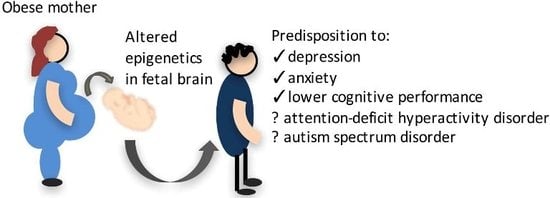
A Review of the Impact of Maternal Obesity on the Cognitive Function and Mental Health of the Offspring
Abstract
:1. Introduction
2. Cognitive Function and Mental Health in Offspring Born to Obese Mothers
2.1. Animal Studies
2.2. Depression and Anxiety
2.3. Learning and Memory
2.4. Motivation and Attention
2.5. Dementia and Neurodegeneration
3. Human Studies
3.1. Depression, Anxiety, and Internalizing and Externalizing Behaviours
3.2. Cognitive Function and Intelligence Quotient (IQ)
3.3. Attention-Deficit Hyperactivity Disorder (ADHD)
3.4. Autism Spectrum Disorders
4. Epigenetic Mechanisms Induced by Maternal Obesity in the Offspring Brain
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Ng, M.; Fleming, T.; Robinson, M.; Thomson, B.; Graetz, N.; Margono, C.; Mullany, E.C.; Biryukov, S.; Abbafati, C.; Abera, S.F.; et al. Global, regional, and national prevalence of overweight and obesity in children and adults during 1980–2013: A systematic analysis for the global burden of disease study 2013. Lancet 2014, 384, 766–781. [Google Scholar] [CrossRef]
- Heslehurst, N.; Rankin, J.; Wilkinson, J.R.; Summerbell, C.D. A nationally representative study of maternal obesity in england, uk: Trends in incidence and demographic inequalities in 619 323 births, 1989–2007. Int. J. Obes. 2010, 34, 420–428. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.Y.; Dietz, P.M.; England, L.; Morrow, B.; Callaghan, W.M. Trends in pre-pregnancy obesity in nine states, 1993–2003. Obesity 2007, 15, 986–993. [Google Scholar] [CrossRef] [PubMed]
- Gregor, L.; Remington, P.L.; Lindberg, S.; Ehrenthal, D. Prevalence of pre-pregnancy obesity, 2011–2014. WMJ 2016, 115, 228–232. [Google Scholar] [PubMed]
- Lindberg, S.; Anderson, C.; Pillai, P.; Tandias, A.; Arndt, B.; Hanrahan, L. Prevalence and predictors of unhealthy weight gain in pregnancy. WMJ 2016, 115, 233–237. [Google Scholar] [PubMed]
- Siega-Riz, A.M.; Gray, G.L. Gestational weight gain recommendations in the context of the obesity epidemic. Nutr. Rev. 2013, 71 (Suppl. S1), S26–S30. [Google Scholar] [CrossRef] [PubMed]
- Moussa, H.N.; Alrais, M.A.; Leon, M.G.; Abbas, E.L.; Sibai, B.M. Obesity epidemic: Impact from preconception to postpartum. Future Sci. OA 2016, 2, FSO137. [Google Scholar] [CrossRef] [PubMed]
- Huda, S.S.; Brodie, L.E.; Sattar, N. Obesity in pregnancy: Prevalence and metabolic consequences. Semin. Fetal Neonatal Med. 2010, 15, 70–76. [Google Scholar] [CrossRef] [PubMed]
- Linabery, A.M.; Nahhas, R.W.; Johnson, W.; Choh, A.C.; Towne, B.; Odegaard, A.O.; Czerwinski, S.A.; Demerath, E.W. Stronger influence of maternal than paternal obesity on infant and early childhood body mass index: The fels longitudinal study. Pediatr. Obes. 2013, 8, 159–169. [Google Scholar] [CrossRef] [PubMed]
- Naess, M.; Holmen, T.L.; Langaas, M.; Bjorngaard, J.H.; Kvaloy, K. Intergenerational transmission of overweight and obesity from parents to their adolescent offspring—The hunt study. PLoS ONE 2016, 11, e0166585. [Google Scholar] [CrossRef] [PubMed]
- Eriksson, J.G.; Sandboge, S.; Salonen, M.; Kajantie, E.; Osmond, C. Maternal weight in pregnancy and offspring body composition in late adulthood: Findings from the helsinki birth cohort study (HBCS). Ann. Med. 2015, 47, 94–99. [Google Scholar] [CrossRef] [PubMed]
