Development of Fluorinated Non-Peptidic Ghrelin Receptor Ligands for Potential Use in Molecular Imaging
Abstract
:1. Introduction
2. Results
2.1. Chemistry
2.2. Biological Investigations
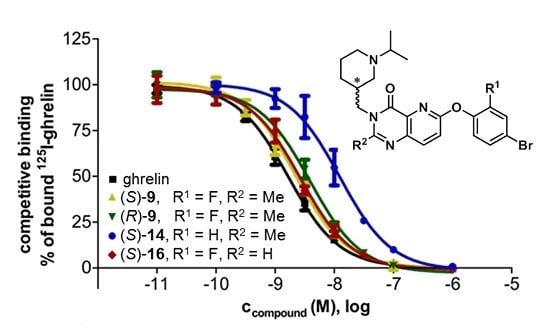
2.2.1. Binding Affinities at the Human Ghrelin Receptor (hGhrR)
2.2.2. Biological Activity at the hGhrR
2.2.3. Selectivity Studies
2.2.4. Distribution Coefficient and Non-Specific Binding
3. Discussion
4. Materials and Methods
4.1. General Methods
4.2. Procedures and Compound Characterization
4.3. Biology In Vitro Characterization of Compounds
4.3.1. Radioligand Binding Assay
4.3.2. Inositol Phosphate Accumulation Assay
4.3.3. Determination of CHI IAM Values
4.3.4. Selectivity Studies
- (a)
- rat σ1 receptor = rat brain homogenate, [3H]pentazocine, 50 mM TRIS-HCl, pH 7.4, 120 mM NaCl, 5 mM KCl, 2 mM CaCl2, 1 mM MgCl2, 120 min incubation at rt;
- (b)
- rat σ2 receptor = rat liver homogenate, [3H]DTG + 1 µM dextrallorphan, 50 mM TRIS-HCl, pH 7.4, 120 mM NaCl, 5 mM KCl, 2 mM CaCl2, 1 mM MgCl2, 120 min incubation at rt;
- (c)
- rat VAChT = homogenates of transfected PC12 cells (by courtesy of Ali Roghani, Texas Tech University, Lubbock, TX, USA), [3H]vesamicol, 50 mM TRIS-HCl, pH 7.4, 120 mM NaCl, 5 mM KCl, 2 mM CaCl2, 1 mM MgCl2, 60 min incubation at rt;
- (d)
- human oxytocin receptor = homogenates of transfected CHO cells (by courtesy of Bice Chini, Isituto di Neuroscienze, Milano, Italy), [3H]oxytocin, 50 mM TRIS-HCl, pH 7.4, 5 mM MgCl2, 0.1% BSA, 60 min incubation at rt;
- (e)
- human nAChR = homogenates of transfected HEK (α4β2, α3β4) and SH-SY5Y (α7) cells (by courtesy of Dominik Feuerbach, Novartis, Basel, Switzerland), [3H]epibatidine (α4β2, α3β4) or [3H]MLA (α7), 50 mM TRIS-HCl, pH 7.4, 120 mM NaCl, 5 mM KCl, 2 mM CaCl2, 1 mM MgCl2, 60 min incubation at rt;
- (f)
- human adenosine receptors = homogenates of transfected CHO cells (by courtesy of Karl-Norbert Klotz, Julius-Maximilians-Universität, Würzburg, Germany), [3H]DPCPX (A1) or [3H]ZM241385 (A2A), 50 mM TRIS-HCl, pH 7.4, 1 mM MgCl2 (A1) or 50 mM TRIS-HCl, pH 7.4, 100 mM NaCl, 5 mM MgCl2, 1 M EDTA (A2A), 120 min (A1) or 60 min (A2A) incubation at rt.
