Advances in Skin Regeneration Using Tissue Engineering
Abstract
:1. Introduction
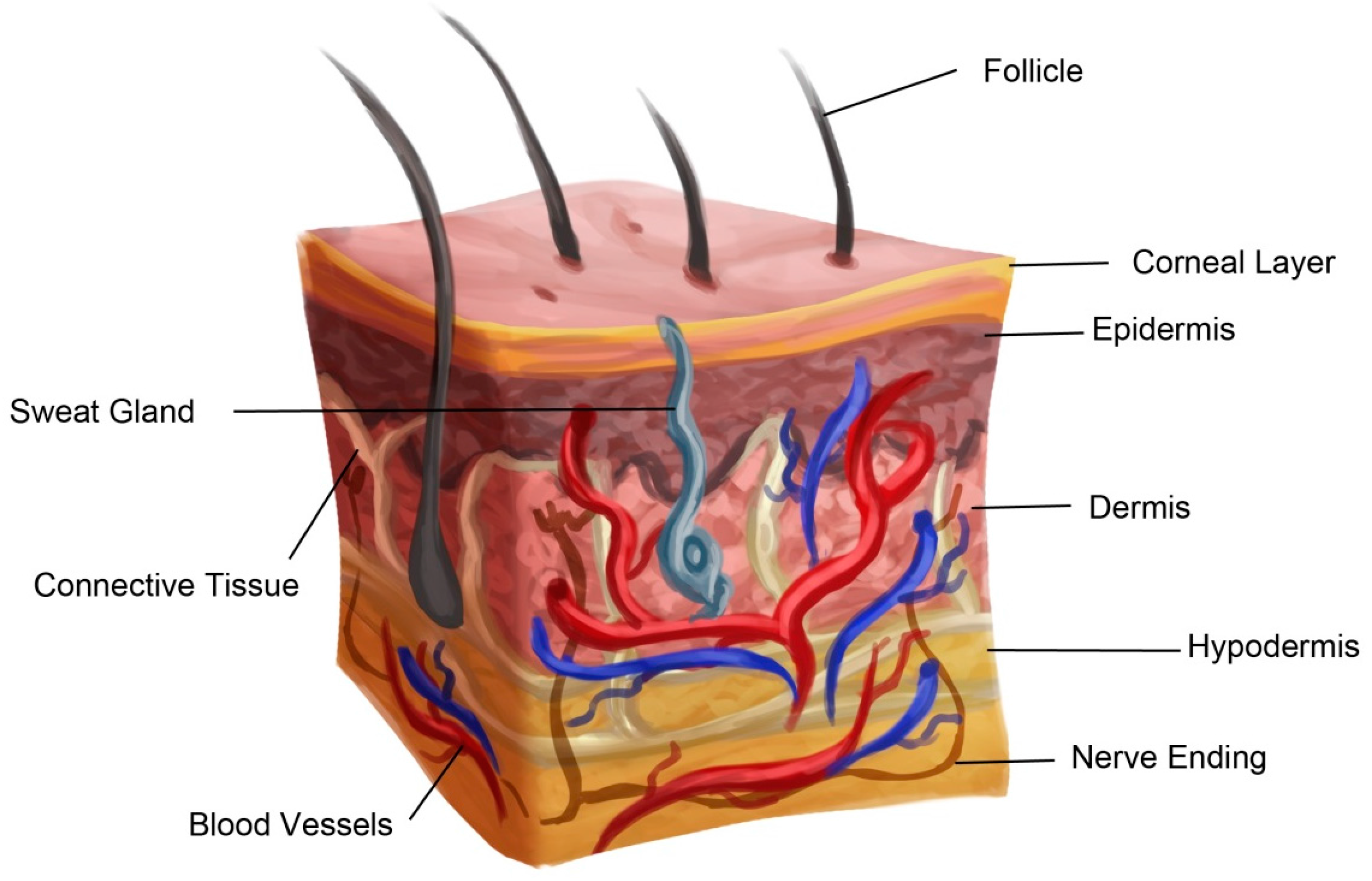
2. Anatomy of Skin
3. Wounds
4. Process of Skin Wound Healing
5. Conventional Treatments for Wound Healing
5.1. Skin Grafting with Autograft
5.2. Skin Allografts
5.3. Xenografts
5.4. Amnion
6. Newer Approaches for Tissue Engineering
6.1. Cell Cocultures
6.2. Cultured Epithelial Autografts
6.3. Tissue Engineered Skin Substitutes
7. Types of Skin Substitutes
7.1. Acellular Skin Substitutes
7.2. Cellular Allogenic Skin Substitutes
7.3. Cellular Autologous Skin Substitutes
7.4. Commercially Available Skin Substitutes
8. Limitations of Commercially Available Skin Substitutes
9. Future Perspectives
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Herndon, D.N.; Barrow, R.E.; Rutan, R.L.; Rutan, T.C.; Desai, M.H.; Abston, S. A comparison of conservative versus early excision. Therapies in severely burned patients. Ann. Surg. 1989, 209, 547–552. [Google Scholar] [CrossRef] [PubMed]
- Rheinwald, J.G. Human epidermal keratinocyte cell culture and xenograft systems: Applications in the detection of potential chemical carcinogens and the study of epidermal transformation. Prog. Clin. Biol. Res. 1989, 298, 113–125. [Google Scholar] [PubMed]
- Alonso, L.; Fuchs, E. Stem cells of the skin epithelium. Proc. Natl. Acad. Sci. USA 2003, 100, 11830–11835. [Google Scholar] [CrossRef] [PubMed]
- Clark, R.A.; Ghosh, K.; Tonnesen, M.G. Tissue engineering for cutaneous wounds. J. Investig. Dermatol. 2007, 127, 1018–1029. [Google Scholar] [CrossRef] [PubMed]
- De Mel, A.; Seifalian, A.M.; Birchall, M.A. Orchestrating cell/material interactions for tissue engineering of surgical implants. Macromol. Biosci. 2012, 12, 1010–1021. [Google Scholar] [CrossRef] [PubMed]
- Harvey, C. Wound healing. Orthop. Nurs. 2005, 24, 143–157. [Google Scholar] [CrossRef] [PubMed]
- Boyce, S.T.; Warden, G.D. Principles and practices for treatment of cutaneous wounds with cultured skin substitutes. Am. J. Surg. 2002, 183, 445–456. [Google Scholar] [CrossRef]
- Phillips, T.J. Current approaches to venous ulcers and compression. Dermatol. Surg. 2001, 27, 611–621. [Google Scholar] [PubMed]
- Braddock, M.; Campbell, C.J.; Zuder, D. Current therapies for wound healing: Electrical stimulation, biological therapeutics, and the potential for gene therapy. Int. J. Dermatol. 1999, 38, 808–817. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Chen, L.; Scott, P.G.; Tredget, E.E. Mesenchymal stem cells enhance wound healing through differentiation and angiogenesis. Stem Cells 2007, 25, 2648–2659. [Google Scholar] [CrossRef] [PubMed]
- Atiyeh, B.S.; Ioannovich, J.; Al-Amm, C.A.; El-Musa, K.A. Management of acute and chronic open wounds: The importance of moist environment in optimal wound healing. Curr. Pharm. Biotechnol. 2002, 3, 179–195. [Google Scholar] [CrossRef] [PubMed]
- Cole-King, A.; Harding, K.G. Psychological factors and delayed healing in chronic wounds. Psychosom. Med. 2001, 63, 216–220. [Google Scholar] [CrossRef] [PubMed]
- Walburn, J.; Vedhara, K.; Hankins, M.; Rixon, L.; Weinman, J. Psychological stress and wound healing in humans: A systematic review and meta-analysis. J. Psychosom. Res. 2009, 67, 253–271. [Google Scholar] [CrossRef] [PubMed]
- Rhett, J.M.; Ghatnekar, G.S.; Palatinus, J.A.; O’Quinn, M.; Yost, M.J.; Gourdie, R.G. Novel therapies for scar reduction and regenerative healing of skin wounds. Trends Biotechnol. 2008, 26, 173–180. [Google Scholar] [CrossRef] [PubMed]
- Janeway, C.A., Jr.; Walport, M.; Travers, P.; Shlomchik, M.J. Immunobiology: The Immune System in Health and Disease, 5th ed.; Garland Science: New York, NY, USA, 2001. [Google Scholar]
- Davis, J.S. Address of the president: The story of plastic surgery. Ann. Surg. 1941, 113, 641–656. [Google Scholar] [CrossRef] [PubMed]
- Akan, M.; Yildirim, S.; Misirlioglu, A.; Ulusoy, G.; Akoz, T.; Avci, G. An alternative method to minimize pain in the split-thickness skin graft donor site. Plast. Reconstr. Surg. 2003, 111, 2243–2249. [Google Scholar] [CrossRef] [PubMed]
- Bello, Y.M.; Falabella, A.F.; Eaglstein, W.H. Tissue-engineered skin. Current status in wound healing. Am. J. Clin. Dermatol. 2001, 2, 305–313. [Google Scholar] [CrossRef] [PubMed]
- Halim, A.S.; Khoo, T.L.; Mohd Yussof, S.J. Biologic and synthetic skin substitutes: An overview. Indian J. Plast. Surg. 2010, 43, 23–28. [Google Scholar] [CrossRef] [PubMed]
