Antiproliferative and Apoptotic Potential of Cyanidin-Based Anthocyanins on Melanoma Cells
Abstract
:1. Introduction
2. Results and Discussion
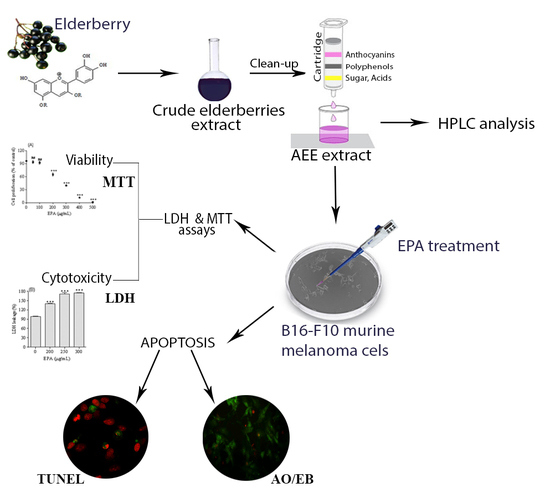
2.1. LC/DAD/ESI-MS Analysis of Anthocyanins Enriched Extract (AEE)
2.2. Melanoma Cell Proliferation
2.3. Assessment of Melanoma Cells Membrane Integrity
2.4. Evaluation of Apoptotic Cell Death
3. Discussion
4. Materials and Methods
4.1. Reagents
4.2. Obtaining the Anthocyanins Extract (AEE)
4.3. LC/DAD/ESI-MS Analysis of AEE
4.4. Cell Culture
4.5. Analysis of Cell Viability
4.6. Detection of LDH Activity
4.7. 96-Well-Based EB/AO Staining
4.8. TUNEL Assay
4.9. Statistical Analysis
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
Abbreviations
| IL-6 | Interleukin 6 |
| TNF-alfa | Tumor Necrosis Factor alfa |
| MAP kinases | Mitogen-activated protein kinases |
| Erk 1/2 | Extracellular signal-regulated kinase 1/2 |
| p38 | Stress-activated protein kinase p38 |
| JNK 1/2 | c-Jun N-terminal kinase |
| MKK4 | Mitogen-activated protein kinase kinase |
| NF-κB | Nuclear factor NF-κB protein |
| AP-1 | Activator protein-1 |
| FBS | Fetal bovine serum |
| DMEM | Dulbecco’s Modified Eagle Medium |
| MTT | 3-(4,5-Dimethylthiazol-2-yl)-2,5-Diphenyltetrazolium bromide |
| MeOH | Methanol |
| EtOAc | Ethylacetate |
| HCl | Hydrochloric acid |
| DMSO | Dimethylsulfoxide |
| EB/AO | Ethidium Bromide/Acridine Orange |
| AEE | Anthocyanins enriched extract |
| LDH | Lactate Dehydrogenase |
| TUNEL assay | Terminal deoxynucleotidyl transferase dUTP nick end labeling assay |
| Draq5 | 1,5-Bis{[2-(di-methylamino) ethyl]amino}-4,8-Dihydroxyanthracene-9,10-Dione |
| PBS | Phosphate Buffered Saline |
| LC/DAD/ESI-MS | Liquid Chromatography/Diode Array/Electrospray Ionization-Mass Spectrometry |
| Cy | Cyanidine |
| Cy-3-O-samb-5-gluc | Cyanidine-3-O-sambubioside-5-glucoside |
| Cy-3,5-O-gluc | Cyanidine-3,5-glucoside |
References
- Afaq, F.; Syed, D.N.; Malik, A.; Hadi, N.; Sarfaraz, S.; Kweon, M.H.; Khan, N.; Zaid, M.A.; Mukhtar, H. Delphinidin, an anthocyanidin in pigmented fruits and vegetables, protects human HaCaT keratinocytes and mouse skin against UVB-mediated oxidative stress and apoptosis. J. Investig. Dermatol. 2007, 127, 222–232. [Google Scholar] [CrossRef] [PubMed]
- Afaq, F.; Zaid, M.A.; Khan, N.; Dreher, M.; Mukhtar, H. Protective effect of pomegranate-derived products on UVB-mediated damage in human reconstituted skin. Exp. Dermatol. 2009, 18, 553–561. [Google Scholar] [CrossRef] [PubMed]
- Chamcheu, J.C.; Afaq, F.; Syed, D.N.; Siddiqui, I.A.; Adhami, V.M.; Khan, N.; Singh, S.; Boylan, B.T.; Wood, G.S.; Mukhtar, H. Delphinidin, a dietary antioxidant, induces human epidermal keratinocyte differentiation but not apoptosis: Studies in submerged and three-dimensional epidermal equivalent models. Exp. Dermatol. 2013, 22, 342–348. [Google Scholar] [CrossRef] [PubMed]
