Dietary Intervention by Phytochemicals and Their Role in Modulating Coding and Non-Coding Genes in Cancer
Abstract
:1. Introduction
2. Phytochemical Compounds Activities Are Determined by Their Structure
3. Antioxidant Benefits of Phytochemicals
4. Phytochemical Compounds and Cancer
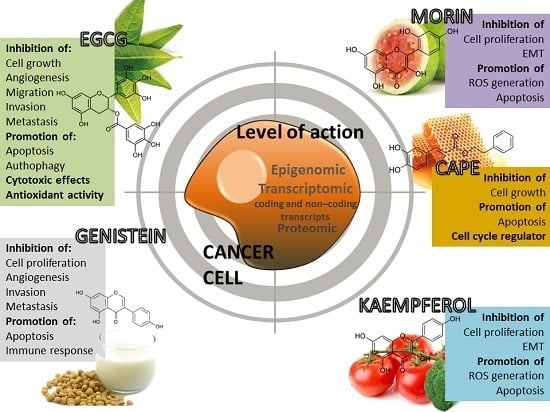
4.1. (−)-Epigallocatechin-3-Gallate (EGCG)
4.2. Morin (3,2′,4′,5,7-Pentahydroxyflavone)
4.3. Caffeic Acid Phenethyl Ester (CAPE)
4.4. Kaempferol
4.5. Genistein
5. Dietary Phytochemicals as Non-Coding Genes Regulators
6. LncRNAs (Long Non-Coding RNAs) as Targets of Dietary Phytochemicals
7. Multidrug Resistance and Polyphenols Relationship
8. Conclusions
Acknowledgments
Conflicts of Interest
References
- Han, X.; Shen, T.; Lou, H. Dietary polyphenols and their biological significance. Int. J. Mol. Sci. 2007, 8, 950–988. [Google Scholar] [CrossRef]
- Tsao, R. Chemistry and biochemistry of dietary polyphenols. Nutrients 2010, 2, 1231–1246. [Google Scholar] [CrossRef] [PubMed]
- De la Rosa, L.A.; Gonzalez-Aguilar, G.A.; Alvarez-Parrilla, E. Fruit and Vegetable Phytochemicals: Chemistry, Nutritional Value and Stability; Wiley-Blackwell: Hoboken, NJ, USA, 2009. [Google Scholar]
- Manach, C.; Scalbert, A.; Morand, C.; Remesy, C.; Jimenez, L. Polyphenols: Food sources and bioavailability. Am. J. Clin. Nutr. 2004, 79, 727–747. [Google Scholar] [PubMed]
- Russo, M.; Spagnuolo, C.; Tedesco, I.; Russo, G.L. Phytochemicals in cancer prevention and therapy: Truth or dare? Toxins 2010, 2, 517–551. [Google Scholar] [CrossRef] [PubMed]
- Petric, R.; Braicu, C.; Raduly, L.; Dragos, N.; Dumitrascu, D.; Berindan-Negoe, I.; Zanoaga, O.; Monroig, P. Phytochemicals modulate carcinogenic signaling pathways in breast and hormone-related cancers. OncoTargets Ther. 2015, 8, 2053–2066. [Google Scholar] [CrossRef] [PubMed]
- Braicu, C.; Pilecki, V.; Balacescu, O.; Irimie, A.; Neagoe, I.B. The relationships between biological activities and structure of flavan-3-ols. Int. J. Mol. Sci. 2011, 12, 9342–9353. [Google Scholar] [CrossRef] [PubMed]
- Baker, M. Deceptive curcumin offers cautionary tale for chemists. Nature 2017, 541, 144–145. [Google Scholar] [CrossRef] [PubMed]
- Smith, T.J. Green Tea Polyphenols in drug discovery—A success or failure? Expert Opin. Drug Discov. 2011, 6, 589–595. [Google Scholar] [CrossRef] [PubMed]
- Irimie, A.I.; Braicu, C.; Cojocneanu-Petric, R.; Berindan-Neagoe, I.; Campian, R.S. Novel technologies for oral squamous carcinoma biomarkers in diagnostics and prognostics. Acta Odontol. Scand. 2015, 73, 161–168. [Google Scholar] [CrossRef] [PubMed]
- Lam, K.S. New aspects of natural products in drug discovery. Trends Microbiol. 2007, 15, 279–289. [Google Scholar] [CrossRef] [PubMed]
- Gu, J.; Gui, Y.; Chen, L.; Yuan, G.; Lu, H.Z.; Xu, X. Use of natural products as chemical library for drug discovery and network pharmacology. PLoS ONE 2013, 8, e62839. [Google Scholar] [CrossRef] [PubMed]
- Bosch, R.; Philips, N.; Suárez-Pérez, J.; Juarranz, A.; Devmurari, A.; Chalensouk-Khaosaat, J.; González, S. Mechanisms of photoaging and cutaneous photocarcinogenesis, and photoprotective strategies with phytochemicals. Antioxidants 2015, 4, 248–268. [Google Scholar] [CrossRef] [PubMed]
- Kusuda, M.; Hatano, T.; Yoshida, T. Water-soluble complexes formed by natural polyphenols and bovine serum albumin: Evidence from gel electrophoresis. Biosci. Biotechnol. Biochem. 2006, 70, 152–160. [Google Scholar] [CrossRef] [PubMed]
- Isaacs, C.E.; Wen, G.Y.; Xu, W.; Jia, J.H.; Rohan, L.; Corbo, C.; di Maggio, V.; Jenkins, E.C.; Hillier, S. Epigallocatechin gallate inactivates clinical isolates of herpes simplex virus. Antimicrob. Agents Chemother. 2008, 52, 962–970. [Google Scholar] [CrossRef] [PubMed]
- Stangl, V.; Dreger, H.; Stangl, K.; Lorenz, M. Molecular targets of tea polyphenols in the cardiovascular system. Cardiovasc. Res. 2007, 73, 348–358. [Google Scholar] [CrossRef] [PubMed]
- Du, G.-J.; Zhang, Z.; Wen, X.-D.; Yu, C.; Calway, T.; Yuan, C.-S.; Wang, C.-Z. Epigallocatechin gallate (EGCG) is the most effective cancer chemopreventive polyphenol in green tea. Nutrients 2012, 4, 1679–1691. [Google Scholar] [CrossRef] [PubMed]
- Cai, S.; Huang, J.; Wang, L.; Dong, Y.; Gong, Y.; Li, J.; Li, Q.; Liu, Z.; Luo, G. Inhibiting effects of epigallocatechin gallate (EGCG) on the formation of age pigment in vitro and in vivo. J. Med. Plants Res. 2011, 5, 5470–5478. [Google Scholar]
- Li, Y.; Tanaka, T.; Kouno, I. Oxidative coupling of the pyrogallol B-ring with a galloyl group during enzymatic oxidation of epigallocatechin 3-O-gallate. Phytochemistry 2007, 68, 1081–1088. [Google Scholar] [CrossRef] [PubMed]
- Muzolf-Panek, M.; Gliszczynska-Swiglo, A.; de Haan, L.; Aarts, J.M.; Szymusiak, H.; Vervoort, J.M.; Tyrakowska, B.; Rietjens, I.M. Role of catechin quinones in the induction of EpRE-mediated gene expression. Chem. Res. Toxicol. 2008, 1, 2352–2360. [Google Scholar] [CrossRef] [PubMed]