- Cardinal, M.; Eisenbud, D.E.; Armstrong, D.G.; Zelen, C.; Driver, V.; Attinger, C.; Phillips, T.; Harding, K. Serial surgical debridement: A retrospective study on clinical outcomes in chronic lower extremity wounds. Wound Repair Regen. 2009, 17, 306–311. [Google Scholar] [CrossRef] [PubMed]
- Rockwell, W.B.; Daane, S.; Zakhireh, M.; Carroll, K.L. Human skin allograft used to treat open wounds after club foot release. Ann. Plast. Surg. 2003, 51, 593–597. [Google Scholar] [CrossRef] [PubMed]
- Nathoo, R.; Howe, N.; Cohen, G. Skin substitutes: An overview of the key players in wound management. J. Clin. Aesthet. Dermatol. 2014, 7, 44–48. [Google Scholar] [PubMed]
- Shores, J.T.; Gabriel, A.; Gupta, S. Skin substitutes and alternatives: A review. Adv. Skin Wound Care 2007, 20, 493–508. [Google Scholar] [CrossRef] [PubMed]
- Bujang-Safawi, E.; Halim, A.S.; Khoo, T.L.; Dorai, A.A. Dried irradiated human amniotic membrane as a biological dressing for facial burns—A 7-year case series. Burns 2010, 36, 876–882. [Google Scholar] [CrossRef] [PubMed]
- Eskandarlou, M.; Azimi, M.; Rabiee, S.; Seif Rabiee, M.A. The healing effect of amniotic membrane in burn patients. World J. Plast. Surg. 2016, 5, 39–44. [Google Scholar] [PubMed]
- Fetterolf, D.E.; Snyder, R.J. Scientific and clinical support for the use of dehydrated amniotic membrane in wound management. Wounds Compend. Clin. Res. Pract. 2012, 24, 299–307. [Google Scholar]
- Thangapazham, R.L.; Darling, T.N.; Meyerle, J. Alteration of skin properties with autologous dermal fibroblasts. Int. J. Mol. Sci. 2014, 15, 8407–8427. [Google Scholar] [CrossRef] [PubMed]
- Li, A.; Pouliot, N.; Redvers, R.; Kaur, P. Extensive tissue-regenerative capacity of neonatal human keratinocyte stem cells and their progeny. J. Clin. Investig. 2004, 113, 390–400. [Google Scholar] [CrossRef] [PubMed]
- Fortunel, N.O.; Vaigot, P.; Cadio, E.; Martin, M.T. Functional investigations of keratinocyte stem cells and progenitors at a single-cell level using multiparallel clonal microcultures. Methods Mol. Biol. 2010, 585, 13–23. [Google Scholar] [PubMed]
- Dong, R.; Liu, X.; Liu, Y.; Deng, Z.; Nie, X.; Wang, X.; Jin, Y. Enrichment of epidermal stem cells by rapid adherence and analysis of the reciprocal interaction of epidermal stem cells with neighboring cells using an organotypic system. Cell Biol. Int. 2007, 31, 733–740. [Google Scholar] [CrossRef] [PubMed]
- King, A.; Balaji, S.; Keswani, S.G.; Crombleholme, T.M. The role of stem cells in wound angiogenesis. Adv. Wound Care 2014, 3, 614–625. [Google Scholar] [CrossRef] [PubMed]
- Dabiri, G.; Heiner, D.; Falanga, V. The emerging use of bone marrow-derived mesenchymal stem cells in the treatment of human chronic wounds. Expert Opin. Emerg. Drugs 2013, 18, 405–419. [Google Scholar] [CrossRef] [PubMed]
- Jackson, W.M.; Nesti, L.J.; Tuan, R.S. Concise review: Clinical translation of wound healing therapies based on mesenchymal stem cells. Stem Cells Transl. Med. 2012, 1, 44–50. [Google Scholar] [CrossRef] [PubMed]
- Nayak, S.; Dey, S.; Kundu, S.C. Skin equivalent tissue-engineered construct: Co-cultured fibroblasts/keratinocytes on 3d matrices of sericin hope cocoons. PLoS ONE 2013, 8, e74779. [Google Scholar] [CrossRef] [PubMed]
- Zuliani, T.; Saiagh, S.; Knol, A.C.; Esbelin, J.; Dreno, B. Fetal fibroblasts and keratinocytes with immunosuppressive properties for allogeneic cell-based wound therapy. PLoS ONE 2013, 8, e70408. [Google Scholar] [CrossRef] [PubMed]
- Dragunova, J.; Kabat, P.; Koller, J. Skin explant cultures as a source of keratinocytes for cultivation. Cell Tissue Bank. 2013, 14, 317–324. [Google Scholar] [CrossRef] [PubMed]
- Guerra, L.; Dellambra, E.; Panacchia, L.; Paionni, E. Tissue engineering for damaged surface and lining epithelia: Stem cells, current clinical applications, and available engineered tissues. Tissue Eng. Part B Rev. 2009, 15, 91–112. [Google Scholar] [CrossRef] [PubMed]
- Boehnke, K.; Mirancea, N.; Pavesio, A.; Fusenig, N.E.; Boukamp, P.; Stark, H.J. Effects of fibroblasts and microenvironment on epidermal regeneration and tissue function in long-term skin equivalents. Eur. J. Cell Biol. 2007, 86, 731–746. [Google Scholar] [CrossRef] [PubMed]
- Sun, T.; Jackson, S.; Haycock, J.W.; MacNeil, S. Culture of skin cells in 3D rather than 2D improves their ability to survive exposure to cytotoxic agents. J. Biotechnol. 2006, 122, 372–381. [Google Scholar] [CrossRef] [PubMed]
- Hill, D.S.; Robinson, N.D.; Caley, M.P.; Chen, M.; O’Toole, E.A.; Armstrong, J.L.; Przyborski, S.; Lovat, P.E. A novel fully humanized 3D skin equivalent to model early melanoma invasion. Mol. Cancer Ther. 2015, 14, 2665–2673. [Google Scholar] [CrossRef] [PubMed]
