethanolamine | 4.58 ± 0.35 | 3.96 ± 0.32 | 3.56 ± 0.21 | 3.30 ± 0.15 | 3.54 ± 0.12 | 3.59 ± 0.07 | 4.01 ± 0.40 | 3.12 ± 0.21 | 3.55 ± 0.12 | 2.63 ± 0.23 | 4.25 ± 0.12 | 4.58 ± 0.12 | 3.65 ± 0.14 | 4.25 ± 0.12 |
| PC: Phosphatydylcholine | 0.33 ± 0.01 | 0.32 ± 0.01 | 0.07 ± 0.001 | 0.05 ± 0.001 | 0.03 ± 0.001 | 0.12 ± 0.011 | 0.45 ± 0.010 | 0.49 ± 0.041 | 0.23 ± 2.58 | 0.14 ± 0.012 | 0.12 ± 0.013 | 0.02 ± 0.0018 | 0.5 ± 0.04 | 0.08 ± 0.007 |
| PI: Phosphatydylinositol | 4.69 ± 0.32 | 4.44 ± 0.12 | 1.54 ± 0.05 | 4.91 ± 0.31 | 4.58 ± 0.40 | 4.98 ± 0.48 | 1.85 ± 0.12 | 3.58 ± 0.21 | 5.05 ± 0.54 | 5.56 ± 0.52 | 3.52 ± 0.24 | 3.63 ± 0.21 | 4.87 ± 0.31 | 4.63 ± 0.41 |
| Fatty acids composition (%) | ||||||||||||||
| C16 | 9.44 ± 0.48 | 10.72 ± 0.03 | 12.20 ± 0.14 | 7.37 ± 0.77 | 9.60 ± 0.091 | 9.42 ± 0.033 | 10.32 ± 0.005 | 9.16 ± 0.05 | 9.70 ± 0.093 | 8.58 ± 0.092 | 9.02 ± 0.003 | 10.80 ± 0.45 | 9.85 ± 0.065 | 9.85 ± 0.003 |
| C18 | 2.92 ± 0.17 | 3.33 ± 0.01 | 3.27 ± 0.006 | 3.43 ± 0.032 | 3.47 ± 0.028 | 3.49 ± 0.010 | 3.96 ± 0.008 | 3.52 ± 0.023 | 3.01 ± 0.036 | 3.52 ± 0.066 | 3.49 ± 0.001 | 4.04+ ± 0.13 | 3.43 ± 0.024 | 3.43 ± 0.001 |
| C16:1n9 | 4.77 ± 0.33 | 5.96 ± 0.020 | 6.24 ± 0.078 | 6.16 ± 0.042 | 5.46 ± 0.047 | 5.42 ± 0.013 | 5.43 ± 0.096 | 5.36 ± 0.035 | 5.62 ± 0.024 | 4.67 ± 0.035 | 5.31 ± 0.002 | 5.60 ± 0.26 | 5.26 ± 0.024 | 5.26 ± 0.002 |
| C18:1n9 | 47.84 ± 2.01 | 50.08 ± 0.16 | 48.45 ± 0.15 | 61.65 ± 0.43 | 51.45 ± 0.44 | 51.64 ± 0.16 | 53.61 ± 0.62 | 51.78 ± 0.35 | 51.02 ± 0.54 | 56.08 ± 1.99 | 53.61 ± 0.11 | 47.89+ ± 0.60 | 51.36 ± 0.30 | 51.36 ± 0.119 |
| C18:2n6 | 32.95 ± 0.59 | 28.67 ± 0.089 | 28.53 ± 0.12 | 30.08 ± 0.19 | 28.76 ± 0.23 | 29.78 ± 0.06 | 25.42 ± 1.06 | 28.90 ± 0.22 | 28.66 ± 0.30 | 26.07 ± 0.23 | 27.45 ± 0.039 | 30.38 ± 1.51 | 28.85 ± 0.16 | 28.85 ± 0.039 |
| C18:3n6 | 2.06 ± 0.031 | 1.21 ± 0.003 | 1.29 ± 0.007 | 1.29 ± 0.007 | 1.23 ± 0.009 | 1.23 ± 0.002 | 1.24 ± 0.025 | 1.25 ± 0.014 | 1.99 ± 0.074 | 1.05 ± 0.003 | 1.09 ± 0.005 | 1.27 ± 0.069 | 1.23 ± 0.005 | 1.23 ± 0.006 |
| Proteins yields (%) | ||||||||||||||
| Aqueous phases | 0.052 ± 0.001 | 0.28 ± 0.012 | 0.326 ± 0.013 | 0.10 ± 0.009 | 0.175 ± 0.015 | 0.205 ± 0.015 | 0.325 ± 0.012 | 0.321 ± 0.014 | 0.324 ± 0.020 | 0.314 ± 0.012 | 0.359 ± 0.034 | 0.211 ± 0.020 | 0.183 ± 0.012 | 0.103 ± 0.001 |