- Hochner, H.; Friedlander, Y.; Calderon-Margalit, R.; Meiner, V.; Sagy, Y.; Avgil-Tsadok, M.; Burger, A.; Savitsky, B.; Siscovick, D.S.; Manor, O. Associations of maternal prepregnancy body mass index and gestational weight gain with adult offspring cardiometabolic risk factors: The jerusalem perinatal family follow-up study. Circulation 2012, 125, 1381–1389. [Google Scholar] [CrossRef] [PubMed]
- Parlee, S.D.; MacDougald, O.A. Maternal nutrition and risk of obesity in offspring: The trojan horse of developmental plasticity. Biochim. Biophys. Acta 2014, 1842, 495–506. [Google Scholar] [CrossRef] [PubMed]
- Reynolds, R.M.; Allan, K.M.; Raja, E.A.; Bhattacharya, S.; McNeill, G.; Hannaford, P.C.; Sarwar, N.; Lee, A.J.; Norman, J.E. Maternal obesity during pregnancy and premature mortality from cardiovascular event in adult offspring: Follow-up of 1 323 275 person years. BMJ 2013, 347, f4539. [Google Scholar] [CrossRef] [PubMed]
- Godfrey, K.M.; Reynolds, R.M.; Prescott, S.L.; Nyirenda, M.; Jaddoe, V.W.; Eriksson, J.G.; Broekman, B.F. Influence of maternal obesity on the long-term health of offspring. Lancet Diabetes Endocrinol. 2017, 5, 53–64. [Google Scholar] [CrossRef]
- Vucetic, Z.; Kimmel, J.; Totoki, K.; Hollenbeck, E.; Reyes, T.M. Maternal high-fat diet alters methylation and gene expression of dopamine and opioid-related genes. Endocrinology 2010, 151, 4756–4764. [Google Scholar] [CrossRef] [PubMed]
- Grissom, N.M.; Herdt, C.T.; Desilets, J.; Lidsky-Everson, J.; Reyes, T.M. Dissociable deficits of executive function caused by gestational adversity are linked to specific transcriptional changes in the prefrontal cortex. Neuropsychopharmacology 2015, 40, 1353–1363. [Google Scholar] [CrossRef] [PubMed]
- Januar, V.; Saffery, R.; Ryan, J. Epigenetics and depressive disorders: A review of current progress and future directions. Int. J. Epidemiol. 2015, 44, 1364–1387. [Google Scholar] [CrossRef] [PubMed]
- Shorter, K.R.; Miller, B.H. Epigenetic mechanisms in schizophrenia. Prog. Biophys. Mol. Biol. 2015, 118, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez, J.S.; Rodriguez-Gonzalez, G.L.; Reyes-Castro, L.A.; Ibanez, C.; Ramirez, A.; Chavira, R.; Larrea, F.; Nathanielsz, P.W.; Zambrano, E. Maternal obesity in the rat programs male offspring exploratory, learning and motivation behavior: Prevention by dietary intervention pre-gestation or in gestation. Int. J. Dev. Neurosci. 2012, 30, 75–81. [Google Scholar] [CrossRef] [PubMed]
- Wright, T.M.; King, M.V.; Davey, W.G.; Langley-Evans, S.C.; Voigt, J.P. Impact of cafeteria feeding during lactation in the rat on novel object discrimination in the offspring. Br. J. Nutr. 2014, 112, 1933–1937. [Google Scholar] [CrossRef] [PubMed]
- Can, O.D.; Ulupinar, E.; Ozkay, U.D.; Yegin, B.; Ozturk, Y. The effect of simvastatin treatment on behavioral parameters, cognitive performance, and hippocampal morphology in rats fed a standard or a high-fat diet. Behav. Pharmacol. 2012, 23, 582–592. [Google Scholar] [CrossRef] [PubMed]
- Hawkes, C.A.; Gentleman, S.M.; Nicoll, J.A.; Carare, R.O. Prenatal high-fat diet alters the cerebrovasculature and clearance of beta-amyloid in adult offspring. J. Pathol. 2015, 235, 619–631. [Google Scholar] [CrossRef] [PubMed]
- White, C.L.; Pistell, P.J.; Purpera, M.N.; Gupta, S.; Fernandez-Kim, S.O.; Hise, T.L.; Keller, J.N.; Ingram, D.K.; Morrison, C.D.; Bruce-Keller, A.J. Effects of high fat diet on morris maze performance, oxidative stress, and inflammation in rats: Contributions of maternal diet. Neurobiol. Dis. 2009, 35, 3–13. [Google Scholar] [CrossRef] [PubMed]