- Gallico, G.G.; O’Connor, N.E.; Compton, C.C.; Kehinde, O.; Green, H. Permanent coverage of large burn wounds with autologous cultured human epithelium. New Engl. J. Med. 1984, 311, 448–451. [Google Scholar] [CrossRef] [PubMed]
- O’Connor, N.E.; Mulliken, J.; Banks-Schlegel, S.; Green, H. Grafting of burns with cultured epithelium prepared from autologous epidermal cells. Lancet 1981, 317, 75–78. [Google Scholar] [CrossRef]
- Rheinwald, J.G.; Green, H. Serial cultivation of strains of human epidermal keratinocytes: The formation of keratinizing colonies from single cells. Cell 1975, 6, 331–343. [Google Scholar] [CrossRef]
- Rheinwald, J.G.; Green, H. Epidermal growth factor and the multiplication of cultured human epidermal keratinocytes. Nature 1977, 265, 421–424. [Google Scholar] [CrossRef] [PubMed]
- Leigh, I.M.; Watt, F.M. The culture of human epidermal keratinocytes. In Keratinocyte Handbook; Cambridge University Press: Cambridge, UK, 1994; pp. 43–51. [Google Scholar]
- Supp, D.M.; Boyce, S.T. Engineered skin substitutes: Practices and potentials. Clin. Dermatol. 2005, 23, 403–412. [Google Scholar] [CrossRef] [PubMed]
- Kroner, E.; Kaiser, J.S.; Fischer, S.C.; Arzt, E. Bioinspired polymeric surface patterns for medical applications. J. Appl. Biomater. Funct. Mater. 2012, 10, 287–292. [Google Scholar] [CrossRef] [PubMed]
- Ho, W.S. Skin substitutes: An overview. Ann. R. Coll. Surg. 2002, 6, 102–108. [Google Scholar] [CrossRef]
- Horch, R.E.; Kopp, J.; Kneser, U.; Beier, J.; Bach, A.D. Tissue engineering of cultured skin substitutes. J. Cell. Mol. Med. 2005, 9, 592–608. [Google Scholar] [CrossRef] [PubMed]
- Leigh, I.M.; Watt, F.M. Keratinocyte Methods; Cambridge University Press: Cambridge, UK, 1994. [Google Scholar]
- MacNeil, S. Progress and opportunities for tissue-engineered skin. Nature 2007, 445, 874–880. [Google Scholar] [CrossRef] [PubMed]
- Metcalfe, A.D.; Ferguson, M.W. Tissue engineering of replacement skin: The crossroads of biomaterials, wound healing, embryonic development, stem cells and regeneration. J. R. Soc. Interface 2007, 4, 413–437. [Google Scholar] [CrossRef] [PubMed]
- Boyce, S.T.; Kagan, R.J.; Meyer, N.A.; Yakuboff, K.P.; Warden, G.D. The 1999 clinical research award. Cultured skin substitutes combined with integra artificial skin to replace native skin autograft and allograft for the closure of excised full-thickness burns. J. Burn Care Rehabil. 1999, 20, 453–461. [Google Scholar] [CrossRef] [PubMed]
- Passaretti, D.; Billmire, D.; Kagan, R.; Corcoran, J.; Boyce, S. Autologous cultured skin substitutes conserve donor autograft in elective treatment of congenital giant melanocytic nevus. Plast. Reconstr. Surg. 2004, 114, 1523–1528. [Google Scholar] [CrossRef] [PubMed]
- Shevchenko, R.V.; James, S.L.; James, S.E. A review of tissue-engineered skin bioconstructs available for skin reconstruction. J. R. Soc. Interface 2010, 7, 229–258. [Google Scholar] [CrossRef] [PubMed]
- Atiyeh, B.S.; Costagliola, M. Cultured epithelial autograft (CEA) in burn treatment: Three decades later. Burns 2007, 33, 405–413. [Google Scholar] [CrossRef] [PubMed]
- Patel, M.; Fisher, J.P. Biomaterial scaffolds in pediatric tissue engineering. Pediatr. Res. 2008, 63, 497–501. [Google Scholar] [CrossRef] [PubMed]
- Shakespeare, P.; Shakespeare, V. Survey: Use of skin substitute materials in UK burn treatment centres. Burns 2002, 28, 295–297. [Google Scholar] [CrossRef]
- Shakespeare, P.G. The role of skin substitutes in the treatment of burn injuries. Clin. Dermatol. 2005, 23, 413–418. [Google Scholar] [CrossRef] [PubMed]
- Melkun, E.T.; Few, J.W. The use of biosynthetic skin substitute (Biobrane) for axillary reconstruction after surgical excision for hidradenitis suppurativa. Plast. Reconstr. Surg. 2005, 115, 1385–1388. [Google Scholar] [CrossRef] [PubMed]
- Curran, M.P.; Plosker, G.L. Bilayered bioengineered skin substitute (Apligraf): A review of its use in the treatment of venous leg ulcers and diabetic foot ulcers. BioDrugs 2002, 16, 439–455. [Google Scholar] [CrossRef] [PubMed]
- Price, R.D.; Myers, S.; Leigh, I.M.; Navsaria, H.A. The role of hyaluronic acid in wound healing: Assessment of clinical evidence. Am. J. Clin. Dermatol. 2005, 6, 393–402. [Google Scholar] [CrossRef] [PubMed]
- Hellman, K.B. Engineered tissues: The regulatory path from concept to market. Adv. Exp. Med. Biol. 2006, 585, 363–376. [Google Scholar] [PubMed]
- Alrubaiy, L.; Al-Rubaiy, K.K. Skin substitutes: A brief review of types and clinical applications. Oman Méd. J. 2009, 24, 4–6. [Google Scholar] [CrossRef] [PubMed]