| Organic phases | 0.0021± 1.10 × 10−4 | 0.0014 ± 10 × 10−4 | 0.0026 ± 2.10 × 10−4 | 0.002 ± 1.10 × 10−4 | 0.015 ± 7.10 × 10−4 | 0.0048 ± 1.10 × 10−4 | 0.0015 ± 1.10 × 10−4 | 0.003 ± 1.10 × 10−4 | 0.0058 ± 1.10 × 10−4 | 0.004 ± 1.10 × 10−4 | 0.0042 ± 1.10 × 10−4 | 0.0036 ± 1.10 × 10−4 | 0.02 ± 1.10 × 10−4 | 0.00025 ± 1.10 × 10−4 |
| Sugars yields (%) | ||||||||||||||
| Aqueous phases | 1.13 ± 0.014 | 1.10 ± 0.09 | 2.15 ± 0.15 | 2.02 ± 0.20 | 1.95 ± 0.14 | 2.05 ± 0.12 | 2.03 ± 0.10 | 2.12 ± 0.18 | 1.93 ± 0.21 | 1.97 ± 0.16 | 2.04 ± 0.17 | 0.87 ± 0.074 | 1.61 ± 0.01 | 1.69 ± 0.012 |
| Organic phases | 0.20 ± 0.001 | 0.10 ± 0.001 | 0.20 ± 0.002 | 0.042 ± 0.001 | 0.023 ± 0.003 | 0.021 ± 0.001 | 0.052 ± 0.001 | 0.045 ± 0.004 | 0.036 ± 0.001 | 0.025 ± 0.001 | 0.021 ± 0.001 | 0.085 ± 0.001 | 0.023 ± 0.002 | 0.20 ± 0.001 |
| Extractions/Molecules | TAG LLL | TAG LOO | TAG OOO | DAG LGL | DAG LGO | DAG OGO | FFA18 1n9 | FFA18 2n6 | FFA 16 | PC LL | PC OL | PE LL | PE OL | Lanosterol | Ergosterol | 1.6 bd Glucan | 1.4 bd Glucan | Chitine | Glycerol | Histidine | Arginine | Glucose |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| D | −9.80 | −10.11 | −10.10 | −5.69 | −7.11 | −6.31 | −2.92 | −2.80 | −2.70 | 0.00 | 0.00 | −4.78 | −4.64 | −4.05 | −3.55 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 |
| E | −5.99 | −6.28 | −6.28 | −3.14 | −4.33 | −3.66 | −0.14 | −0.45 | −0.23 | 0.00 | 0.00 | 0.00 | −0.28 | −2.30 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 |
| F | −3.44 | −3.77 | −3.78 | −0.09 | −2.32 | −0.23 | −0.59 | −0.25 | −0.28 | 0.00 | 0.00 | −0.16 | −0.18 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 |
| G | 0.00 | 0.00 | 0.00 | 0.00 | −0.38 | −0.05 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | −0.21 | −0.27 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 |
| H | −0.04 | −0.05 | −0.05 | 0.00 | −0.07 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | −0.04 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 |
| I | 0.00 | 0.00 | −0.09 | −0.08 | 0.00 | 0.00 | −0.03 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 |
| J | 0.00 | −0.25 | −0.14 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 |
| K | 0.00 | −0.09 | −0.06 | 0.00 | −0.07 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | −0.04 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 |
| L | −6.97 | −6.97 | −6.63 | −3.59 | −4.83 | −4.15 | −0.43 | −0.19 | −0.27 | 0.00 | 0.00 | −2.51 | −2.34 | −2.65 | −2.13 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 |