- Zhang, P.; Tang, Y.; Li, N.-G.; Zhu, Y.; Duan, J.-A. Bioactivity and chemical synthesis of caffeic acid phenethyl ester and its derivatives. Molecules 2014, 19, 16458–16476. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.H.; Chen, C.S.; Huang, S.H.; Yu, S.H.; Lai, Z.Y.; Huang, S.T.; Lin, C.M. Hydrophilic ester-bearing chlorogenic acid binds to a novel domain to inhibit xanthine oxidase. Planta Med. 2009, 75, 1237–1240. [Google Scholar] [CrossRef] [PubMed]
- Polkowski K1, M.A. Biological properties of genistein. A review of in vitro and in vivo data. Acta Pol. Pharm. 2000, 57, 135–155. [Google Scholar]
- Islam, N. Investigation of comparative shielding of morin against oxidative damage by radicals: A DFT study. Cogent Chem. 2015, 1, 1078272. [Google Scholar] [CrossRef]
- Brown, J. Enhanced sensitivity of human oral tumours to the flavonol, morin, during cancer progression: Involvement of the Akt and stress kinase pathways. Carcinogenesis 2003, 24, 171–177. [Google Scholar] [CrossRef] [PubMed]
- Lugasi, A. Natural Antioxidants Chemistry, Health Effects, and Applications. Edited byF. Shahidi. VIII and 432 pages, numerous figures and tables. AOCS Press, Champaign, Illinois, 1997. Price: 105.00 U$. Mol. Nutr. Food Res. 1997, 41, 321. [Google Scholar] [CrossRef]
- Rice-Evans, C. Flavonoid antioxidants. Curr. Med. Chem. 2001, 8, 797–807. [Google Scholar] [CrossRef] [PubMed]
- Boyer, J.; Liu, R.H. Apple phytochemicals and their health benefits. Nutr. J. 2004, 3, 5. [Google Scholar] [CrossRef] [PubMed]
- Ames, B.N.; Shigenaga, M.K.; Hagen, T.M. Oxidants, antioxidants, and the degenerative diseases of aging. Proc. Natl. Acad. Sci. USA 1993, 90, 7915–7922. [Google Scholar] [CrossRef] [PubMed]
- Hollman, P.C.H.; Hollman, M.B. Katan, Absorption, metabolism and health effects of dietary flavonoids in man. Biomed. Pharmacother. 1997, 51, 305–310. [Google Scholar] [CrossRef]
- Liu, R.H. Health benefits of fruit and vegetables are from additive and synergistic combinations of phytochemicals. Am. J. Clin. Nutr. 2003, 78, 517S–520S. [Google Scholar] [PubMed]
- Gan, R.Y.; Li, H.B.; Sui, Z.Q.; Corke, H. Absorption, metabolism, anti-cancer effect and molecular targets of epigallocatechin gallate (EGCG): An updated review. Crit. Rev. Food Sci. Nutr. 2016, 19. [Google Scholar] [CrossRef] [PubMed]
- Seeram, N.P.; Nair, M.G. Inhibition of lipid peroxidation and structure-activity-related studies of the dietary constituents anthocyanins, anthocyanidins, and catechins. J. Agric. Food Chem. 2002, 50, 5308–5312. [Google Scholar] [CrossRef] [PubMed]
- Wei, Y.; Chen, P.; Ling, T.; Wang, Y.; Dong, R.; Zhang, C.; Zhang, L.; Han, M.; Wang, D.; Wan, X.; et al. Certain (−)-epigallocatechin-3-gallate (EGCG) auto-oxidation products (EAOPs) retain the cytotoxic activities of EGCG. Food Chem. 2016, 204, 218–226. [Google Scholar] [CrossRef] [PubMed]
- Song, S.; Huang, Y.W.; Tian, Y.; Wang, X.J.; Sheng, J. Mechanism of action of (−)-epigallocatechin-3-gallate: Auto-oxidation-dependent activation of extracellular signal-regulated kinase 1/2 in Jurkat cells. Chin. J. Natl. Med. 2016, 12, 654–662. [Google Scholar] [CrossRef]
- Lee-Hilz, Y.Y.; Boerboom, A.M.; Westphal, A.H.; Berkel, W.J.; Aarts, J.M.; Rietjens, I.M. Pro-oxidant activity of flavonoids induces EpRE-mediated gene expression. Chem. Res. Toxicol. 2006, 19, 1499–1505. [Google Scholar] [CrossRef] [PubMed]
- Manson, M.M. Cancer prevention—The potential for diet to modulate molecular signalling. Trends Mol. Med. 2003, 9, 11–18. [Google Scholar] [CrossRef]
- Surh, Y.-J. Cancer chemoprevention with dietary phytochemicals. Nat. Rev. Cancer 2003, 3, 768–780. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Chen, C. Emerging applications of metabolomics in studying chemopreventive phytochemicals. AAPS J. 2013, 15, 941–950. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Khor, T.O.; Shu, L.; Su, Z.Y.; Fuentes, F.; Lee, J.H.; Kong, A.N. Plants vs. cancer: A review on natural phytochemicals in preventing and treating cancers and their druggability. Anticancer Agents Med. Chem. 2012, 12, 1281–1305. [Google Scholar] [CrossRef] [PubMed]
- Pandey, K.B.; Rizvi, S.I. Plant polyphenols as dietary antioxidants in human health and disease. Oxid. Med. Cell. Longev. 2009, 2, 270–278. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Lei, Z.; Huang, Z.; Zhang, X.; Zhou, Y.; Luo, Z.; Zeng, W.; Su, J.; Peng, C.; Chen, X. Epigallocatechin-3-gallate(EGCG) suppresses melanoma cell growth and metastasis by targeting TRAF6 activity. Oncotarget 2016, 7, 79557–79571. [Google Scholar] [CrossRef] [PubMed]
- Manjegowda, M.C.; Deb, G.; Kumar, N.; Limaye, A.M. Expression profiling of genes modulated by estrogen, EGCG or both in MCF-7 breast cancer cells. Genom. Data 2015, 5, 210–212. [Google Scholar] [CrossRef] [PubMed]
- Braicu, C.; Gherman, C.D.; Irimie, A.; Berindan-Neagoe, I. Epigallocatechin-3-gallate (egcg) inhibits cell proliferation and migratory behaviour of triple negative breast cancer cells. J. Nanosci. Nanotechnol. 2013, 13, 632–637. [Google Scholar] [CrossRef] [PubMed]