- Gordley, K.; Cole, P.; Hicks, J.; Hollier, L. A comparative, long term assessment of soft tissue substitutes: Alloderm, enduragen, and dermamatrix. JPRAS 2009, 62, 849–850. [Google Scholar] [CrossRef] [PubMed]
- Hodde, J.P.; Ernst, D.M.; Hiles, M.C. An investigation of the long-term bioactivity of endogenous growth factor in oasis wound matrix. J. Wound Care 2005, 14, 23–25. [Google Scholar] [CrossRef] [PubMed]
- Tausche, A.K.; Skaria, M.; Bohlen, L.; Liebold, K.; Hafner, J.; Friedlein, H.; Meurer, M.; Goedkoop, R.J.; Wollina, U.; Salomon, D.; et al. An autologous epidermal equivalent tissue-engineered from follicular outer root sheath keratinocytes is as effective as split-thickness skin autograft in recalcitrant vascular leg ulcers. Wound Repair Regen. 2003, 11, 248–252. [Google Scholar] [CrossRef] [PubMed]
- Haddow, D.B.; Steele, D.A.; Short, R.D.; Dawson, R.A.; Macneil, S. Plasma-polymerized surfaces for culture of human keratinocytes and transfer of cells to an in vitro wound-bed model. J. Biomed. Mater. Res. Part A 2003, 64, 80–87. [Google Scholar] [CrossRef] [PubMed]
- Hüsing, B.; Bührlen, B.; Gaisser, S. Human tissue engineered products: Today’s markets and future prospects. Fraunhofer Inst. Syst. Innov. Res. 2003, 1, 54. [Google Scholar]
- Goedkoop, R.; Juliet, R.; You, P.H.; Daroczy, J.; de Roos, K.P.; Lijnen, R.; Rolland, E.; Hunziker, T. Wound stimulation by growth-arrested human keratinocytes and fibroblasts: Hp802-247, a new-generation allogeneic tissue engineering product. Dermatology 2010, 220, 114–120. [Google Scholar] [CrossRef] [PubMed]
- Kirsner, R.S.; Marston, W.A.; Snyder, R.J.; Lee, T.D.; Cargill, D.I.; Slade, H.B. Spray-applied cell therapy with human allogeneic fibroblasts and keratinocytes for the treatment of chronic venous leg ulcers: A phase 2, multicentre, double-blind, randomised, placebo-controlled trial. Lancet 2012, 380, 977–985. [Google Scholar] [CrossRef]
- Magnusson, M.; Papini, R.P.; Rea, S.M.; Reed, C.C.; Wood, F.M. Cultured autologous keratinocytes in suspension accelerate epithelial maturation in an in vivo wound model as measured by surface electrical capacitance. Plast. Reconstr. Surg. 2007, 119, 495–499. [Google Scholar] [CrossRef] [PubMed]
- Carsin, H.; Ainaud, P.; Le Bever, H.; Rives, J.; Lakhel, A.; Stephanazzi, J.; Lambert, F.; Perrot, J. Cultured epithelial autografts in extensive burn coverage of severely traumatized patients: A five year single-center experience with 30 patients. Burns 2000, 26, 379–387. [Google Scholar] [CrossRef]
- Caravaggi, C.; de Giglio, R.; Pritelli, C.; Sommaria, M.; Dalla Noce, S.; Faglia, E.; Mantero, M.; Clerici, G.; Fratino, P.; Dalla Paola, L.; et al. Hyaff 11-based autologous dermal and epidermal grafts in the treatment of noninfected diabetic plantar and dorsal foot ulcers: A prospective, multicenter, controlled, randomized clinical trial. Diabetes Care 2003, 26, 2853–2859. [Google Scholar] [CrossRef] [PubMed]
- Dietz, J.; Lundgren, P.; Veeramani, A.; O’Rourke, C.; Bernard, S.; Djohan, R.; Larson, J.; Isakov, R.; Yetman, R. Autologous inferior dermal sling (Autoderm) with concomitant skin-envelope reduction mastectomy: An excellent surgical choice for women with macromastia and clinically significant ptosis. Ann. Surg. Oncol. 2012, 19, 3282–3288. [Google Scholar] [CrossRef] [PubMed]
- Harding, K.G.; Krieg, T.; Eming, S.A.; Flour, M.L.; Jawien, A.; Cencora, A.; Kaszuba, A.; Noszcyk, W.; Willems, P.; de Deene, A.; et al. Efficacy and safety of the freeze-dried cultured human keratinocyte lysate, lyphoderm 0.9%, in the treatment of hard-to-heal venous leg ulcers. Wound Repair Regen. 2005, 13, 138–147. [Google Scholar] [CrossRef] [PubMed]
- Paquet, P.; Quatresooz, P.; Braham, C.; Pierard, G.E. Tapping into the influence of keratinocyte allografts and biocenosis on healing of chronic leg ulcers: Split-ulcer controlled pilot study. Dermatol. Surg. 2005, 31, 431–435. [Google Scholar] [CrossRef] [PubMed]
- Varkey, M.; Ding, J.; Tredget, E.E. Advances in skin substitutes-potential of tissue engineered skin for facilitating anti-fibrotic healing. J. Funct. Biomater. 2015, 6, 547–563. [Google Scholar] [CrossRef] [PubMed]
- Limova, M. Active wound coverings: Bioengineered skin and dermal substitutes. Surg. Clin. N. Am. 2010, 90, 1237–1255. [Google Scholar] [CrossRef] [PubMed]
- Uccioli, L. A clinical investigation on the characteristics and outcomes of treating chronic lower extremity wounds using the tissuetech autograft system. Int. J. Low. Extremity Wounds 2003, 2, 140–151. [Google Scholar] [CrossRef] [PubMed]