- Page, K.C.; Jones, E.K.; Anday, E.K. Maternal and postweaning high-fat diets disturb hippocampal gene expression, learning, and memory function. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2014, 306, R527–R537. [Google Scholar] [CrossRef] [PubMed]
- Lepinay, A.L.; Larrieu, T.; Joffre, C.; Acar, N.; Garate, I.; Castanon, N.; Ferreira, G.; Langelier, B.; Guesnet, P.; Bretillon, L.; et al. Perinatal high-fat diet increases hippocampal vulnerability to the adverse effects of subsequent high-fat feeding. Psychoneuroendocrinology 2015, 53, 82–93. [Google Scholar] [CrossRef] [PubMed]
- Sullivan, E.L.; Grayson, B.; Takahashi, D.; Robertson, N.; Maier, A.; Bethea, C.L.; Smith, M.S.; Coleman, K.; Grove, K.L. Chronic consumption of a high-fat diet during pregnancy causes perturbations in the serotonergic system and increased anxiety-like behavior in nonhuman primate offspring. J. Neurosci. 2010, 30, 3826–3830. [Google Scholar] [CrossRef] [PubMed]
- Bilbo, S.D.; Tsang, V. Enduring consequences of maternal obesity for brain inflammation and behavior of offspring. FASEB J. 2010, 24, 2104–2115. [Google Scholar] [CrossRef] [PubMed]
- Peleg-Raibstein, D.; Luca, E.; Wolfrum, C. Maternal high-fat diet in mice programs emotional behavior in adulthood. Behav. Brain Res. 2012, 233, 398–404. [Google Scholar] [CrossRef] [PubMed]
- Sasaki, A.; de Vega, W.C.; St-Cyr, S.; Pan, P.; McGowan, P.O. Perinatal high fat diet alters glucocorticoid signaling and anxiety behavior in adulthood. Neuroscience 2013, 240, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Kang, S.S.; Kurti, A.; Fair, D.A.; Fryer, J.D. Dietary intervention rescues maternal obesity induced behavior deficits and neuroinflammation in offspring. J. Neuroinflamm. 2014, 11, 156. [Google Scholar] [CrossRef] [PubMed]
- Sasaki, A.; de Vega, W.; Sivanathan, S.; St-Cyr, S.; McGowan, P.O. Maternal high-fat diet alters anxiety behavior and glucocorticoid signaling in adolescent offspring. Neuroscience 2014, 272, 92–101. [Google Scholar] [CrossRef] [PubMed]
- Speight, A.; Davey, W.G.; McKenna, E.; Voigt, J.W. Exposure to a maternal cafeteria diet changes open-field behaviour in the developing offspring. Int. J. Dev. Neurosci. 2017, 57, 34–40. [Google Scholar] [CrossRef] [PubMed]
- Wright, T.; Langley-Evans, S.C.; Voigt, J.P. The impact of maternal cafeteria diet on anxiety-related behaviour and exploration in the offspring. Physiol. Behav. 2011, 103, 164–172. [Google Scholar] [CrossRef] [PubMed]
- Balsevich, G.; Baumann, V.; Uribe, A.; Chen, A.; Schmidt, M.V. Prenatal exposure to maternal obesity alters anxiety and stress coping behaviors in aged mice. Neuroendocrinology 2016, 103, 354–368. [Google Scholar] [CrossRef] [PubMed]
- Champagne, F.; Meaney, M.J. Like mother, like daughter: Evidence for non-genomic transmission of parental behavior and stress responsivity. Prog. Brain Res. 2001, 133, 287–302. [Google Scholar] [PubMed]
- Clouard, C.; Gerrits, W.J.; Kemp, B.; Val-Laillet, D.; Bolhuis, J.E. Perinatal exposure to a diet high in saturated fat, refined sugar and cholesterol affects behaviour, growth, and feed intake in weaned piglets. PLoS ONE 2016, 11, e0154698. [Google Scholar] [CrossRef] [PubMed]
- Lin, C.; Shao, B.; Huang, H.; Zhou, Y.; Lin, Y. Maternal high fat diet programs stress-induced behavioral disorder in adult offspring. Physiol. Behav. 2015, 152, 119–127. [Google Scholar] [CrossRef] [PubMed]