- Tudoran, O.; Soritau, O.; Balacescu, O.; Balacescu, L.; Braicu, C.; Rus, M.; Gherman, C.; Virag, P.; Irimie, F.; Berindan-Neagoe, I. Early transcriptional pattern of angiogenesis induced by EGCG treatment in cervical tumour cells. J. Cell. Mol. Med. 2012, 16, 520–530. [Google Scholar] [CrossRef] [PubMed]
- Irimie, A.I.; Braicu, C.; Pileczki, V.; Petrushev, B.; Soritau, O.; Campian, R.S.; Berindan-Neagoe, I. Knocking down of p53 triggers apoptosis and autophagy, concomitantly with inhibition of migration on SSC-4 oral squamous carcinoma cells. Mol. Cell. Biochem. 2016, 419, 75–82. [Google Scholar] [CrossRef] [PubMed]
- Braicu, C.; Gherman, C. Epigallocatechin gallate induce cell death and apoptosis in triple negative breast cancer cells Hs578T. J. Drug Target. 2012, 21. [Google Scholar] [CrossRef] [PubMed]
- Irimie, A.I.; Braicu, C.; Zanoaga, O.; Pileczki, V.; Gherman, C.; Berindan-Neagoe, I.; Campian, R.S. Epigallocatechin-3-gallate suppresses cell proliferation and promotes apoptosis and autophagy in oral cancer SSC-4 cells. OncoTargets Ther. 2015, 8, 461–470. [Google Scholar]
- Iriti, M.; Varoni, E. Chemopreventive potential of flavonoids in oral squamous cell carcinoma in human studies. Nutrients 2013, 5, 2564–2576. [Google Scholar] [CrossRef] [PubMed]
- Lee, U.-L.; Choi, S.-W. The chemopreventive properties and therapeutic modulation of green tea polyphenols in oral squamous cell carcinoma. ISRN Oncol. 2011, 2011, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Zou, C.; Liu, H.; Feugang, J.M.; Hao, Z.; Chow, H.H.S.; Garcia, F. Green tea compound in chemoprevention of cervical cancer. Int. J. Gynecol. Cancer 2010, 20, 617–624. [Google Scholar] [CrossRef] [PubMed]
- Shim, J.H.; Su, Z.Y.; Chae, J.I.; Kim, D.J.; Zhu, F.; Ma, W.Y.; Bode, A.M.; Yang, C.S.; Dong, Z. Epigallocatechin gallate suppresses lung cancer cell growth through Ras-GTPase-activating protein SH3 domain-binding protein 1. Cancer Prev. Res. 2010, 3, 670–679. [Google Scholar] [CrossRef] [PubMed]
- Jin, H.; Gong, W.; Zhang, C.; Wang, S. Epigallocatechin gallate inhibits the proliferation of colorectal cancer cells by regulating Notch signaling. OncoTargets Ther. 2013, 6, 145–153. [Google Scholar] [CrossRef] [PubMed]
- Albrecht, D.S.; Clubbs, E.A.; Ferruzzi, M.; Bomser, J.A. Epigallocatechin-3-gallate (EGCG) inhibits PC-3 prostate cancer cell proliferation via MEK-independent ERK1/2 activation. Chemi. Biol. Interact. 2008, 171, 89–95. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Tang, J.; Du, Y.; Ding, J.; Liu, J.Y. The green tea polyphenol EGCG potentiates the antiproliferative activity of sunitinib in human cancer cells. Tumour Biol. 2016, 37, 8555–8566. [Google Scholar] [CrossRef] [PubMed]
- Cerezo-Guisado, M.I.; Zur, R.; Lorenzo, M.J.; Risco, A.; Martin-Serrano, M.A.; Alvarez-Barrientos, A.; Cuenda, A.; Centeno, F. Implication of Akt, ERK1/2 and alternative p38MAPK signalling pathways in human colon cancer cell apoptosis induced by green tea EGCG. Food Chem. Toxicol. 2015, 84, 125–132. [Google Scholar] [CrossRef] [PubMed]
- Siddiqui, I.A.; Asim, M.; Hafeez, B.B.; Adhami, V.M.; Tarapore, R.S.; Mukhtar, H. Green tea polyphenol EGCG blunts androgen receptor function in prostate cancer. FASEB J. 2011, 25, 1198–1207. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.H.; Kwak, J.; Choi, H.K.; Choi, K.C.; Kim, S.; Lee, J.; Jun, W.; Park, H.J.; Yoon, H.G. EGCG suppresses prostate cancer cell growth modulating acetylation of androgen receptor by anti-histone acetyltransferase activity. Int. J. Mol. Med. 2012, 30, 69–74. [Google Scholar] [PubMed]
- Kawabata, K.; Tanaka, T.; Honjo, S.; Kakumoto, M.; Hara, A.; Makita, H.; Tatematsu, N.; Ushida, J.; Tsuda, H.; Mori, H. Chemopreventive effect of dietary flavonoid morin on chemically induced rat tongue carcinogenesis. Int. J. Cancer 1999, 83, 381–386. [Google Scholar] [CrossRef]
- Karimi, R.; Parivar, K.; Roudbari, N.H.; Sadeghi, S.V.; Hashemi, M.; Hayat, P. Anti-proliferative and apoptotic effects of morin in human Leukemia cell lines (HUT-78). Int. J. Cell. Mol. Biotechnol. 2013, 2013, 1–13. [Google Scholar] [CrossRef]
- Hsiang, C.Y.; Wu, S.L.; Ho, T.Y. Morin inhibits 12-O-tetradecanoylphorbol-13-acetate-induced hepatocellular transformation via activator protein 1 signaling pathway and cell cycle progression. Biochem. Pharmacol. 2005, 69, 1603–1611. [Google Scholar] [CrossRef] [PubMed]
- Kuo, H.M.; Chang, L.S.; Lin, Y.L.; Lu, H.F.; Yang, J.S.; Lee, J.H.; Chung, J.G. Morin inhibits the growth of human leukemia HL-60 cells via cell cycle arrest and induction of apoptosis through mitochondria dependent pathway. Anticancer Res. 2007, 27, 395–405. [Google Scholar] [PubMed]
- Kondath, S.; Raghavan, B.S.; Anantanarayanan, R.; Rajaram, R. Synthesis and characterisation of morin reduced gold nanoparticles and its cytotoxicity in MCF-7 cells. Chem. Biol. Int. 2014, 224, 78–88. [Google Scholar] [CrossRef] [PubMed]