- Noordenbos, J.; Dore, C.; Hansbrough, J.F. Safety and efficacy of transcyte for the treatment of partial-thickness burns. J. Burn Care Rehabil. 1999, 20, 275–281. [Google Scholar] [CrossRef] [PubMed]
- Groeber, F.; Holeiter, M.; Hampel, M.; Hinderer, S.; Schenke-Layland, K. Skin tissue engineering—In vivo and in vitro applications. Adv. Drug Deliv. Rev. 2011, 63, 352–366. [Google Scholar] [CrossRef] [PubMed]
- Ng, K.W.; Hutmacher, D.W. Reduced contraction of skin equivalent engineered using cell sheets cultured in 3D matrices. Biomaterials 2006, 27, 4591–4598. [Google Scholar] [CrossRef] [PubMed]
- Myers, S.R.; Partha, V.N.; Soranzo, C.; Price, R.D.; Navsaria, H.A. Hyalomatrix: A temporary epidermal barrier, hyaluronan delivery, and neodermis induction system for keratinocyte stem cell therapy. Tissue Eng. 2007, 13, 2733–2741. [Google Scholar] [CrossRef] [PubMed]
- Cullen, B. The role of oxidized regenerated cellulose/collagen in chronic wound repair. Part 2. Ostomy Wound Manag. 2002, 48, 8–13. [Google Scholar]
- Stark, H.J.; Boehnke, K.; Mirancea, N.; Willhauck, M.J.; Pavesio, A.; Fusenig, N.E.; Boukamp, P. Epidermal homeostasis in long-term scaffold-enforced skin equivalents. J. Investig. Dermatol. Symp. Proc. 2006, 11, 93–105. [Google Scholar] [CrossRef] [PubMed]
- Ackermann, K.; Borgia, S.L.; Korting, H.C.; Mewes, K.R.; Schafer-Korting, M. The phenion full-thickness skin model for percutaneous absorption testing. Skin Pharmacol. Physiol. 2010, 23, 105–112. [Google Scholar] [CrossRef] [PubMed]
- Still, J.; Glat, P.; Silverstein, P.; Griswold, J.; Mozingo, D. The use of a collagen sponge/living cell composite material to treat donor sites in burn patients. Burns 2003, 29, 837–841. [Google Scholar] [CrossRef]
- Lindberg, K.; Badylak, S.F. Porcine small intestinal submucosa (SIS): A bioscaffold supporting in vitro primary human epidermal cell differentiation and synthesis of basement membrane proteins. Burns 2001, 27, 254–266. [Google Scholar] [CrossRef]
- Rockwood, D.N.; Woodhouse, K.A.; Fromstein, J.D.; Chase, D.B.; Rabolt, J.F. Characterization of biodegradable polyurethane microfibers for tissue engineering. J. Biomater. Sci. Polym. 2007, 18, 743–758. [Google Scholar] [CrossRef] [PubMed]
- Van Dorp, A.G.; Verhoeven, M.C.; Koerten, H.K.; van Blitterswijk, C.A.; Ponec, M. Bilayered biodegradable poly (ethylene glycol)/poly (butylene terephthalate) copolymer (polyactive) as substrate for human fibroblasts and keratinocytes. J. Biomed. Mater. Res. 1999, 47, 292–300. [Google Scholar] [CrossRef]
- Dodson, B.P.; Levine, A.D. Challenges in the translation and commercialization of cell therapies. BMC Biotechnol. 2015, 15, 70. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, M.C.; Paggiaro, A.O.; Isaac, C.; Teixeira Neto, N.; dos Santos, G.B. Skin substitutes: Current concepts and a new classification system. Rev. Bras. Cir. Plást. 2011, 26, 696–702. [Google Scholar] [CrossRef]
- Centanni, J.M.; Straseski, J.A.; Wicks, A.; Hank, J.A.; Rasmussen, C.A.; Lokuta, M.A.; Schurr, M.J.; Foster, K.N.; Faucher, L.D.; Caruso, D.M.; et al. Stratagraft skin substitute is well-tolerated and is not acutely immunogenic in patients with traumatic wounds: Results from a prospective, randomized, controlled dose escalation trial. Ann. Surg. 2011, 253, 672–683. [Google Scholar] [CrossRef] [PubMed]
- Schurr, M.J.; Foster, K.N.; Centanni, J.M.; Comer, A.R.; Wicks, A.; Gibson, A.L.; Thomas-Virnig, C.L.; Schlosser, S.J.; Faucher, L.D.; Lokuta, M.A.; et al. Phase I/II clinical evaluation of stratagraft: A consistent, pathogen-free human skin substitute. J. Trauma 2009, 66, 866–873. [Google Scholar] [CrossRef] [PubMed]
- Supp, D.M.; Karpinski, A.C.; Boyce, S.T. Expression of human β-defensins HBD-1, HBD-2, and HBD-3 in cultured keratinocytes and skin substitutes. Burns 2004, 30, 643–648. [Google Scholar] [CrossRef] [PubMed]
- Cheah, A.K.W.; Chong, S.J.; Tan, B.K. Early experience with biobrane™ in singapore in the management of partial thickness burns. Proc. Singap. Healthc. 2014, 23, 196–200. [Google Scholar] [CrossRef]
- Chua, A.W.; Khoo, Y.C.; Tan, B.K.; Tan, K.C.; Foo, C.L.; Chong, S.J. Skin tissue engineering advances in severe burns: Review and therapeutic applications. Burns Trauma 2016, 4, 3. [Google Scholar] [CrossRef] [PubMed]
- Greenwood, J.E.; Clausen, J.; Kavanagh, S. Experience with biobrane: Uses and caveats for success. Eplasty 2009, 9, e25. [Google Scholar] [PubMed]
- Tan, H.; Wasiak, J.; Paul, E.; Cleland, H. Effective use of biobrane as a temporary wound dressing prior to definitive split-skin graft in the treatment of severe burn: A retrospective analysis. Burns 2015, 41, 969–976. [Google Scholar] [CrossRef] [PubMed]