| M | −4.88 | −4.87 | −4.51 | −0.12 | −3.26 | −2.58 | −0.46 | −0.28 | −0.39 | 0.00 | 0.00 | −0.18 | −0.15 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | −0.08 | 0.00 | 0.00 | |
| N | −0.03 | 0.00 | −0.02 | −0.40 | −0.03 | −0.21 | −0.01 | 0.00 | −0.01 | 0.00 | 0.00 | 0.00 | 0.00 | −0.04 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 |
| Water | −23.96 | −24.57 | −24.55 | −15.09 | −17.26 | −16.23 | −8.18 | −7.99 | −7.67 | −7.86 | −5.92 | −14.56 | −14.13 | −10.05 | −9.19 | 0.00 | −1.01 | −0.21 | 0.00 | 0.00 | 0.00 | 0.00 |
| Ethanol | −2.41 | −2.40 | −2.26 | 0.00 | −0.03 | −0.08 | 0.00 | 0.00 | 0.00 | 0.00 | −0.27 | −0.16 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 |
| Ethyl acetate | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | −7.86 | −5.92 | −14.56 | −14.13 | −17.48 | −20.22 | 0.00 |
| Water | −23.96 | −24.57 | −24.55 | −15.09 | −17.26 | −16.23 | −8.18 | −7.99 | −7.67 | −7.86 | −5.92 | −14.56 | −14.13 | −10.05 | −9.19 | 0.00 | −1.01 | −0.21 | 0.00 | 0.00 | 0.00 | 0.00 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Breil, C.; Abert Vian, M.; Zemb, T.; Kunz, W.; Chemat, F. “Bligh and Dyer” and Folch Methods for Solid–Liquid–Liquid Extraction of Lipids from Microorganisms. Comprehension of Solvatation Mechanisms and towards Substitution with Alternative Solvents. Int. J. Mol. Sci. 2017, 18, 708. https://doi.org/10.3390/ijms18040708
Breil C, Abert Vian M, Zemb T, Kunz W, Chemat F. “Bligh and Dyer” and Folch Methods for Solid–Liquid–Liquid Extraction of Lipids from Microorganisms. Comprehension of Solvatation Mechanisms and towards Substitution with Alternative Solvents. International Journal of Molecular Sciences. 2017; 18(4):708. https://doi.org/10.3390/ijms18040708
Chicago/Turabian StyleBreil, Cassandra, Maryline Abert Vian, Thomas Zemb, Werner Kunz, and Farid Chemat. 2017. "“Bligh and Dyer” and Folch Methods for Solid–Liquid–Liquid Extraction of Lipids from Microorganisms. Comprehension of Solvatation Mechanisms and towards Substitution with Alternative Solvents" International Journal of Molecular Sciences 18, no. 4: 708. https://doi.org/10.3390/ijms18040708
APA StyleBreil, C., Abert Vian, M., Zemb, T., Kunz, W., & Chemat, F. (2017). “Bligh and Dyer” and Folch Methods for Solid–Liquid–Liquid Extraction of Lipids from Microorganisms. Comprehension of Solvatation Mechanisms and towards Substitution with Alternative Solvents. International Journal of Molecular Sciences, 18(4), 708. https://doi.org/10.3390/ijms18040708