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
Abbreviations
| AcOH | Acetic acid |
| GhrR | Ghrelin receptor |
| BOP | Benzotriazole-1-yl-oxy-tris-(dimethylamino)-phosphonium hexafluorophosphate |
| BBB | Blood-brain barrier |
| Boc | tert-Butyloxycarbonyl |
| CHI IAM | Chromatographic hydrophobicity index on immobilized artificial membranes |
| DCM | Dichloromethane |
| DMF | N,N-Dimethylformamide |
| EA | Ethyl acetate |
| HPES | 4-(2-Hydroxyethyl)-1-piperazineethanesulfonic acid |
| HPLC | High-performance liquid chromatography |
| HRMS | High resolution mass spectrometry |
| IC50 | The half maximal inhibitory concentration |
| IH | Petroleum ether (40–65 °C) |
| M | Molarity |
| MW | Molecular weight |
| PET | Positron emission tomography |
| SPECT | Single-photon emission computed tomography |
| TBAF | Tetrabutylammonium fluoride |
| Et3N | Triethylamine |
| TFA | Trifluoroacetic acid |
References
- Howard, A.D.; Feighner, S.D.; Cully, D.F.; Arena, J.P.; Liberator, P.A.; Rosenblum, C.I.; Hamelin, M.; Hreniuk, D.L.; Palyha, O.C.; Anderson, J.; et al. A receptor in pituitary and hypothalamus that functions in growth hormone release. Science 1996, 273, 974–977. [Google Scholar] [CrossRef] [PubMed]
- Kojima, M.; Hosoda, H.; Date, Y.; Nakazato, M.; Matsuo, H.; Kangawa, K. Ghrelin is a growth-hormone-releasing acylated peptide from stomach. Nature 1999, 402, 656–660. [Google Scholar] [CrossRef] [PubMed]
- Lopez, M.; Lage, R.; Saha, A.K.; Perez-Tilve, D.; Vazquez, M.J.; Varela, L.; Sangiao-Alvarellos, S.; Tovar, S.; Raghay, K.; Rodriguez-Cuenca, S.; et al. Hypothalamic fatty acid metabolism mediates the orexigenic action of ghrelin. Cell. Metab. 2008, 7, 389–399. [Google Scholar] [CrossRef] [PubMed]
- Takaya, K.; Ariyasu, H.; Kanamoto, N.; Iwakura, H.; Yoshimoto, A.; Harada, M.; Mori, K.; Komatsu, Y.; Usui, T.; Shimatsu, A.; et al. Ghrelin strongly stimulates growth hormone release in humans. J. Clin. Endocrinol. Metab. 2000, 85, 4908–4911. [Google Scholar] [CrossRef] [PubMed]
- Müller, T.D.; Nogueiras, R.; Andermann, M.L.; Andrews, Z.B.; Anker, S.D.; Argente, J.; Batterham, R.L.; Benoit, S.C.; Bowers, C.Y.; Broglio, F.; et al. Ghrelin. Mol. Metab. 2015, 4, 437–460. [Google Scholar] [CrossRef] [PubMed]
- Howick, K.; Griffin, B.; Cryan, J.; Schellekens, H. From belly to brain: Targeting the ghrelin receptor in appetite and food intake regulation. Int. J. Mol. Sci. 2017, 18, 273. [Google Scholar] [CrossRef] [PubMed]
- Cowley, M.A.; Smith, R.G.; Diano, S.; Tschöp, M.; Pronchuk, N.; Grove, K.L.; Strasburger, C.J.; Bidlingmaier, M.; Esterman, M.; Heiman, M.L.; et al. The distribution and mechanism of action of ghrelin in the CNS demonstrates a novel hypothalamic circuit regulating energy homeostasis. Neuron 2003, 37, 649–661. [Google Scholar] [CrossRef]
- Hou, Z.; Miao, Y.; Gao, L.; Pan, H.; Zhu, S. Ghrelin-containing neuron in cerebral cortex and hypothalamus linked with the DVC of brainstem in rat. Regul. Pept. 2006, 134, 126–131. [Google Scholar] [CrossRef] [PubMed]
- DiGruccio, M.R.; Mawla, A.M.; Donaldson, C.J.; Noguchi, G.M.; Vaughan, J.; Cowing-Zitron, C.; van der Meulen, T.; Huising, M.O. Comprehensive alpha, beta and delta cell transcriptomes reveal that ghrelin selectively activates delta cells and promotes somatostatin release from pancreatic islets. Mol. Metab. 2016, 5, 449–458. [Google Scholar] [CrossRef] [PubMed]