- Park, C.; Lee, W.S.; Go, S.; Nagappan, A.; Han, M.H.; Hong, S.H.; Kim, G.S.; Kim, G.Y.; Kwon, T.K.; Ryu, C.H.; et al. Morin, a flavonoid from moraceae, induces apoptosis by induction of BAD protein in human Leukemic cells. Int. J. Mol. Sci. 2015, 16, 645–659. [Google Scholar] [CrossRef] [PubMed]
- Hyun, H.B.; Lee, W.S.; Go, S.I.; Nagappan, A.; Park, C.; Han, M.H.; Hong, S.H.; Kim, G.; Kim, G.Y.; Cheong, J.; et al. The flavonoid morin from Moraceae induces apoptosis by modulation of Bcl-2 family members and Fas receptor in HCT 116 cells. Int. J. Oncol. 2015, 46, 2670–2678. [Google Scholar] [CrossRef] [PubMed]
- Jin, H.; Lee, W.S.; Eun, S.Y.; Jung, J.H.; Park, H.S.; Kim, G.; Choi, Y.H.; Ryu, C.H.; Jung, J.M.; Hong, S.C.; et al. Morin, a flavonoid from Moraceae, suppresses growth and invasion of the highly metastatic breast cancer cell line MDA-MB231 partly through suppression of the Akt pathway. Int. J. Oncol. 2014, 45, 1629–1637. [Google Scholar] [PubMed]
- Lee, J.; Jin, H.; Lee, W.S.; Nagappan, A.; Choi, Y.H.; Kim, G.S.; Jung, J.; Ryu, C.H.; Shin, S.C.; Hong, S.C.; et al. Morin, a flavonoid from moraceae, inhibits cancer cell adhesion to endothelial cells and EMT by downregulating VCAM1 and ncadherin. Asian Pac. J. Cancer Prev. 2016, 17, 3071–3075. [Google Scholar] [PubMed]
- Son, S.; Lewis, B.A. Free radical scavenging and antioxidative activity of caffeic acid amide and ester analogues: Structure−activity relationship. J. Agric. Food Chem. 2002, 50, 468–472. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Omene, C.; Karkoszka, J.; Bosland, M.; Eckard, J.; Klein, C.B.; Frenkel, K. Caffeic acid phenethyl ester (CAPE), derived from a honeybee product propolis, exhibits a diversity of anti-tumor effects in pre-clinical models of human breast cancer. Cancer Lett. 2011, 308, 43–53. [Google Scholar] [CrossRef] [PubMed]
- Rzepecka-Stojko, A.; Kabała-Dzik, A.; Moździerz, A.; Kubina, R.; Wojtyczka, R.; Stojko, R.; Dziedzic, A.; Jastrzębska-Stojko, Ż.; Jurzak, M.; Buszman, E.; et al. Caffeic acid phenethyl ester and ethanol extract of propolis induce the complementary cytotoxic effect on triple-negative breast cancer cell lines. Molecules 2015, 20, 9242–9262. [Google Scholar] [CrossRef] [PubMed]
- Cho, M.S.; Park, W.S.; Jung, W.-K.; Qian, Z.-J.; Lee, D.-S.; Choi, J.-S.; Lee, D.-Y.; Park, S.-G.; Seo, S.-K.; Kim, H.-J.; et al. Caffeic acid phenethyl ester promotes anti-inflammatory effects by inhibiting MAPK and NF-κB signaling in activated HMC-1 human mast cells. Pharm. Biol. 2014, 52, 926–932. [Google Scholar] [CrossRef] [PubMed]
- Altuntaş, A.; Yılmaz, H.R.; Altuntaş, A.; Uz, E.; Demir, M.; Gökçimen, A.; Aksu, O.; Bayram, D.Ş.; Sezer, M.T. Caffeic acid phenethyl ester protects against amphotericin B induced nephrotoxicity in rat model. BioMed Res. Int. 2014, 2014, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Zhou, K.; Li, X.; Du, Q.; Li, D.; Hu, M.; Yang, X.; Jiang, Q.; Li, Z. A CAPE analogue as novel antiplatelet agent efficiently inhibits collagen-induced platelet aggregation. Pharmazie 2014, 69, 615–620. [Google Scholar] [PubMed]
- Gherman, C.; Braicu, O.L.; Zanoaga, O.; Jurj, A.; Pileczki, V.; Maralani, M.; Drigla, F.; Braicu, C.; Budisan, L.; Achimas-Cadariu, P.; et al. Caffeic acid phenethyl ester activates pro-apoptotic and epithelial-mesenchymal transition-related genes in ovarian cancer cells A2780 and A2780cis. Mol. Cell. Biochem. 2016, 413, 189–198. [Google Scholar] [CrossRef] [PubMed]
- Xiang, D.; Wang, D.; He, Y.; Xie, J.; Zhong, Z.; Li, Z.; Xie, J. Caffeic acid phenethyl ester induces growth arrest and apoptosis of colon cancer cells via the β-catenin/T-cell factor signaling. Anticancer Drugs 2006, 17, 753–762. [Google Scholar] [CrossRef] [PubMed]
- Omene, C.O.; Wu, J.; Frenkel, K. Caffeic acid phenethyl ester (CAPE) derived from propolis, a honeybee product, inhibits growth of breast cancer stem cells. Investig. New Drugs 2012, 30, 1279–1288. [Google Scholar] [CrossRef] [PubMed]
- Lin, H.-P.; Jiang, S.S.; Chuu, C.-P. Caffeic acid phenethyl ester causes p21Cip1 induction, Akt signaling reduction, and growth inhibition in PC-3 human prostate cancer cells. PLoS ONE 2012, 7, e31286. [Google Scholar] [CrossRef] [PubMed]
- Kuo, Y.-Y.; Jim, W.-T.; Su, L.-C.; Chung, C.-J.; Lin, C.-Y.; Huo, C.; Tseng, J.-C.; Huang, S.-H.; Lai, C.-J.; Chen, B.-C.; et al. Caffeic acid phenethyl ester is a potential therapeutic agent for oral cancer. Int. J. Mol. Sci. 2015, 16, 10748–10766. [Google Scholar] [CrossRef] [PubMed]
- Watabe, M.; Hishikawa, K.; Takayanagi, A.; Shimizu, N.; Nakaki, T. Caffeic acid phenethyl ester induces apoptosis by inhibition of NFκB and activation of Fas in human breast cancer MCF-7 cells. J. Biol. Chem. 2004, 279, 6017–6026. [Google Scholar] [CrossRef] [PubMed]
- Hsu, T.-H.; Chu, C.-C.; Hung, M.-W.; Lee, H.-J.; Hsu, H.-J.; Chang, T.-C. Caffeic acid phenethyl ester induces E2F-1-mediated growth inhibition and cell-cycle arrest in human cervical cancer cells. FEBS J. 2013, 280, 2581–2593. [Google Scholar] [CrossRef] [PubMed]