- Chinembiri, T.N.; Du Plessis, L.H.; Gerber, M.; Hamman, J.H.; Du Plessis, J. Review of natural compounds for potential skin cancer treatment. Molecules 2014, 19, 11679–11721. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.E.; Kwon, J.Y.; Seo, S.K.; Son, J.E.; Jung, S.K.; Min, S.Y.; Hwang, M.K.; Heo, Y.S.; Lee, K.W.; Lee, H.J. Cyanidin suppresses ultraviolet B-induced COX-2 expression in epidermal cells by targeting MKK4, MEK1, and Raf-1. Biochem. Pharmacol. 2010, 79, 1473–1482. [Google Scholar] [CrossRef] [PubMed]
- Murapa, P.; Dai, J.; Chung, M.; Mumper, R.J.; D’Orazio, J. Anthocyanin-rich fractions of blackberry extracts reduce UV-induced free radicals and oxidative damage in keratinocytes. Phytother. Res. 2012, 26, 106–112. [Google Scholar] [CrossRef] [PubMed]
- Pratheeshkumar, P.; Son, Y.O.; Wang, X.; Divya, S.P.; Joseph, B.; Hitron, J.A.; Wang, L.; Kim, D.; Yin, Y.; Roy, R.V.; et al. Cyanidin-3-glucoside inhibits UVB-induced oxidative damage and inflammation by regulating MAP kinase and NF-κB signaling pathways in SKH-1 hairless mice skin. Toxicol. Appl. Pharmacol. 2014, 280, 127–137. [Google Scholar] [CrossRef] [PubMed]
- Kwon, J.Y.; Lee, K.W.; Kim, J.E.; Jung, S.K.; Kang, N.J.; Hwang, M.K.; Heo, Y.S.; Bode, A.M.; Dong, Z.; Lee, H.J. Delphinidin suppresses ultraviolet B-induced cyclooxygenases-2 expression through inhibition of MAPKK4 and PI-3 kinase. Carcinogenesis 2009, 30, 1932–1940. [Google Scholar] [CrossRef] [PubMed]
- Felgines, C.; Talavera, S.; Texier, O.; Gil-Izquierdo, A.; Lamaison, J.L.; Remesy, C. Blackberry anthocyanins are mainly recovered from urine as methylated and glucuronidated conjugates in humans. J. Agric. Food Chem. 2005, 53, 7721–7727. [Google Scholar] [CrossRef] [PubMed]
- Frank, T.; Janssen, M.; Netzet, G.; Christian, B.; Bitsch, I.; Netzel, M. Absorption and excretion of elderberry (Sambucus nigra L.) anthocyanins in healthy humans. Methods Find. Exp. Clin. Pharmacol. 2007, 29, 525–533. [Google Scholar] [CrossRef] [PubMed]
- Kay, C.D.; Mazza, G.J.; Holub, B.J. Anthocyanins exist in the circulation primarily as metabolites in adult men. J. Nutr. 2005, 135, 2582–2588. [Google Scholar] [PubMed]
- Shipp, J.; Abdel-Aal, E.S.M. Food applications and physiological effects of anthocyanins as functional food ingredients. Open Food Sci. J. 2010, 4, 7–22. [Google Scholar] [CrossRef]
- Duymus, H.G.; Goger, F.; Baser, K.H. In vitro antioxidant properties and anthocyanin compositions of elderberry extracts. Food Chem. 2014, 155, 112–119. [Google Scholar] [CrossRef] [PubMed]
- Vlachojannis, C.; Zimmermann, B.F.; Chrubasik-Hausmann, S. Quantification of anthocyanins in elderberry and chokeberry dietary supplements. Phytother. Res. 2015, 29, 561–565. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.; Gu, L.; Prior, R.L.; McKay, S. Characterization of Anthocyanins and Proanthocyanidins in Some Cultivars of Ribes, Aronia, and Sambucus and Their Antioxidant Capacity. J. Agric. Food Chem. 2004, 52, 7846–7856. [Google Scholar] [CrossRef] [PubMed]