- Broglio, F.; Arvat, E.; Benso, A.; Gottero, C.; Muccioli, G.; Papotti, M.; van der Lely, A.J.; Deghenghi, R.; Ghigo, E. Ghrelin, a natural GH secretagogue produced by the stomach, induces hyperglycemia and reduces insulin secretion in humans. J. Clin. Endocrinol. Metab. 2001, 86, 5083–5086. [Google Scholar] [CrossRef] [PubMed]
- Nass, R.; Pezzoli, S.S.; Oliveri, M.C.; Patrie, J.T.; Harrell, F.E., Jr.; Clasey, J.L.; Heymsfield, S.B.; Bach, M.A.; Vance, M.L.; Thorner, M.O. Effects of an oral ghrelin mimetic on body composition and clinical outcomes in healthy older adults: A randomized trial. Ann. Intern. Med. 2008, 149, 601–611. [Google Scholar] [CrossRef] [PubMed]
- Neary, N.M.; Small, C.J.; Wren, A.M.; Lee, J.L.; Druce, M.R.; Palmieri, C.; Frost, G.S.; Ghatei, M.A.; Coombes, R.C.; Bloom, S.R. Ghrelin increases energy intake in cancer patients with impaired appetite: Acute, randomized, placebo-controlled trial. J. Clin. Endocrinol. Metab. 2004, 89, 2832–2836. [Google Scholar] [CrossRef] [PubMed]
- Schmid, D.A.; Held, K.; Ising, M.; Uhr, M.; Weikel, J.C.; Steiger, A. Ghrelin stimulates appetite, imagination of food, GH, ACTH, and cortisol, but does not affect leptin in normal controls. Neuropsychopharmacology 2005, 30, 1187–1192. [Google Scholar] [CrossRef] [PubMed]
- Tong, J.; Prigeon, R.L.; Davis, H.W.; Bidlingmaier, M.; Kahn, S.E.; Cummings, D.E.; Tschöp, M.H.; D’Alessio, D. Ghrelin suppresses glucose-stimulated insulin secretion and deteriorates glucose tolerance in healthy humans. Diabetes 2010, 59, 2145–2151. [Google Scholar] [CrossRef] [PubMed]
- Tschöp, M.; Smiley, D.L.; Heiman, M.L. Ghrelin induces adiposity in rodents. Nature 2000, 407, 908–913. [Google Scholar] [CrossRef] [PubMed]
- Rizzo, M.; Rizvi, A.A.; Sudar, E.; Soskic, S.; Obradovic, M.; Montalto, G.; Boutjdir, M.; Mikhailidis, D.P.; Isenovic, E.R. A review of the cardiovascular and anti-atherogenic effects of ghrelin. Curr. Pharm. Des. 2013, 19, 4953–4963. [Google Scholar] [CrossRef] [PubMed]
- Guan, X.M.; Yu, H.; Palyha, O.C.; McKee, K.K.; Feighner, S.D.; Sirinathsinghji, D.J.; Smith, R.G.; van der Ploeg, L.H.; Howard, A.D. Distribution of mRNA encoding the growth hormone secretagogue receptor in brain and peripheral tissues. Brain Res. Mol. Brain Res. 1997, 48, 23–29. [Google Scholar] [CrossRef]
- Dickson, S.L.; Luckman, S.M. Induction of c-fos messenger ribonucleic acid in neuropeptide Y and growth hormone (GH)-releasing factor neurons in the rat arcuate nucleus following systemic injection of the GH secretagogue, GH-releasing peptide-6. Endocrinology 1997, 138, 771–777. [Google Scholar] [CrossRef] [PubMed]
- Kern, A.; Albarran-Zeckler, R.; Walsh, H.E.; Smith, R.G. Apo-ghrelin receptor forms heteromers with DRD2 in hypothalamic neurons and is essential for anorexigenic effects of DRD2 agonism. Neuron 2012, 73, 317–332. [Google Scholar] [CrossRef] [PubMed]
- Willesen, M.G.; Kristensen, P.; Romer, J. Co-localization of growth hormone secretagogue receptor and NPY mRNA in the arcuate nucleus of the rat. Neuroendocrinology 1999, 70, 306–316. [Google Scholar] [CrossRef] [PubMed]