- Huang, W.-W.; Chiu, Y.-J.; Fan, M.-J.; Lu, H.-F.; Yeh, H.-F.; Li, K.-H.; Chen, P.-Y.; Chung, J.-G.; Yang, J.-S. Kaempferol induced apoptosis via endoplasmic reticulum stress and mitochondria-dependent pathway in human osteosarcoma U-2 OS cells. Mol. Nutr. Food Res. 2010, 54, 1585–1595. [Google Scholar] [CrossRef] [PubMed]
- Kim, B.-W.; Lee, E.-R.; Min, H.-M.; Jeong, H.-S.; Ahn, J.-Y.; Kim, J.-H.; Choi, H.-Y.; Choi, H.; Kim, E.Y.; Park, S.P.; et al. Sustained ERK activation is involved in the kaempferol-induced apoptosis of breast cancer cells and is more evident under 3-D culture condition. Cancer Biol. Ther. 2008, 7, 1080–1089. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.M.; Lee, D.E.; Seo, S.K.; Hwang, M.K.; Heo, Y.S.; Lee, K.W.; Lee, H.J. Phosphatidylinositol 3-kinase, a novel target molecule for the inhibitory effects of kaempferol on neoplastic cell transformation. Carcinogenesis 2010, 31, 1338–1343. [Google Scholar] [CrossRef] [PubMed]
- Ackland, M.L.; van de Waarsenburg, S.; Jones, R. Synergistic antiproliferative action of the flavonols quercetin and kaempferol in cultured human cancer cell lines. In Vivo 2005, 19, 69–76. [Google Scholar] [PubMed]
- Luo, H.; Daddysman, M.K.; Rankin, G.O.; Jiang, B.-H.; Chen, Y.C. Kaempferol enhances cisplatin’s effect on ovarian cancer cells through promoting apoptosis caused by down regulation of cMyc. Cancer Cell. Int. 2010, 10, 16. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Chen, A.Y.; Li, M.; Chen, C.; Yao, Q. Ginkgo biloba extract kaempferol inhibits cell proliferation and induces apoptosis in pancreatic cancer cells. J. Surg. Res. 2008, 148, 17–23. [Google Scholar] [CrossRef] [PubMed]
- Lin, C.-W.; Chen, P.-N.; Chen, M.-K.; Yang, W.-E.; Tang, C.-H.; Yang, S.-F.; Hsieh, Y.-S. Kaempferol reduces matrix metalloproteinase-2 expression by down-regulating ERK1/2 and the activator protein-1 signaling pathways in oral cancer cells. PLoS ONE 2013, 8, e80883. [Google Scholar] [CrossRef] [PubMed]
- Huang, W.W.; Tsai, S.C.; Peng, S.F.; Lin, M.W.; Chiang, J.H.; Chiu, Y.J.; Fushiya, S.; Tseng, M.T.; Yang, J.S. Kaempferol induces autophagy through AMPK and AKT signaling molecules and causes G2/M arrest via downregulation of CDK1/cyclin B in SK-HEP-1 human hepatic cancer cells. Int. J. Oncol. 2013, 42, 2069–2077. [Google Scholar] [PubMed]
- Choi, E.J.; Ahn, W.S. Kaempferol induced the apoptosis via cell cycle arrest in human breast cancer MDA-MB-453 cells. Nutr. Res. Pract. 2008, 2, 322. [Google Scholar] [CrossRef] [PubMed]
- Tsiklauri, L.; An, G.; Ruszaj, D.M.; Alaniya, M.; Kemertelidze, E.; Morris, M.E. Simultaneous determination of the flavonoids robinin and kaempferol in human breast cancer cells by liquid chromatography-tandem mass spectrometry. J. Pharm. Biom. Anal. 2011, 55, 109–113. [Google Scholar] [CrossRef] [PubMed]
- Jeong, J.C.; Kim, M.S.; Kim, T.H.; Kim, Y.K. Kaempferol induces cell death through ERK and Akt-dependent down-regulation of XIAP and survivin in human glioma cells. Neurochem. Res. 2008, 34, 991–1001. [Google Scholar] [CrossRef] [PubMed]
- Marfe, G.; Tafani, M.; Indelicato, M.; Sinibaldi-Salimei, P.; Reali, V.; Pucci, B.; Fini, M.; Russo, M.A. Kaempferol induces apoptosis in two different cell lines via Akt inactivation, Bax and SIRT3 activation, and mitochondrial dysfunction. J. Cell. Biochem. 2009, 106, 643–650. [Google Scholar] [CrossRef] [PubMed]
- Xie, F.; Su, M.; Qiu, W.; Zhang, M.; Guo, Z.; Su, B.; Liu, J.; Li, X.; Zhou, L. Kaempferol promotes apoptosis in human bladder cancer cells by inducing the tumor suppressor, PTEN. Int. J. Mol. Sci. 2013, 14, 21215–21226. [Google Scholar] [CrossRef] [PubMed]
- Dang, Q.; Song, W.; Xu, D.; Ma, Y.; Li, F.; Zeng, J.; Zhu, G.; Wang, X.; Chang, L.S.; He, D.; Li, L. Kaempferol suppresses bladder cancer tumor growth by inhibiting cell proliferation and inducing apoptosis. Mol. Carcinog. 2015, 54, 831–840. [Google Scholar] [CrossRef] [PubMed]
- Lee, G.A.; Choi, K.C.; Hwang, K.A. Kaempferol, a phytoestrogen, suppressed triclosan-induced epithelial-mesenchymal transition and metastatic-related behaviors of MCF-7 breast cancer cells. Environ. Toxicol. Pharm. 2017, 49, 48–57. [Google Scholar] [CrossRef] [PubMed]
- Jo, E.; Park, S.J.; Choi, Y.S.; Jeon, W.K.; Kim, B.C. Kaempferol suppresses transforming growth factor-β1-induced epithelial-to-mesenchymal transition and migration of A549 lung cancer cells by inhibiting Akt1-mediated phosphorylation of Smad3 at Threonine-179. Neoplasia 2015, 17, 525–537. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Kong, D.; Bao, B.; Ahmad, A.; Sarkar, F.H. Induction of cancer cell death by isoflavone: The role of multiple signaling pathways. Nutrients 2011, 3, 877–896. [Google Scholar] [CrossRef] [PubMed]
- Zhu, J.; Zhang, C.; Qing, Y.; Cheng, Y.; Jiang, X.; Li, M.; Yang, Z.; Wang, D. Genistein induces apoptosis by stabilizing intracellular p53 protein through an APE1-mediated pathway. Free Radic. Biol. Med. 2015, 86, 209–218. [Google Scholar] [CrossRef] [PubMed]