- Mikulic-Petkovsek, M.; Schmitzer, V.; Slatnar, A.; Todorovic, B.; Veberic, R.; Stampar, F.; Ivancic, A. Investigation of anthocyanin profile of four elderberry species and interspecific hybrids. J. Agric. Food Chem. 2014, 62, 5573–5580. [Google Scholar] [CrossRef] [PubMed]
- Veberic, R.; Jakopic, J.; Stampar, F.; Schmitzer, V. European elderberry (Sambucus nigra L.) rich in sugars, organic acids, anthocyanins and selected polyphenols. Food Chem. 2009, 114, 511–515. [Google Scholar] [CrossRef]
- Lee, J.; Finn, C.E. Anthocyanins and other polyphenolics in American elderberry (Sambucus canadensis) and European elderberry (S. nigra) cultivars. J. Sci. Food Agric. 2007, 87, 2665–2675. [Google Scholar] [CrossRef] [PubMed]
- Bunea, A.; Rugina, D.; Sconta, Z.; Pop, R.M.; Pintea, A.; Socaciu, C.; Tabaran, F.; Grootaert, C.; Struijs, K.; VanCamp, J. Anthocyanin determination in blueberry extracts from various cultivars and their antiproliferative and apoptotic properties in B16-F10 metastatic murine melanoma cells. Phytochemistry 2013, 95, 436–444. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Zhang, W.; Jing, H.; Popovich, D.G. Bog bilberry (Vaccinium uliginosum L.) extract reduces cultured Hep-G2, Caco-2, and 3T3-L1 cell viability, affects cell cycle progression, and has variable effects on membrane permeability. J. Food Sci. 2010, 75, 103–107. [Google Scholar] [CrossRef] [PubMed]
- Yi, W.; Fischer, J.; Krewer, G.; Akoh, C.C. Phenolic compounds from blueberries can inhibit colon cancer cell proliferation and induce apoptosis. J. Agric. Food Chem. 2005, 53, 7320–7329. [Google Scholar] [CrossRef] [PubMed]
- Reddivari, L.; Vanamala, J.; Chintharlapalli, S.; Safe, S.H.; Miller, J.C., Jr. Anthocyanin fraction from potato extracts is cytotoxic to prostate cancer cells through activation of caspase-dependent and caspase-independent pathways. Carcinogenesis 2007, 28, 2227–2235. [Google Scholar] [CrossRef] [PubMed]
- Li, D.; Wang, P.; Luo, Y.; Zhao, M.; Chen, F. Health Benefits of Anthocyanins and Molecular Mechanisms: Update from Recent Decade. Crit. Rev. Food Sci. Nutr. 2015, 57, 1729–1741. [Google Scholar] [CrossRef] [PubMed]
- Vareed, S.K.; Schutzki, R.E.; Nair, M.G. Lipid peroxidation, cyclooxygenase enzyme and tumor cell proliferation inhibitory compounds in Cornus kousa fruits. Phytomedicine 2007, 14, 706–709. [Google Scholar] [CrossRef] [PubMed]
- Konczak-Islam, I.; Yoshimoto, M.; Hou, D.X.; Terahara, N.; Yamakawa, O. Potential chemopreventive properties of anthocyanin-rich aqueous extracts from in vitro produced tissue of sweetpotato (Ipomoea batatas L.). J. Agric. Food Chem. 2003, 51, 5916–5922. [Google Scholar] [CrossRef] [PubMed]