- Gnanapavan, S.; Kola, B.; Bustin, S.A.; Morris, D.G.; McGee, P.; Fairclough, P.; Bhattacharya, S.; Carpenter, R.; Grossman, A.B.; Korbonits, M. The tissue distribution of the mRNA of ghrelin and subtypes of its receptor, GHSR, in humans. J. Clin. Endocrinol. Metab. 2002, 87, 2988. [Google Scholar] [CrossRef] [PubMed]
- Grouselle, D.; Chaillou, E.; Caraty, A.; Bluet-Pajot, M.T.; Zizzari, P.; Tillet, Y.; Epelbaum, J. Pulsatile cerebrospinal fluid and plasma ghrelin in relation to growth hormone secretion and food intake in the sheep. J. Neuroendocrinol. 2008, 20, 1138–1146. [Google Scholar] [CrossRef] [PubMed]
- Holst, B.; Schwartz, T.W. Constitutive ghrelin receptor activity as a signaling set-point in appetite regulation. Trends Pharmacol. Sci. 2004, 25, 113–117. [Google Scholar] [CrossRef] [PubMed]
- Hou, J.; Charron, C.L.; Fowkes, M.M.; Luyt, L.G. Bridging computational modeling with amino acid replacements to investigate GHSR1a-peptidomimetic recognition. Eur. J. Med. Chem. 2016, 123, 822–833. [Google Scholar] [CrossRef] [PubMed]
- M’Kadmi, C.; Leyris, J.P.; Onfroy, L.; Gales, C.; Sauliere, A.; Gagne, D.; Damian, M.; Mary, S.; Maingot, M.; Denoyelle, S.; et al. Agonism, antagonism, and inverse agonism bias at the ghrelin receptor signaling. J. Biol. Chem. 2015, 290, 27021–27039. [Google Scholar] [CrossRef] [PubMed]
- Wettschureck, N.; Moers, A.; Wallenwein, B.; Parlow, A.F.; Maser-Gluth, C.; Offermanns, S. Loss of Gq/11 family G proteins in the nervous system causes pituitary somatotroph hypoplasia and dwarfism in mice. Mol. Cell. Biol. 2005, 25, 1942–1948. [Google Scholar] [CrossRef] [PubMed]
- Schellekens, H.; Dinan, T.G.; Cryan, J.F. Taking two to tango: A role for ghrelin receptor heterodimerization in stress and reward. Front. Neurosci. 2013, 7, 148. [Google Scholar] [CrossRef] [PubMed]
- Jerlhag, E.; Egecioglu, E.; Landgren, S.; Salome, N.; Heilig, M.; Moechars, D.; Datta, R.; Perrissoud, D.; Dickson, S.L.; Engel, J.A. Requirement of central ghrelin signaling for alcohol reward. Proc. Natl. Acad. Sci. USA 2009, 106, 11318–11323. [Google Scholar] [CrossRef] [PubMed]
- Engel, J.A.; Nylander, I.; Jerlhag, E. A ghrelin receptor (GHSR1a) antagonist attenuates the rewarding properties of morphine and increases opioid peptide levels in reward areas in mice. Eur. Neuropsychopharmacol. 2015, 25, 2364–2371. [Google Scholar] [CrossRef] [PubMed]
- Chuang, J.C.; Zigman, J.M. Ghrelin’s roles in stress, mood, and anxiety regulation. Int. J. Pept. 2010, 2010. [Google Scholar] [CrossRef] [PubMed]
- Kunath, N.; Müller, N.C.; Tonon, M.; Konrad, B.N.; Pawlowski, M.; Kopczak, A.; Elbau, I.; Uhr, M.; Kuhn, S.; Repantis, D.; et al. Ghrelin modulates encoding-related brain function without enhancing memory formation in humans. Neuroimage 2016, 142, 465–473. [Google Scholar] [CrossRef] [PubMed]
- Spitznagel, M.B.; Benitez, A.; Updegraff, J.; Potter, V.; Alexander, T.; Glickman, E.; Gunstad, J. Serum ghrelin is inversely associated with cognitive function in a sample of non-demented elderly. Psychiatry Clin. Neurosci. 2010, 64, 608–611. [Google Scholar] [CrossRef] [PubMed]