- Woodroof, E.A. The search for an ideal temporary skin substitute: Awbat. Eplasty 2009, 9, e10. [Google Scholar] [PubMed]
- Conconi, M.T.; de Coppi, P.; di Liddo, R.; Vigolo, S.; Zanon, G.F.; Parnigotto, P.P.; Nussdorfer, G.G. Tracheal matrices, obtained by a detergent-enzymatic method, support in vitro the adhesion of chondrocytes and tracheal epithelial cells. Transplant Int. 2005, 18, 727–734. [Google Scholar] [CrossRef] [PubMed]
- Shahrokhi, S.; Arno, A.; Jeschke, M.G. The use of dermal substitutes in burn surgery: Acute phase. Wound Repair Regen. 2014, 22, 14–22. [Google Scholar] [CrossRef] [PubMed]
- Dickinson, L.E.; Gerecht, S. Engineered biopolymeric scaffolds for chronic wound healing. Front. Physiol. 2016, 7, 341. [Google Scholar] [CrossRef] [PubMed]
- Lev-Tov, H.; Li, C.S.; Dahle, S.; Isseroff, R.R. Cellular versus acellular matrix devices in treatment of diabetic foot ulcers: Study protocol for a comparative efficacy randomized controlled trial. Trials 2013, 14, 8. [Google Scholar] [CrossRef] [PubMed]
- Raguse, J.D.; Gath, H.J. The buccal fad pad lined with a metabolic active dermal replacement (dermagraft) for treatment of defects of the buccal plane. Br. J. Plast. Surg. 2004, 57, 764–768. [Google Scholar] [CrossRef] [PubMed]
- Armstrong, D.G.; Lepow, B.D.; Downey, M.; Yurgelon, J.; Klassen, L. Bioengineered tissues in wound healing a progress report. Expert Rev. Dermatol. 2011, 6, 255–262. [Google Scholar]
- Kim, P.J.; Heilala, M.; Steinberg, J.S.; Weinraub, G.M. Bioengineered alternative tissues and hyperbaric oxygen in lower extremity wound healing. Clin. Podiatr. Med. Surg. 2007, 24, 529–546. [Google Scholar] [CrossRef] [PubMed]
- Ryssel, H.; Gazyakan, E.; Germann, G.; Ohlbauer, M. The use of matriderm in early excision and simultaneous autologous skin grafting in burns—A pilot study. Burns 2008, 34, 93–97. [Google Scholar] [CrossRef] [PubMed]
- Kolokythas, P.; Aust, M.C.; Vogt, P.M.; Paulsen, F. Dermal subsitute with the collagen-elastin matrix matriderm in burn injuries: A comprehensive review. Handchir. Mikrochir. Plast. Chir. 2008, 40, 367–371. [Google Scholar] [CrossRef] [PubMed]
- Van Zuijlen, P.P.; van Trier, A.J.; Vloemans, J.F.; Groenevelt, F.; Kreis, R.W.; Middelkoop, E. Graft survival and effectiveness of dermal substitution in burns and reconstructive surgery in a one-stage grafting model. Plast. Reconstr. Surg. 2000, 106, 615–623. [Google Scholar] [CrossRef] [PubMed]
- Greenberg, J.E.; Falabella, A.F.; Bello, Y.M.; Schachner, L.A. Tissue-engineered skin in the healing of wound stumps from limb amputations secondary to purpura fulminans. Pediatr. Dermatol. 2003, 20, 169–172. [Google Scholar] [CrossRef] [PubMed]
- Akkouch, A.; Shi, G.; Zhang, Z.; Rouabhia, M. Bioactivating electrically conducting polypyrrole with fibronectin and bovine serum albumin. J. Biomed. Mater. Res. Part A 2010, 92, 221–231. [Google Scholar] [CrossRef] [PubMed]
- Cui, X.; Hetke, J.F.; Wiler, J.A.; Anderson, D.J.; Martin, D.C. Electrochemical deposition and characterization of conducting polymer polypyrrole/PSS on multichannel neural probes. Sens. Actuators A Phys. 2001, 93, 8–18. [Google Scholar] [CrossRef]
- Li, Y.; Neoh, K.G.; Kang, E.T. Plasma protein adsorption and thrombus formation on surface functionalized polypyrrole with and without electrical stimulation. J. Colloid Interface Sci. 2004, 275, 488–495. [Google Scholar] [CrossRef] [PubMed]
- Austin, R.E.; Merchant, N.; Shahrokhi, S.; Jeschke, M.G. A comparison of biobrane and cadaveric allograft for temporizing the acute burn wound: Cost and procedural time. Burns 2015, 41, 749–753. [Google Scholar] [CrossRef] [PubMed]
- Cooper, M.L.; Andree, C.; Hansbrough, J.F.; Zapata-Sirvent, R.L.; Spielvogel, R.L. Direct comparison of a cultured composite skin substitute containing human keratinocytes and fibroblasts to an epidermal sheet graft containing human keratinocytes on athymic mice. J. Investig. Dermatol. 1993, 101, 811–819. [Google Scholar] [CrossRef] [PubMed]
- Farroha, A.; Frew, Q.; El-Muttardi, N.; Philp, B.; Dziewulski, P. The use of biobrane® to dress split-thickness skin graft in paediatric burns. Ann. Burns Fire Disasters 2013, 26, 94–97. [Google Scholar] [PubMed]
- Pandya, A.N.; Woodward, B.; Parkhouse, N. The use of cultured autologous keratinocytes with integra in the resurfacing of acute burns. Plast. Reconstr. Sur. 1998, 102, 825–828. [Google Scholar] [CrossRef]