- Giriko, C.A.; Andreoli, C.A.; Mennitti, L.V.; Hosoume, L.F.; Souto Tdos, S.; Silva, A.V.; Mendes-da-Silva, C. Delayed physical and neurobehavioral development and increased aggressive and depression-like behaviors in the rat offspring of dams fed a high-fat diet. Int. J. Dev. Neurosci. 2013, 31, 731–739. [Google Scholar] [CrossRef] [PubMed]
- Johnson, S.A.; Javurek, A.B.; Painter, M.S.; Murphy, C.R.; Conard, C.M.; Gant, K.L.; Howald, E.C.; Ellersieck, M.R.; Wiedmeyer, C.E.; Vieira-Potter, V.J.; et al. Effects of a maternal high-fat diet on offspring behavioral and metabolic parameters in a rodent model. J. Dev. Orig. Health Dis. 2017, 8, 75–88. [Google Scholar] [CrossRef] [PubMed]
- Clouard, C.; Kemp, B.; Val-Laillet, D.; Gerrits, W.J.; Bartels, A.C.; Bolhuis, J.E. Prenatal, but not early postnatal, exposure to a western diet improves spatial memory of pigs later in life and is paired with changes in maternal prepartum blood lipid levels. FASEB J. 2016, 30, 2466–2475. [Google Scholar] [CrossRef] [PubMed]
- Val-Laillet, D.; Besson, M.; Guerin, S.; Coquery, N.; Randuineau, G.; Kanzari, A.; Quesnel, H.; Bonhomme, N.; Bolhuis, J.E.; Kemp, B.; et al. A maternal western diet during gestation and lactation modifies offspring’s microbiota activity, blood lipid levels, cognitive responses, and hippocampal neurogenesis in yucatan pigs. FASEB J. 2017, 31, 2037–2049. [Google Scholar] [CrossRef] [PubMed]
- Tozuka, Y.; Kumon, M.; Wada, E.; Onodera, M.; Mochizuki, H.; Wada, K. Maternal obesity impairs hippocampal bdnf production and spatial learning performance in young mouse offspring. Neurochem. Int. 2010, 57, 235–247. [Google Scholar] [CrossRef] [PubMed]
- Graf, A.E.; Lallier, S.W.; Waidyaratne, G.; Thompson, M.D.; Tipple, T.E.; Hester, M.E.; Trask, A.J.; Rogers, L.K. Maternal high fat diet exposure is associated with increased hepcidin levels, decreased myelination, and neurobehavioral changes in male offspring. Brain Behav. Immun. 2016, 58, 369–378. [Google Scholar] [CrossRef] [PubMed]
- Romani-Perez, M.; Lepinay, A.L.; Alonso, L.; Rincel, M.; Xia, L.; Fanet, H.; Caille, S.; Cador, M.; Laye, S.; Vancassel, S.; et al. Impact of perinatal exposure to high-fat diet and stress on responses to nutritional challenges, food-motivated behaviour and mesolimbic dopamine function. Int. J. Obes. 2017, 41, 502–509. [Google Scholar] [CrossRef] [PubMed]
- Naef, L.; Moquin, L.; Dal Bo, G.; Giros, B.; Gratton, A.; Walker, C.D. Maternal high-fat intake alters presynaptic regulation of dopamine in the nucleus accumbens and increases motivation for fat rewards in the offspring. Neuroscience 2011, 176, 225–236. [Google Scholar] [CrossRef] [PubMed]
- Ong, Z.Y.; Muhlhausler, B.S. Maternal “junk-food” feeding of rat dams alters food choices and development of the mesolimbic reward pathway in the offspring. FASEB J. 2011, 25, 2167–2179. [Google Scholar] [CrossRef] [PubMed]
- Carlin, J.; George, R.; Reyes, T.M. Methyl donor supplementation blocks the adverse effects of maternal high fat diet on offspring physiology. PLoS ONE 2013, 8, e63549. [Google Scholar] [CrossRef] [PubMed]
- McKee, S.E.; Grissom, N.M.; Herdt, C.T.; Reyes, T.M. Methyl donor supplementation alters cognitive performance and motivation in female offspring from high-fat diet-fed dams. FASEB J. 2017. [Google Scholar] [CrossRef] [PubMed]
- Wu, T.; Deng, S.; Li, W.G.; Yu, Y.; Li, F.; Mao, M. Maternal obesity caused by overnutrition exposure leads to reversal learning deficits and striatal disturbance in rats. PLoS ONE 2013, 8, e78876. [Google Scholar] [CrossRef] [PubMed]