- Kluge, M.; Schüssler, P.; Bleninger, P.; Kleyer, S.; Uhr, M.; Weikel, J.C.; Yassouridis, A.; Zuber, V.; Steiger, A. Ghrelin alone or co-administered with GHRH or CRH increases non-REM sleep and decreases REM sleep in young males. Psychoneuroendocrinology 2008, 33, 497–506. [Google Scholar] [CrossRef] [PubMed]
- Wittekind, D.A.; Kluge, M. Ghrelin in psychiatric disorders - a review. Psychoneuroendocrinology 2015, 52, 176–194. [Google Scholar] [CrossRef] [PubMed]
- Brust, P.; van den Hoff, J.; Steinbach, J. Development of 18F-labeled radiotracers for neuroreceptor imaging with positron emission tomography. Neurosci. Bull. 2014, 30, 777–811. [Google Scholar] [CrossRef] [PubMed]
- Potter, R.; Horti, A.G.; Ravert, H.T.; Holt, D.P.; Finley, P.; Scheffel, U.; Dannals, R.F.; Wahl, R.L. Synthesis and in vivo evaluation of (S)-6-(4-fluorophenoxy)-3-((1-[11C]methylpiperidin-3-yl)methyl)-2-O-tolylquinazol in-4(3H)-one, a potential PET tracer for growth hormone secretagogue receptor (GHSR). Bioorg. Med. Chem. 2011, 19, 2368–2372. [Google Scholar] [CrossRef] [PubMed]
- Chollet, C.; Bergmann, R.; Pietzsch, J.; Beck-Sickinger, A.G. Design, evaluation, and comparison of ghrelin receptor agonists and inverse agonists as suitable radiotracers for PET imaging. Bioconjug. Chem. 2012, 23, 771–784. [Google Scholar] [CrossRef] [PubMed]
- Koźmiński, P.; Gniazdowska, E. Synthesis and in vitro/in vivo evaluation of novel mono- and trivalent technetium-99m labeled ghrelin peptide complexes as potential diagnostic radiopharmaceuticals. Nucl. Med. Biol. 2015, 42, 28–37. [Google Scholar] [CrossRef] [PubMed]
- Rosita, D.; DeWit, M.A.; Luyt, L.G. Fluorine and rhenium substituted ghrelin analogues as potential imaging probes for the growth hormone secretagogue receptor. J. Med. Chem. 2009, 52, 2196–2203. [Google Scholar] [CrossRef] [PubMed]
- Vodnik, M.; Strukelj, B.; Lunder, M. Ghrelin receptor ligands reaching clinical trials: From peptides to peptidomimetics; from agonists to antagonists. Horm. Metab. Res. 2016, 48, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Schaeffer, M.; Langlet, F.; Lafont, C.; Molino, F.; Hodson, D.J.; Roux, T.; Lamarque, L.; Verdié, P.; Bourrier, E.; Dehouck, B.; et al. Rapid sensing of circulating ghrelin by hypothalamic appetite-modifying neurons. Proc. Natl. Acad. Sci. USA 2013, 110, 1512–1517. [Google Scholar] [CrossRef] [PubMed]
- Cabral, A.; Valdivia, S.; Fernandez, G.; Reynaldo, M.; Perello, M. Divergent neuronal circuitries underlying acute orexigenic effects of peripheral or central ghrelin: Critical role of brain accessibility. J. Neuroendocrinol. 2014, 26, 542–554. [Google Scholar] [CrossRef] [PubMed]
- Cameron, K.O.; Bhattacharya, S.K.; Loomis, A.K. Small molecule ghrelin receptor inverse agonists and antagonists. J. Med. Chem. 2014, 57, 8671–8691. [Google Scholar] [CrossRef] [PubMed]
- Hanrahan, P.; Bell, J.; Bottomley, G.; Bradley, S.; Clarke, P.; Curtis, E.; Davis, S.; Dawson, G.; Horswill, J.; Keily, J.; et al. Substituted azaquinazolinones as modulators of GHSR1a for the treatment of type II diabetes and obesity. Bioorg. Med. Chem. Lett. 2012, 22, 2271–2278. [Google Scholar] [CrossRef] [PubMed]