- Pham, C.; Greenwood, J.; Cleland, H.; Woodruff, P.; Maddern, G. Bioengineered skin substitutes for the management of burns: A systematic review. Burns 2007, 33, 946–957. [Google Scholar] [CrossRef] [PubMed]
- Rue, L.W., 3rd; Cioffi, W.G.; McManus, W.F.; Pruitt, B.A. Wound closure and outcome in extensively burned patients treated with cultured autologous keratinocytes. J. Trauma 1993, 34, 662–667. [Google Scholar] [CrossRef] [PubMed]
- Lovett, M.; Lee, K.; Edwards, A.; Kaplan, D.L. Vascularization strategies for tissue engineering. Tissue Eng. Part B Rev. 2009, 15, 353–370. [Google Scholar] [CrossRef] [PubMed]


| Substitute Type | Product | Company | Components | References |
|---|---|---|---|---|
| Acellular | Alloderm® | LifeCell Inc., Branchburg, NJ, USA | Human acellular lyophilized dermis | [18,22,46,49,65] |
| SureDerm | HANS BIOMED Corporation, Daejeon, Korea | Human acellular lyophilized dermis | - | |
| OASIS Wound Matrix | Cook Biotech Inc., West Lafayette, IN, USA | Porcine acellular lyophilized small intestine submucosa | [66] | |
| Biobrane® | Mylan Bertek Pharmaceuticals, USA | Ultrathin silicone as epidermal analog film and 3D nylon filament as dermal analog with type I collagen peptides | [46,49,55] | |
| Integra® DRT (dermal regeneration template) | Integra® LifeSciences Corp., USA | Dermal analog—bovine collagen and chondroitin-6-sulfate GAG; epidermal analog—silicone polymer polysiloxane | [18,46,49,55] | |
| Epidermal | ||||
| Autologous | EpiDex | Modex Therapeutiques, Lausanne, Switzerland | Cultured keratinocytes from outer root sheath of scalp hair follicles (confluent cell sheet) | [67] |
| EPIBASE | Laboratoires Genevrier, Antibes, France | Cultured keratinocytes (confluent cell sheet) | - | |
| MySkin | CellTran Ltd., UK | Cultured keratinocytes (subconfluent cell sheet) silicone support layer with a specially formulated surface coating | [68] | |
| Bioseed-S | BioTissue Technologies GmbH, Germany | Cultured keratinocytes (subconfluent cell suspension) fibrin sealant | [69] | |
| CellSpray | Clinical Cell Culture (C3), Australia | Non-/cultured keratinocytes (subconfluent cell suspension) | [70,71,72] | |
| Epicel® | Genzyme Biosurgery, USA | Sheets of autologous keratinocytes attached to petrolatum gauze support | [73] | |
| Laserskin® or Vivoderm | Fidia Advanced Biopolymers Srl, Italy | Autologous keratinocytes and fibroblasts, grown on microperforated hyaluronic acid membranes | [74] | |
| Autoderm (Autologous Inferior Dermal Sling) | XCELLentis NV, Belgium | Cultured keratinocytes | [75] | |
| TransDerm | XCELLentis NV, Belgium | Cultured keratinocytes | [75] | |
| Lyphoderm | XCELLentis NV, Belgium | Lyophilized neonatal keratinocytes | [76] | |
| Cryoceal | XCELLentis NV, Belgium | Cryopreserved keratinocytes | [77] | |
| Dermal | ||||
| Autologous | denovoDerm™ | EUROSKINGRAFT, Switzerland | Autologous dermal substitute | [78] |
| Pelnac Standard/Fortified | Gunze Ltd., Japan | Porcine tendon derived atelocollagen type I, sponge layer with silicone film | - | |
| Hyalomatrix PA | Fidia Advanced Biopolymers, Italy | HYAFF (an ester of hyaluronic acid) layered on silicone membrane | - | |
| Hyalograft 3D | Fidia Advanced Biopolymers, Italy | Cultured fibroblasts hyaluronic acid membrane (HAM) | [79,80] | |
| Allogenic | Dermagraft® | Advanced BioHealing, Inc., USA | Bioabsorbable polygalactin mesh matrix seeded with human neonatal fibroblasts and cryopreserved | [78] |
| TransCyte® | Advanced BioHealing, Inc., USA | Collagen-coated nylon mesh seeded with allogenic neonatal human foreskin fibroblasts | [81] | |
| Terudermis | Olympus Terumo Biomaterial Corp., Japan | Silicone, bovine lyophilized crosslinked collagen sponge made of heat-denatured collagen | [82] | |
| Cyzact (ICX-PRO) | Intercytex, St John’s Innovation Center, UK | Cultured allogeneic human dermal fibroblasts embedded in a human fibrin gel matrix | - | |
| ICX-SKN skin graft replacement | Intercytex, St John’s Innovation Center, UK | Cultured dermal fibroblasts natural human collagen matrix | - | |
| Polycaprolactone collagen nanofibrous membrane | National University of Singapore, Singapore | Cultured dermal fibroblasts polycaprolactone-blended collagen electrospun nanofibrous membrane | [83] | |
| Tegaderm-nanofibre construct | National University of Singapore, Singapore | Cultured dermal fibroblasts poly(e -caprolactone)/gelatin nanofibrous scaffold electrospun on polyurethane dressing | - | |
| Collagen–glycosaminoglycan–chitosan dermal matrix seeded with fibroblasts | INSERM, France | Cultured dermal fibroblasts bovine collagen I/chondroitin-4/6-sulfate/chitosan lyophilized dermal matrix | [46,49,55] | |