- Mangialasche, F.; Kivipelto, M.; Solomon, A.; Fratiglioni, L. Dementia prevention: Current epidemiological evidence and future perspective. Alzheimer’s Res. Ther. 2012, 4, 6. [Google Scholar] [CrossRef] [PubMed]
- Martin, S.A.; Jameson, C.H.; Allan, S.M.; Lawrence, C.B. Maternal high-fat diet worsens memory deficits in the triple-transgenic (3xTgAD) mouse model of Alzheimer’s disease. PLoS ONE 2014, 9, e99226. [Google Scholar] [CrossRef] [PubMed]
- Hsiao, K.; Chapman, P.; Nilsen, S.; Eckman, C.; Harigaya, Y.; Younkin, S.; Yang, F.; Cole, G. Correlative memory deficits, abeta elevation, and amyloid plaques in transgenic mice. Science 1996, 274, 99–102. [Google Scholar] [CrossRef] [PubMed]
- Nizari, S.; Carare, R.O.; Hawkes, C.A. Increased abeta pathology in aged tg2576 mice born to mothers fed a high fat diet. Sci. Rep. 2016, 6, 21981. [Google Scholar] [CrossRef] [PubMed]
- Gardener, H.; Munger, K.L.; Chitnis, T.; Michels, K.B.; Spiegelman, D.; Ascherio, A. Prenatal and perinatal factors and risk of multiple sclerosis. Epidemiology 2009, 20, 611–618. [Google Scholar] [CrossRef] [PubMed]
- Lahiri, D.K.; Maloney, B. The “learn” (latent early-life associated regulation) model integrates environmental risk factors and the developmental basis of Alzheimer’s disease, and proposes remedial steps. Exp. Gerontol. 2010, 45, 291–296. [Google Scholar] [CrossRef] [PubMed]
- Edlow, A.G. Maternal obesity and neurodevelopmental and psychiatric disorders in offspring. Prenat. Diagn. 2017, 37, 95–110. [Google Scholar] [CrossRef] [PubMed]
- Rivera, H.M.; Christiansen, K.J.; Sullivan, E.L. The role of maternal obesity in the risk of neuropsychiatric disorders. Front. Neurosci. 2015, 9, 194. [Google Scholar] [CrossRef] [PubMed]
- Veena, S.R.; Gale, C.R.; Krishnaveni, G.V.; Kehoe, S.H.; Srinivasan, K.; Fall, C.H. Association between maternal nutritional status in pregnancy and offspring cognitive function during childhood and adolescence; a systematic review. BMC Pregnancy Childbirth 2016, 16, 220. [Google Scholar] [CrossRef] [PubMed]
- Richards, M.; Huppert, F.A. Do positive children become positive adults? Evidence from a longitudinal birth cohort study. J. Posit. Psychol. 2011, 6, 75–87. [Google Scholar] [CrossRef] [PubMed]
- Schaefer, J.D.; Caspi, A.; Belsky, D.W.; Harrington, H.; Houts, R.; Horwood, L.J.; Hussong, A.; Ramrakha, S.; Poulton, R.; Moffitt, T.E. Enduring mental health: Prevalence and prediction. J. Abnorm. Psychol. 2017, 126, 212–224. [Google Scholar] [CrossRef] [PubMed]
- Gustafsson, H.C.; Kuzava, S.E.; Werner, E.A.; Monk, C. Maternal dietary fat intake during pregnancy is associated with infant temperament. Dev. Psychobiol. 2016, 58, 528–535. [Google Scholar] [CrossRef] [PubMed]
- Steenweg-de Graaff, J.; Tiemeier, H.; Steegers-Theunissen, R.P.; Hofman, A.; Jaddoe, V.W.; Verhulst, F.C.; Roza, S.J. Maternal dietary patterns during pregnancy and child internalising and externalising problems. The generation r study. Clin. Nutr. 2014, 33, 115–121. [Google Scholar] [CrossRef] [PubMed]
- Bergmann, S.; Schlesier-Michel, A.; Wendt, V.; Grube, M.; Keitel-Korndorfer, A.; Gausche, R.; von Klitzing, K.; Klein, A.M. Maternal weight predicts children’s psychosocial development via parenting stress and emotional availability. Front. Psychol. 2016, 7, 1156. [Google Scholar] [CrossRef] [PubMed]
- Mina, T.H.; Lahti, M.; Drake, A.J.; Raikkonen, K.; Minnis, H.; Denison, F.C.; Norman, J.E.; Reynolds, R.M. Prenatal exposure to very severe maternal obesity is associated with adverse neuropsychiatric outcomes in children. Psychol. Med. 2017, 47, 353–362. [Google Scholar] [CrossRef] [PubMed]