- Rudolph, J.; Esler, W.P.; O’Connor, S.; Coish, P.D.; Wickens, P.L.; Brands, M.; Bierer, D.E.; Bloomquist, B.T.; Bondar, G.; Chen, L.; et al. Quinazolinone derivatives as orally available ghrelin receptor antagonists for the treatment of diabetes and obesity. J. Med. Chem. 2007, 50, 5202–5216. [Google Scholar] [CrossRef] [PubMed]
- Lipinski, C.A. Drug-like properties and the causes of poor solubility and poor permeability. J. Pharmacol. Toxicol. Methods 2000, 44, 235–249. [Google Scholar] [CrossRef]
- Esler, W.P.; Rudolph, J.; Claus, T.H.; Tang, W.; Barucci, N.; Brown, S.E.; Bullock, W.; Daly, M.; Decarr, L.; Li, Y.; et al. Small-molecule ghrelin receptor antagonists improve glucose tolerance, suppress appetite, and promote weight loss. Endocrinology 2007, 148, 5175–5185. [Google Scholar] [CrossRef] [PubMed]
- Katugampola, S.D.; Pallikaros, Z.; Davenport, A.P. [125I-His9]-ghrelin, a novel radioligand for localizing GHS orphan receptors in human and rat tissue: Up-regulation of receptors with athersclerosis. Br. J. Pharmacol. 2001, 134, 143–149. [Google Scholar] [CrossRef] [PubMed]
- Els, S.; Schild, E.; Petersen, P.S.; Kilian, T.M.; Mokrosinski, J.; Frimurer, T.M.; Chollet, C.; Schwartz, T.W.; Holst, B.; Beck-Sickinger, A.G. An aromatic region to induce a switch between agonism and inverse agonism at the ghrelin receptor. J. Med. Chem. 2012, 55, 7437–7449. [Google Scholar] [CrossRef] [PubMed]
- Els-Heindl, S.; Bellmann-Sickert, K.; Beck-Sickinger, A.G. C-terminus of a hexapeptidic ghrelin receptor inverse agonist can switch peptide behavior from inverse agonism to agonism. Biopolymers 2016, 106, 101–108. [Google Scholar] [CrossRef] [PubMed]
- Valko, K.; Du, C.M.; Bevan, C.D.; Reynolds, D.P.; Abraham, M.H. Rapid-gradient HPLC method for measuring drug interactions with immobilized artificial membrane: Comparison with other lipophilicity measures. J. Pharm. Sci. 2000, 89, 1085–1096. [Google Scholar] [CrossRef]
- Pardridge, W.M. Drug transport across the blood–brain barrier. J. Cereb. Blood Flow Metab. 2012, 32, 1959–1972. [Google Scholar] [CrossRef] [PubMed]
- Auberson, Y.P.; Briard, E.; Sykes, D.; Reilly, J.; Healy, M. Ligand specific efficiency (LSE) index for PET tracer optimization. ChemMedChem 2016, 11, 1415–1427. [Google Scholar] [CrossRef] [PubMed]
- Kong, J.; Chuddy, J.; Stock, I.A.; Loria, P.M.; Straub, S.V.; Vage, C.; Cameron, K.O.; Bhattacharya, S.K.; Lapham, K.; McClure, K.F.; et al. Pharmacological characterization of the first in class clinical candidate PF-05190457: A selective ghrelin receptor competitive antagonist with inverse agonism that increases vagal afferent firing and glucose-dependent insulin secretion ex vivo. Br. J. Pharmacol. 2016, 173, 1452–1464. [Google Scholar] [CrossRef] [PubMed]
- Sivertsen, B.; Holliday, N.; Madsen, A.N.; Holst, B. Functionally biased signalling properties of 7TM receptors - opportunities for drug development for the ghrelin receptor. Br. J. Pharmacol. 2013, 170, 1349–1362. [Google Scholar] [CrossRef] [PubMed]
- Perdona, E.; Faggioni, F.; Buson, A.; Sabbatini, F.M.; Corti, C.; Corsi, M. Pharmacological characterization of the ghrelin receptor antagonist, GSK1614343 in rat RC-4b/c cells natively expressing GHS type 1a receptors. Eur. J. Pharmacol. 2011, 650, 178–183. [Google Scholar] [CrossRef] [PubMed]