- Mahmoud, A.M.; Zhu, T.; Parray, A.; Siddique, H.R.; Yang, W.; Saleem, M.; Bosland, M.C. Differential effects of genistein on prostate cancer cells depend on mutational status of the androgen receptor. PLoS ONE 2013, 8, e78479. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.; Tai, Y.-C.; Wong, C.-H.S.; Tai, L.-K.; Koay, E.S.-C.; Chen, C.-S. Molecular response of leukemia HL-60 cells to genistein treatment, a proteomics study. Leuk. Res. 2007, 31, 75–82. [Google Scholar] [CrossRef] [PubMed]
- Narasimhan, K.; Lee, Y.M.; Lim, T.K.; Port, S.A.; Han, J.-H.; Chen, C.-S.; Lin, Q. Genistein exerts anti-leukemic effects on genetically different acute myeloid leukemia cell lines by inhibiting protein synthesis and cell proliferation while inducing apoptosis—Molecular insights from an iTRAQ™ quantitative proteomics study. Oncoscience 2015, 2, 111. [Google Scholar] [CrossRef] [PubMed]
- Pavese, J.M.; Krishna, S.N.; Bergan, R.C. Genistein inhibits human prostate cancer cell detachment, invasion, and metastasis. Am. J. Clin. Nutr. 2014, 100, 431S–436S. [Google Scholar] [CrossRef] [PubMed]
- Pons, D.G.; Nadal-Serrano, M.; Torrens-Mas, M.; Oliver, J.; Roca, P. The phytoestrogen genistein affects breast cancer cells treatment depending on the ERα/ERβ ratio. J. Cell. Biochem. 2015, 117, 218–229. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.F.; Wong, M.S. Genistein enhances insulin-like growth factor signaling pathway in human breast cancer (MCF-7) cells. J. Clin. Endocrinol. Metab. 2004, 89, 2351–2359. [Google Scholar] [CrossRef] [PubMed]
- Karsli-Ceppioglu, S.; Ngollo, M.; Judes, G.; Penault-Llorca, F.; Bignon, Y.-J.; Guy, L.; Bernard-Gallon, D. The Role of Soy Phytoestrogens on Genetic and Epigenetic Mechanisms of Prostate Cancer. In Mechanism of the Anticancer Effect of Phytochemicals; Elsevier BV: Amsterdan, The Netherlands, 2015; pp. 193–221. [Google Scholar]
- Russo, M.; Russo, G.L.; Daglia, M.; Kasi, P.D.; Ravi, S.; Nabavi, S.F.; Nabavi, S.M. Understanding genistein in cancer: The “good” and the “bad” effects: A review. Food Chem. 2016, 196, 589–600. [Google Scholar] [CrossRef] [PubMed]
- Qin, J.; Teng, J.; Zhu, Z.; Chen, J.; Huang, W.J. Genistein induces activation of the mitochondrial apoptosis pathway by inhibiting phosphorylation of Akt in colorectal cancer cells. Pharm. Biol. 2016, 54, 74–79. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Duan, Y.; Zhang, X.; Ye, Y.; Ge, B.; Chen, J. Genistein induces apoptosis by the inactivation of the IGF-1R/p-Akt signaling pathway in MCF-7 human breast cancer cells. Food Funct. 2015, 6, 995–1000. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Yang, B.; Zhou, K.; Li, H.; Li, D.; Gao, H.; Zhang, T.; Wei, D.; Li, Z.; Diao, Y. Potential therapeutic mechanism of genistein in breast cancer involves inhibition of cell cycle regulation. Mol. Med. Rep. 2015, 11, 1820–1826. [Google Scholar] [CrossRef] [PubMed]
- Xiao, X.; Liu, Z.; Wang, R.; Wang, J.; Zhang, S.; Cai, X.; Wu, K.; Bergan, R.C.; Xu, L.; Fan, D. Genistein suppresses FLT4 and inhibits human colorectal cancer metastasis. Oncotarget 2015, 6, 225–239. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.J.; Lin, C.M.; Lee, C.Y.; Shih, N.C.; Peng, S.F.; Tsuzuki, M.; Amagaya, S.; Huang, W.W.; Yang, J.S. Kaempferol suppresses cell metastasis via inhibition of the ERK-p38-JNK and AP-1 signaling pathways in U-2 OS human osteosarcoma cells. Oncol. Rep. 2013, 30, 925–932. [Google Scholar] [PubMed]
- Mahmoud, A.M.; Al-Alem, U.; Ali, M.M.; Bosland, M.C. Genistein increases estrogen receptor beta expression in prostate cancer via reducing its promoter methylation. J. Steroid Biochem. Mol. Biol. 2015, 152, 62–75. [Google Scholar] [CrossRef] [PubMed]
- Mineva, N.D.; Paulson, K.E.; Naber, S.P.; Yee, A.S.; Sonenshein, G.E. Epigallocatechin-3-gallate inhibits stem-like inflammatory breast cancer cells. PLoS ONE 2013, 8, e73464. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.; Du, W.; Yang, D. Inhibition of green tea polyphenol EGCG((−)-epigallocatechin-3-gallate) on the proliferation of gastric cancer cells by suppressing canonical Wnt/β-catenin signalling pathway. Int. J. Food Sci. Nutr. 2016, 67, 818–827. [Google Scholar] [CrossRef] [PubMed]
- Kuo, Y.Y.; Lin, H.P.; Huo, C.; Su, L.C.; Yang, J.; Hsiao, P.H.; Chiang, H.C.; Chung, C.J.; Wang, H.D.; Chang, J.Y.; et al. Caffeic acid phenethyl ester suppresses proliferation and survival of TW2.6 human oral cancer cells via inhibition of Akt signaling. Int. J. Mol. Sci. 2013, 14, 8801–8817. [Google Scholar] [CrossRef] [PubMed]
- Chuu, C.P.; Lin, H.P.; Ciaccio, M.F.; Kokontis, J.M.; Hause, R.J.; Hiipakka, R.A., Jr.; Liao, S.; Jones, R.B. Caffeic acid phenethyl ester suppresses the proliferation of human prostate cancer cells through inhibition of p70S6K and Akt signaling networks. Cancer Prev. Res. 2012, 5, 788–797. [Google Scholar] [CrossRef] [PubMed]