- Robinson, M.; Zubrick, S.R.; Pennell, C.E.; Van Lieshout, R.J.; Jacoby, P.; Beilin, L.J.; Mori, T.A.; Stanley, F.J.; Newnham, J.P.; Oddy, W.H. Pre-pregnancy maternal overweight and obesity increase the risk for affective disorders in offspring. J. Dev. Orig. Health Dis. 2013, 4, 42–48. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez, A. Maternal pre-pregnancy obesity and risk for inattention and negative emotionality in children. J. Child Psychol. Psychiatry 2010, 51, 134–143. [Google Scholar] [CrossRef] [PubMed]
- Van Lieshout, R.J.; Robinson, M.; Boyle, M.H. Maternal pre-pregnancy body mass index and internalizing and externalizing problems in offspring. Can. J. Psychiatry 2013, 58, 151–159. [Google Scholar] [CrossRef] [PubMed]
- Basatemur, E.; Gardiner, J.; Williams, C.; Melhuish, E.; Barnes, J.; Sutcliffe, A. Maternal prepregnancy bmi and child cognition: A longitudinal cohort study. Pediatrics 2013, 131, 56–63. [Google Scholar] [CrossRef] [PubMed]
- Bliddal, M.; Olsen, J.; Stovring, H.; Eriksen, H.L.; Kesmodel, U.S.; Sorensen, T.I.; Nohr, E.A. Maternal pre-pregnancy bmi and intelligence quotient (IQ) in 5-year-old children: A cohort based study. PLoS ONE 2014, 9, e94498. [Google Scholar] [CrossRef] [PubMed]
- Brion, M.J.; Zeegers, M.; Jaddoe, V.; Verhulst, F.; Tiemeier, H.; Lawlor, D.A.; Smith, G.D. Intrauterine effects of maternal prepregnancy overweight on child cognition and behavior in 2 cohorts. Pediatrics 2011, 127, e202–e211. [Google Scholar] [CrossRef] [PubMed]
- Casas, M.; Chatzi, L.; Carsin, A.E.; Amiano, P.; Guxens, M.; Kogevinas, M.; Koutra, K.; Lertxundi, N.; Murcia, M.; Rebagliato, M.; et al. Maternal pre-pregnancy overweight and obesity, and child neuropsychological development: Two southern european birth cohort studies. Int. J. Epidemiol. 2013, 42, 506–517. [Google Scholar] [CrossRef] [PubMed]
- Craig, W.Y.; Palomaki, G.E.; Neveux, L.M.; Haddow, J.E. Maternal body mass index during pregnancy and offspring neurocognitive development. Obstet. Med. 2013, 6, 20–25. [Google Scholar] [PubMed]
- Eriksen, H.L.; Kesmodel, U.S.; Underbjerg, M.; Kilburn, T.R.; Bertrand, J.; Mortensen, E.L. Predictors of intelligence at the age of 5: Family, pregnancy and birth characteristics, postnatal influences, and postnatal growth. PLoS ONE 2013, 8, e79200. [Google Scholar] [CrossRef] [PubMed]
- Hinkle, S.N.; Schieve, L.A.; Stein, A.D.; Swan, D.W.; Ramakrishnan, U.; Sharma, A.J. Associations between maternal prepregnancy body mass index and child neurodevelopment at 2 years of age. Int. J. Obes. 2012, 36, 1312–1319. [Google Scholar] [CrossRef] [PubMed]
- Huang, L.; Yu, X.; Keim, S.; Li, L.; Zhang, L.; Zhang, J. Maternal prepregnancy obesity and child neurodevelopment in the collaborative perinatal project. Int. J. Epidemiol. 2014, 43, 783–792. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Fallin, M.D.; Riley, A.; Landa, R.; Walker, S.O.; Silverstein, M.; Caruso, D.; Pearson, C.; Kiang, S.; Dahm, J.L.; et al. The association of maternal obesity and diabetes with autism and other developmental disabilities. Pediatrics 2016, 137, e20152206. [Google Scholar] [CrossRef] [PubMed]
- Neggers, Y.H.; Goldenberg, R.L.; Ramey, S.L.; Cliver, S.P. Maternal prepregnancy body mass index and psychomotor development in children. Acta Obstet. Gynecol. Scand. 2003, 82, 235–240. [Google Scholar] [CrossRef] [PubMed]