- Sabbatini, F.M.; Di Fabio, R.; Corsi, M.; Cavanni, P.; Bromidge, S.M.; St-Denis, Y.; D’Adamo, L.; Contini, S.; Rinaldi, M.; Guery, S.; et al. Discovery process and characterization of novel carbohydrazide derivatives as potent and selective GHSR1a antagonists. Sigma2 2010, 5, 1450–1455. [Google Scholar] [CrossRef] [PubMed]
- Kostelnik, K.B.; Els-Heindl, S.; Kloting, N.; Baumann, S.; von Bergen, M.; Beck-Sickinger, A.G. High metabolic in vivo stability and bioavailability of a palmitoylated ghrelin receptor ligand assessed by mass spectrometry. Bioorg. Med. Chem. 2015, 23, 3925–3932. [Google Scholar] [CrossRef] [PubMed]



 | ||||||
|---|---|---|---|---|---|---|
| Compound | R1 | R2 | R3 | MW | IC50 [nM] | pIC50 1 |
| Ghrelin | 1.7 | 8.78 ± 0.04 | ||||
| (S)-9 | F |  | Me | 489 | 2.2 | 8.66 ± 0.05 |
| (R)-9 | F |  | Me | 489 | 3.9 | 8.41 ± 0.05 |
| (S)-10 | F |  | Me | 493 | 69 | 7.16 ± 0.11 |
| (S)-11 | F |  | Me | 507 | 66 | 7.18 ± 0.09 |
| (S)-12 | F |  | Me | 521 | 574 | 6.24 ± 0.12 |
| (S)-13 | F |  | Me | 507 | 13 | 7.90 ± 0.13 |
| (S)-14 | H |  | Me | 471 | 12 | 7.91 ± 0.10 |
| (S)-16 | F |  | H | 475 | 2.7 | 8.57 ± 0.06 |
| (S)-17 | F |  | F | 493 | 151 | 6.82 ± 0.09 |
| Compound | EC50 (nM) | pEC50 1 | Emax 1 (%) 2 | n 3 |
|---|---|---|---|---|
| (S)-9 | 0.7 | 9.17 ± 0.19 | 63 ± 6 | ≥3 |
| (R)-9 | 0.6 | 9.26 ± 0.21 | 59 ± 6 | ≥3 |
| (S)-10 | 1.6 | 8.80 ± 0.14 | 64 ± 5 | ≥3 |
| (S)-11 | 2.9 | 8.54 ± 0.15 | 58 ± 4 | ≥3 |
| (S)-13 | 1.4 | 8.87 ± 0.13 | 57 ± 4 | ≥3 |
| (S)-14 | 2.1 | 8.67 ± 0.20 | 42 ± 4 | ≥3 |
| (S)-16 | 1.0 | 8.99 ± 0.20 | 40 ± 4 | ≥3 |
| Compound | cLogD7.4 1 | CHI IAM |
|---|---|---|
| (S)-9 | 2.1 | 43.0 |
| (R)-9 | 2.1 | 42.8 |
| (S)-10 | 3.4 | 39.6 |
| (S)-11 | 3.1 | 42.2 |
| (S)-12 | 3.0 | 43.3 |
| (S)-13 | 3.1 | 42.8 |
| (S)-14 | 2.4 | 42.8 |
| (S)-16 | 2.9 | 42.3 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Moldovan, R.-P.; Els-Heindl, S.; Worm, D.J.; Kniess, T.; Kluge, M.; Beck-Sickinger, A.G.; Deuther-Conrad, W.; Krügel, U.; Brust, P. Development of Fluorinated Non-Peptidic Ghrelin Receptor Ligands for Potential Use in Molecular Imaging. Int. J. Mol. Sci. 2017, 18, 768. https://doi.org/10.3390/ijms18040768
Moldovan R-P, Els-Heindl S, Worm DJ, Kniess T, Kluge M, Beck-Sickinger AG, Deuther-Conrad W, Krügel U, Brust P. Development of Fluorinated Non-Peptidic Ghrelin Receptor Ligands for Potential Use in Molecular Imaging. International Journal of Molecular Sciences. 2017; 18(4):768. https://doi.org/10.3390/ijms18040768
Chicago/Turabian StyleMoldovan, Rareş-Petru, Sylvia Els-Heindl, Dennis J. Worm, Torsten Kniess, Michael Kluge, Annette G. Beck-Sickinger, Winnie Deuther-Conrad, Ute Krügel, and Peter Brust. 2017. "Development of Fluorinated Non-Peptidic Ghrelin Receptor Ligands for Potential Use in Molecular Imaging" International Journal of Molecular Sciences 18, no. 4: 768. https://doi.org/10.3390/ijms18040768
APA StyleMoldovan, R.-P., Els-Heindl, S., Worm, D. J., Kniess, T., Kluge, M., Beck-Sickinger, A. G., Deuther-Conrad, W., Krügel, U., & Brust, P. (2017). Development of Fluorinated Non-Peptidic Ghrelin Receptor Ligands for Potential Use in Molecular Imaging. International Journal of Molecular Sciences, 18(4), 768. https://doi.org/10.3390/ijms18040768