- Lin, H.P.; Lin, C.Y.; Huo, C.; Hsiao, P.H.; Su, L.C.; Jiang, S.S.; Chan, T.M.; Chang, C.H.; Chen, L.T.; Kung, H.J.; et al. Caffeic acid phenethyl ester induced cell cycle arrest and growth inhibition in androgen-independent prostate cancer cells via regulation of Skp2, p53, p21Cip1 and p27Kip1. Oncotarget 2015, 6, 6684–6707. [Google Scholar] [CrossRef] [PubMed]
- Braicu, C.; Tomuleasa, C.; Monroig, P.; Cucuianu, A.; Berindan-Neagoe, I.; Calin, G.A. Exosomes as divine messengers: Are they the Hermes of modern molecular oncology? Cell Death Differ. 2015, 22, 34–45. [Google Scholar] [CrossRef] [PubMed]
- Berindan-Neagoe, I.; Calin, G.A. Molecular pathways: MicroRNAs, cancer cells, and microenvironment. Clin. Cancer Res. 2014, 20, 6247–6253. [Google Scholar] [CrossRef] [PubMed]
- Berindan-Neagoe, I.; Pdel, C.M.; Pasculli, B.; Calin, G.A. MicroRNAome genome: A treasure for cancer diagnosis and therapy. CA Cancer J. Clin. 2014, 64, 311–336. [Google Scholar] [CrossRef] [PubMed]
- Srivastava, S.K.; Arora, S.; Averett, C.; Singh, S.; Singh, A.P. Modulation of microRNAs by phytochemicals in cancer: Underlying mechanisms and translational significance. BioMed Res. Int. 2015, 2015, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Thakur, V.S.; Deb, G.; Babcook, M.A.; Gupta, S. Plant phytochemicals as epigenetic modulators: Role in cancer chemoprevention. AAPS J. 2014, 16, 151–163. [Google Scholar] [CrossRef] [PubMed]
- Masika, J.; Hescheler, J.; Liang, H.; Zhao, Y. Modulation of miRNAs by natural agents: Nature’s way of dealing with cancer. RNA Discov. 2016, 3, 2375–2467. [Google Scholar]
- Biersack, B. Non-coding RNA/microRNA-modulatory dietary factors and natural products for improved cancer therapy and prevention: Alkaloids, organosulfur compounds, aliphatic carboxylic acids and water-soluble vitamins. Non-Coding RNA Res. 2016, 1, 51–63. [Google Scholar] [CrossRef]
- Shankar, S.; Kumar, D.; Srivastava, R.K. Epigenetic modifications by dietary phytochemicals: Implications for personalized nutrition. Pharmacol. Ther. 2013, 138, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Naselli, F.; Belshaw, N.J.; Gentile, C.; Tutone, M.; Tesoriere, L.; Livrea, M.A.; Caradonna, F. Phytochemical indicaxanthin inhibits colon cancer cell growth and affects the DNA methylation status by influencing epigenetically modifying enzyme expression and activity. J. Nutr. Nutr. 2015, 8, 114–127. [Google Scholar] [CrossRef] [PubMed]
- Nakazawa, T.; Hayashi, K.; Naitoh, I.; Miyabe, K.; Shimizu, S.; Kondo, H.; Nishi, Y.; Yoshida, M.; Umemura, S.; Hori, Y.; et al. Chemopreventive effect of resveratrol and apocynin on pancreatic carcinogenesis via modulation of nuclear phosphorylated GSK3β and ERK1/2. Oncotarget 2015, 6, 42963–42975. [Google Scholar]
- Johnson, S.M.; Grosshans, H.; Shingara, J.; Byrom, M.; Jarvis, R.; Cheng, A.; Labourier, E.; Reinert, K.L.; Brown, D.; Slack, F.J. RAS is regulated by the let-7 microRNA family. Cell 2005, 120, 635–647. [Google Scholar] [CrossRef] [PubMed]
- Barh, D.; Malhotra, R.; Ravi, B.; Sindhurani, P. Microrna let-7: An emerging next-generation cancer therapeutic. Curr. Oncol. 2010, 17, 70–80. [Google Scholar] [CrossRef] [PubMed]
- Takamizawa, J. Reduced expression of the let-7 microRNAs in human lung cancers in association with shortened postoperative survival. Cancer Res. 2004, 64, 3753–3756. [Google Scholar] [CrossRef] [PubMed]
- Brueckner, B.; Stresemann, C.; Kuner, R.; Mund, C.; Musch, T.; Meister, M.; Sultmann, H.; Lyko, F. The Human let-7a-3 locus contains an epigenetically regulated microRNA gene with oncogenic function. Cancer Res. 2007, 67, 1419–1423. [Google Scholar] [CrossRef] [PubMed]
- Xia, X.-M. Clinical significance and the correlation of expression between Let-7 and K-ras in non-small cell lung cancer. Oncol. Lett. 2010, 1, 1045–1047. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Deng, C.; Wang, J.; Xiao, J.; Gatalica, Z.; Recker, R.R.; Xiao, G.G. Let-7 family miRNAs regulate estrogen receptor α signaling in estrogen receptor positive breast cancer. Breast Cancer Res. Treat. 2011, 127, 69–80. [Google Scholar] [CrossRef] [PubMed]
- Cimmino, A.; Calin, G.A.; Fabbri, M.; Iorio, M.V.; Ferracin, M.; Shimizu, M.; Wojcik, S.E.; Aqeilan, R.I.; Zupo, S.; Dono, M.; et al. miR-15 and miR-16 induce apoptosis by targeting BCL2. Proc. Natl. Acad. Sci. USA 2005, 102, 13944–13949. [Google Scholar] [CrossRef] [PubMed]
- Aqeilan, R.I.; Calin, G.A.; Croce, C.M. miR-15a and miR-16–1 in cancer: Discovery, function and future perspectives. Cell Death Differ. 2009, 17, 215–220. [Google Scholar] [CrossRef] [PubMed]
- Chang, T.-C.; Wentzel, E.A.; Kent, O.A.; Ramachandran, K.; Mullendore, M.; Lee, K.H.; Feldmann, G.; Yamakuchi, M.; Ferlito, M.; Lowenstein, C.J.; et al. Transactivation of miR-34a by p53 broadly influences gene expression and promotes apoptosis. Mol. Cell 2007, 26, 745–752. [Google Scholar] [CrossRef] [PubMed]