- Buss, C.; Entringer, S.; Davis, E.P.; Hobel, C.J.; Swanson, J.M.; Wadhwa, P.D.; Sandman, C.A. Impaired executive function mediates the association between maternal pre-pregnancy body mass index and child adhd symptoms. PLoS ONE 2012, 7, e37758. [Google Scholar] [CrossRef] [PubMed]
- Tanda, R.; Salsberry, P.J.; Reagan, P.B.; Fang, M.Z. The impact of prepregnancy obesity on children’s cognitive test scores. Matern. Child Health J. 2013, 17, 222–229. [Google Scholar] [CrossRef] [PubMed]
- Tavris, D.R.; Read, J.A. Effect of maternal weight gain on fetal, infant, and childhood death and on cognitive development. Obstet. Gynecol. 1982, 60, 689–694. [Google Scholar] [PubMed]
- Gage, S.H.; Lawlor, D.A.; Tilling, K.; Fraser, A. Associations of maternal weight gain in pregnancy with offspring cognition in childhood and adolescence: Findings from the avon longitudinal study of parents and children. Am. J. Epidemiol. 2013, 177, 402–410. [Google Scholar] [CrossRef] [PubMed]
- Heikura, U.; Taanila, A.; Hartikainen, A.L.; Olsen, P.; Linna, S.L.; von Wendt, L.; Jarvelin, M.R. Variations in prenatal sociodemographic factors associated with intellectual disability: A study of the 20-year interval between two birth cohorts in northern finland. Am. J. Epidemiol. 2008, 167, 169–177. [Google Scholar] [CrossRef] [PubMed]
- Koenen, K.C.; Moffitt, T.E.; Roberts, A.L.; Martin, L.T.; Kubzansky, L.; Harrington, H.; Poulton, R.; Caspi, A. Childhood iq and adult mental disorders: A test of the cognitive reserve hypothesis. Am. J. Psychiatry 2009, 166, 50–57. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez, A.; Miettunen, J.; Henriksen, T.B.; Olsen, J.; Obel, C.; Taanila, A.; Ebeling, H.; Linnet, K.M.; Moilanen, I.; Jarvelin, M.R. Maternal adiposity prior to pregnancy is associated with adhd symptoms in offspring: Evidence from three prospective pregnancy cohorts. Int. J. Obes 2008, 32, 550–557. [Google Scholar] [CrossRef] [PubMed]
- Tanda, R.; Salsberry, P.J. Racial differences in the association between maternal prepregnancy obesity and children’s behavior problems. J. Dev. Behav. Pediatr. 2014, 35, 118–127. [Google Scholar] [CrossRef] [PubMed]
- Chen, Q.; Sjolander, A.; Langstrom, N.; Rodriguez, A.; Serlachius, E.; D’Onofrio, B.M.; Lichtenstein, P.; Larsson, H. Maternal pre-pregnancy body mass index and offspring attention deficit hyperactivity disorder: A population-based cohort study using a sibling-comparison design. Int. J. Epidemiol. 2014, 43, 83–90. [Google Scholar] [CrossRef] [PubMed]
- Bilder, D.A.; Bakian, A.V.; Viskochil, J.; Clark, E.A.; Botts, E.L.; Smith, K.R.; Pimentel, R.; McMahon, W.M.; Coon, H. Maternal prenatal weight gain and autism spectrum disorders. Pediatrics 2013, 132, e1276–e1283. [Google Scholar] [CrossRef] [PubMed]
- Dodds, L.; Fell, D.B.; Shea, S.; Armson, B.A.; Allen, A.C.; Bryson, S. The role of prenatal, obstetric and neonatal factors in the development of autism. J. Autism Dev. Disord. 2011, 41, 891–902. [Google Scholar] [CrossRef] [PubMed]
- Krakowiak, P.; Walker, C.K.; Bremer, A.A.; Baker, A.S.; Ozonoff, S.; Hansen, R.L.; Hertz-Picciotto, I. Maternal metabolic conditions and risk for autism and other neurodevelopmental disorders. Pediatrics 2012, 129, e1121–e1128. [Google Scholar] [CrossRef] [PubMed]
- Gardner, R.M.; Lee, B.K.; Magnusson, C.; Rai, D.; Frisell, T.; Karlsson, H.; Idring, S.; Dalman, C. Maternal body mass index during early pregnancy, gestational weight gain, and risk of autism spectrum disorders: Results from a swedish total population and discordant sibling study. Int. J. Epidemiol. 2015, 44, 870–883. [Google Scholar] [CrossRef] [PubMed]