| Human hair keratincollagen sponge | Southern Medical University, China | Cryomilled porcine acellular diisocyanite cross-linked dermis | - | |
| Hyaluronan-FNfds hydrogel matrix | SUNY at Stony Brook, USA | Hyaluronan coupled with fibronectin functional domains | [84] | |
| Composite nano-titanium oxide–chitosan artificial skin (NTCAS) | Cardinal Tien College of Healthcare and Management, Taiwan | Composite nano-titanium oxide–chitosan with gelatin and hyaluronic acid | - | |
| Xenogeneic | Permacol Surgical Implant | Tissue Science Laboratories plc, UK | Porcine acellular diisocyanite crosslinked dermis | [79,85] |
| Matriderm | Dr Suwelack Skin and HealthCare AG, Germany | Bovine non-cross-linked lyophilized dermis, coated with a-elastin hydrolysate | [79,85] | |
| EZ DermTM | Brennen Medical Inc., USA | Porcine aldehyde cross-linked reconstituted dermal collagen | [18] | |
| Bovine collagen cross-linked with microbial transglutaminase | National University of Ireland, Ireland | Freeze-dried bovine collagen scaffold cross-linked with microbial transglutaminase | [86,87,88] | |
| Collatamp | SYNTACOLL AG, Switzerland | Multilayer bovine collagen matrix | - | |
| Synthetic | Hybrid nanofibrous PLGA/chitosan membrane | Tianjin University, China | PLGA/chitosan hybrid electrospun nanofibrous membrane | [83,89] |
| Biodegradable polyurethane microfibers | University of Delaware, USA | Biodegradable polyurethane microfibres | [90] | |
| Epidermal/Dermal (Composite) | ||||
| Autologous | Permaderm™ (Cincinnati Shriners Skin Substitute) | Regeninic Inc.USA | Autologous fibroblasts and keratinocytes in culture with bovine collagen and GAG substrates | [78] |
| Tiscover™ (A-Skin) | Advanced Tissue Medicinal Product, Netherlands | Autologous full thickness cultured skin | [78] | |
| denovoSkin™ | EUROSKINGRAFT, Univ. of Zurich, Switzerland | Autologous full thickness substitute consisting of dermal and epidermal layers | [78] | |
| PolyActive | HC Implants BV, Netherlands | Cultured keratinocytes and fibroblasts polyethylene oxide terephthalate (PEO)/polybutylene terephthalate (PBT) | [91] | |
| TissueTech Autograft System (Laserskin and Hyalograft 3D) | Fidia Advanced Biopolymers, Italy | Cultured keratinocytes and fibroblasts microperforated hyaluronic acid membrane (HAM) | [74,79] | |
| Allogenic | Apligraf® | Organogenesis Inc., USA | Bovine collagen matrix seeded with neonatal foreskin fibroblasts and keratinocytes | [19,63,92,93] |
| OrCel® | Ortec International Inc., USA | Type I collagen matrix seeded with neonatal foreskin fibroblasts and keratinocytes | [86,87,88] | |
| Karoskin (Karocells) | Karocell Tissue Engineering AB, Sweden | Native human cadaver skin with dermal and epidermal cells | [55] | |
| CeladermTM | Celadon Science LLC, USA | Sheets of cells derived from neonatal (allogenic) foreskin | [19,63,92,93] | |
| StrataGraft™ | Stratatech Corporation, USA | Full thickness skin substitute with dermal and fully differentiated epidermal layers | [79,94,95,96] | |
| AcuDress | DFB Pharmaceuticals, Inc., USA | Cultured keratinocytes fibrin substrate | - | |
| Allox | DFB Pharmaceuticals, Inc., USA | Sprayed suspension of allogeneic keratinocytes and fibroblasts in fibrin substrate | - | |
| Xenogeneic | Oasis® | Healthpoint Biotherapeutics, USA | Intact matrix from porcine small-intestine submucosa and intended for wound closure stimulation in acute, chronic and burns wounds | [79,85] |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vig, K.; Chaudhari, A.; Tripathi, S.; Dixit, S.; Sahu, R.; Pillai, S.; Dennis, V.A.; Singh, S.R. Advances in Skin Regeneration Using Tissue Engineering. Int. J. Mol. Sci. 2017, 18, 789. https://doi.org/10.3390/ijms18040789
Vig K, Chaudhari A, Tripathi S, Dixit S, Sahu R, Pillai S, Dennis VA, Singh SR. Advances in Skin Regeneration Using Tissue Engineering. International Journal of Molecular Sciences. 2017; 18(4):789. https://doi.org/10.3390/ijms18040789
Chicago/Turabian StyleVig, Komal, Atul Chaudhari, Shweta Tripathi, Saurabh Dixit, Rajnish Sahu, Shreekumar Pillai, Vida A. Dennis, and Shree R. Singh. 2017. "Advances in Skin Regeneration Using Tissue Engineering" International Journal of Molecular Sciences 18, no. 4: 789. https://doi.org/10.3390/ijms18040789
APA StyleVig, K., Chaudhari, A., Tripathi, S., Dixit, S., Sahu, R., Pillai, S., Dennis, V. A., & Singh, S. R. (2017). Advances in Skin Regeneration Using Tissue Engineering. International Journal of Molecular Sciences, 18(4), 789. https://doi.org/10.3390/ijms18040789