- Rugină, D.; Sconţa, Z.; Leopold, L.; Pintea, A.; Bunea, A.; Socaciu, C. Antioxidant activities of chokeberry extracts and the cytotoxic action of their anthocyanin fraction on HeLa human cervical tumor cells. J. Med. Food 2012, 15, 700–706. [Google Scholar] [CrossRef] [PubMed]
- Zhao, C.; Giusti, M.M.; Malik, M.; Moyer, M.P.; Magnuson, B.A. Effects of commercial anthocyanin-rich on colonic cancer and nontumorigenic colonic cell growth. J. Agric. Food. Chem. 2004, 52, 6122–6128. [Google Scholar] [CrossRef] [PubMed]
- Jing, P.; Bomser, J.A.; Schwartz, S.J.; He, J.; Magnuson, B.A.; Giusti, M.M. Structure-Function Relationships of Anthocyanins from Various Anthocyanin-Rich Extracts on the Inhibition of Colon Cancer Cell Growth. J. Agric. Food Chem. 2008, 56, 9391–9398. [Google Scholar] [CrossRef] [PubMed]
- Ribble, D.; Goldstein, N.B.; Norris, D.A.; Shellman, Y.G. A simple technique for quantifying apoptosis in 96-well plates. BMC Biotechnol. 2005, 5, 12. [Google Scholar] [CrossRef] [PubMed]




| Peak | HPLC Rt (min) | λmax (nm) | Molecular Ion (MS) m/z+ | Fragment (MS-MS) m/z+ | Identification of Anthocyanins | Anthocyanins Content (mg/100g FW) |
|---|---|---|---|---|---|---|
| 1 | 4.7 | 275 (525) | 743 | 581,449,287 | Cy-3-O-samb-5-gluc | 48.49 ± 0.74 |
| 2 | 6.8 | 275 (516) | 611 | 449,287 | Cy-3,5-digluc | 84.57 ± 1.53 |
| 3 | 10.4 | 271 (518) | 581 | 449,287 | Cy-hexoside-pentoside | 35.34 ± 1.42 |
| 4 | 12.2 | 279 (518) | 581 | 449,287 | Cy-3-O-samb | 255.56 ± 2.23 |
| 5 | 13.0 | 275 (517) | 449 | 287 | Cy-3-O-gluc | 71.18 ± 1.83 |
| Total | 495.16 ± 7.75 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rugină, D.; Hanganu, D.; Diaconeasa, Z.; Tăbăran, F.; Coman, C.; Leopold, L.; Bunea, A.; Pintea, A. Antiproliferative and Apoptotic Potential of Cyanidin-Based Anthocyanins on Melanoma Cells. Int. J. Mol. Sci. 2017, 18, 949. https://doi.org/10.3390/ijms18050949
Rugină D, Hanganu D, Diaconeasa Z, Tăbăran F, Coman C, Leopold L, Bunea A, Pintea A. Antiproliferative and Apoptotic Potential of Cyanidin-Based Anthocyanins on Melanoma Cells. International Journal of Molecular Sciences. 2017; 18(5):949. https://doi.org/10.3390/ijms18050949
Chicago/Turabian StyleRugină, Dumitriţa, Daniela Hanganu, Zoriţa Diaconeasa, Flaviu Tăbăran, Cristina Coman, Loredana Leopold, Andrea Bunea, and Adela Pintea. 2017. "Antiproliferative and Apoptotic Potential of Cyanidin-Based Anthocyanins on Melanoma Cells" International Journal of Molecular Sciences 18, no. 5: 949. https://doi.org/10.3390/ijms18050949
APA StyleRugină, D., Hanganu, D., Diaconeasa, Z., Tăbăran, F., Coman, C., Leopold, L., Bunea, A., & Pintea, A. (2017). Antiproliferative and Apoptotic Potential of Cyanidin-Based Anthocyanins on Melanoma Cells. International Journal of Molecular Sciences, 18(5), 949. https://doi.org/10.3390/ijms18050949