- Getz, K.D.; Anderka, M.T.; Werler, M.M.; Jick, S.S. Maternal pre-pregnancy body mass index and autism spectrum disorder among offspring: A population-based case-control study. Paediatr. Perinat. Epidemiol. 2016, 30, 479–487. [Google Scholar] [CrossRef] [PubMed]
- Hatanaka, Y.; Kabuta, T.; Wada, K. Disturbance in maternal environment leads to abnormal synaptic instability during neuronal circuitry development. Front. Neurosci. 2017, 11, 35. [Google Scholar] [CrossRef] [PubMed]
- Tozuka, Y.; Wada, E.; Wada, K. Diet-induced obesity in female mice leads to peroxidized lipid accumulations and impairment of hippocampal neurogenesis during the early life of their offspring. FASEB J. 2009, 23, 1920–1934. [Google Scholar] [CrossRef] [PubMed]
- Yu, M.; Jiang, M.; Yang, C.; Wu, Y.; Liu, Y.; Cui, Y.; Huang, G. Maternal high-fat diet affects msi/notch/hes signaling in neural stem cells of offspring mice. J. Nutr. Biochem. 2014, 25, 227–231. [Google Scholar] [CrossRef] [PubMed]
- Nazki, F.H.; Sameer, A.S.; Ganaie, B.A. Folate: Metabolism, genes, polymorphisms and the associated diseases. Gene 2014, 533, 11–20. [Google Scholar] [CrossRef] [PubMed]
- Mohd-Shukri, N.A.; Duncan, A.; Denison, F.C.; Forbes, S.; Walker, B.R.; Norman, J.E.; Reynolds, R.M. Health behaviours during pregnancy in women with very severe obesity. Nutrients 2015, 7, 8431–8443. [Google Scholar] [CrossRef] [PubMed]
- Edlow, A.G.; Guedj, F.; Pennings, J.L.; Sverdlov, D.; Neri, C.; Bianchi, D.W. Males are from mars, and females are from venus: Sex-specific fetal brain gene expression signatures in a mouse model of maternal diet-induced obesity. Am. J. Obstet. Gynecol. 2016, 214, 623 e610–623 e621. [Google Scholar] [PubMed]
- Fuemmeler, B.F.; Lee, C.T.; Soubry, A.; Iversen, E.S.; Huang, Z.; Murtha, A.P.; Schildkraut, J.M.; Jirtle, R.L.; Murphy, S.K.; Hoyo, C. DNA methylation of regulatory regions of imprinted genes at birth and its relation to infant temperament. Genet. Epigenet. 2016, 8, 59–67. [Google Scholar] [CrossRef] [PubMed]
- Nardelli, C.; Iaffaldano, L.; Ferrigno, M.; Labruna, G.; Maruotti, G.M.; Quaglia, F.; Capobianco, V.; Di Noto, R.; Del Vecchio, L.; Martinelli, P.; et al. Characterization and predicted role of the microrna expression profile in amnion from obese pregnant women. Int. J. Obes. 2014, 38, 466–469. [Google Scholar] [CrossRef] [PubMed]
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Contu, L.; Hawkes, C.A. A Review of the Impact of Maternal Obesity on the Cognitive Function and Mental Health of the Offspring. Int. J. Mol. Sci. 2017, 18, 1093. https://doi.org/10.3390/ijms18051093
Contu L, Hawkes CA. A Review of the Impact of Maternal Obesity on the Cognitive Function and Mental Health of the Offspring. International Journal of Molecular Sciences. 2017; 18(5):1093. https://doi.org/10.3390/ijms18051093
Chicago/Turabian StyleContu, Laura, and Cheryl A. Hawkes. 2017. "A Review of the Impact of Maternal Obesity on the Cognitive Function and Mental Health of the Offspring" International Journal of Molecular Sciences 18, no. 5: 1093. https://doi.org/10.3390/ijms18051093
APA StyleContu, L., & Hawkes, C. A. (2017). A Review of the Impact of Maternal Obesity on the Cognitive Function and Mental Health of the Offspring. International Journal of Molecular Sciences, 18(5), 1093. https://doi.org/10.3390/ijms18051093