- Xu, L. miR-203 regulates the proliferation, apoptosis and cell cycle progression of pancreatic cancer cells by targeting survivin. Mol. Med. Rep. 2013, 8, 379–384. [Google Scholar] [CrossRef] [PubMed]
- Sonkoly, E.; Lovén, J.; Xu, N.; Meisgen, F.; Wei, T.; Brodin, P.; Jaks, V.; Kasper, M.; Shimokawa, T.; Harada, M.; et al. MicroRNA-203 functions as a tumor suppressor in basal cell carcinoma. Oncogenesis 2012, 1, e3. [Google Scholar] [CrossRef] [PubMed]
- He, L.; Thomson, J.M.; Hemann, M.T.; Hernando-Monge, E.; Mu, D.; Goodson, S.; Powers, S.; Cordon-Cardo, C.; Lowe, S.W.; Hannon, G.J.; et al. A microRNA polycistron as a potential human oncogene. Nature 2005, 435, 828–833. [Google Scholar] [CrossRef] [PubMed]
- Rinaldi, A.; Poretti, G.; Kwee, I.; Zucca, E.; Catapano, C.V.; Tibiletti, M.G.; Bertoni, F. Concomitant MYC and microRNA cluster miR-17–92 (C13orf25) amplification in human mantle cell lymphoma. Leuk. Lymphoma 2007, 48, 410–412. [Google Scholar] [CrossRef] [PubMed]
- Mendell, J.T. miRiad roles for the miR-17–92 cluster in development and disease. Cell 2008, 133, 217–222. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Huang, J.; Yang, N.; Greshock, J.; Megraw, M.S.; Giannakakis, A.; Liang, S.; Naylor, T.L.; Barchetti, A.; Ward, M.R.; et al. microRNAs exhibit high frequency genomic alterations in human cancer. Proc. Natl. Acad. Sci. USA 2006, 103, 9136–9141. [Google Scholar] [CrossRef] [PubMed]
- Tang, R.; Liang, L.; Luo, D.; Feng, Z.; Huang, Q.; He, R.; Gan, T.; Yang, L.; Chen, G. Downregulation of miR-30a is associated with poor prognosis in lung cancer. Med. Sci. Monit. 2015, 21, 2514–2520. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Lai, N.; Liao, K.; Sun, J.; Lin, Y. microRNA-210 regulates cell proliferation and apoptosis by targeting regulator of differentiation 1 in glioblastoma cells. Folia Neuropathol. 2015, 3, 236–244. [Google Scholar] [CrossRef] [PubMed]
- Thomas, R.; Kim, M.H. Epigallocatechin gallate inhibits HIF-1α degradation in prostate cancer cells. Biochem. Biophys. Res. Commun. 2005, 26, 543–548. [Google Scholar] [CrossRef] [PubMed]
- Zhu, K.; Wang, W. Green tea polyphenol EGCG suppresses osteosarcoma cell growth through upregulating miR-1. Tumour Biol. 2016, 37, 4373–4382. [Google Scholar] [CrossRef] [PubMed]
- Arola-Arnal, A.; Blade, C. Proanthocyanidins modulate microRNA expression in human HepG2 cells. PLoS ONE 2011, 6, e25982. [Google Scholar] [CrossRef] [PubMed]
- Zhou, D.H.; Wang, X.; Feng, Q. EGCG enhances the efficacy of cisplatin by downregulating hsa-miR-98-5p in NSCLC A549 cells. Nutr. Cancer 2014, 66, 636–644. [Google Scholar] [CrossRef] [PubMed]
- Kumazaki, M.; Noguchi, S.; Yasui, Y.; Iwasaki, J.; Shinohara, H.; Yamada, N.; Akao, Y. Anti-cancer effects of naturally occurring compounds through modulation of signal transduction and miRNA expression in human colon cancer cells. J. Nutr. Biochem. 2013, 24, 1849–1858. [Google Scholar] [CrossRef] [PubMed]
- Tsang, W.P.; Kwok, T.T. Epigallocatechin gallate up-regulation of miR-16 and induction of apoptosis in human cancer cells. J. Nutr. Biochem. 2010, 21, 140–146. [Google Scholar] [CrossRef] [PubMed]
- Jang, J.-Y.; Lee, J.-K.; Jeon, Y.-K.; Kim, C.-W. Exosome derived from epigallocatechin gallate treated breast cancer cells suppresses tumor growth by inhibiting tumor-associated macrophage infiltration and M2 polarization. BMC Cancer 2013, 13, 421. [Google Scholar] [CrossRef] [PubMed]
- Chakrabarti, M.; Khandkar, M.; Banik, N.L.; Ray, S.K. Alterations in expression of specific microRNAs by combination of 4-HPR and EGCG inhibited growth of human malignant neuroblastoma cells. Brain Res. 2012, 1454, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Farooqi, A.A.; Gadaleta, C.D.; Ranieri, G.; Fayyaz, S.; Marech, I. New frontiers in promoting TRAIL-mediated cell death: Focus on natural sensitizers, miRNAs, and nanotechnological advancements. Cell Biochem. Biophys. 2016, 74, 3–10. [Google Scholar] [CrossRef] [PubMed]
- Noratto, G.D.; Kim, Y.; Talcott, S.T.; Mertens-Talcott, S.U. Flavonol-rich fractions of yaupon holly leaves (Ilex vomitoria, Aquifoliaceae) induce microRNA-146a and have anti-inflammatory and chemopreventive effects in intestinal myofribroblast CCD-18Co cells. Fitoterapia 2011, 82, 557–569. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Jiang, F.; Chen, L.; Yang, Y.; Cao, S.; Ye, Y.; Wang, X.; Mu, J.; Li, Z.; Li, L. Blockage of TGFβ-SMAD2 by demethylation-activated miR-148a is involved in caffeic acid-induced inhibition of cancer stem cell-like properties in vitro and in vivo. FEBS Open Bio 2015, 5, 466–475. [Google Scholar] [CrossRef] [PubMed]
- Xu, L.; Xiang, J.; Shen, J.; Zou, X.; Zhai, S.; Yin, Y.; Li, P.; Wang, X.; Sun, Q. Oncogenic microRNA-27a is a target for genistein in ovarian cancer cells. Anticancer Agents Med. Chem. 2013, 13, 1126–1132. [Google Scholar] [CrossRef] [PubMed]
- Xia, J.; Cheng, L.; Mei, C.; Ma, J.; Shi, Y.; Zeng, F.; Wang, Z.; Wang, Z. Genistein inhibits cell growth and invasion through regulation of miR-27a in pancreatic cancer cells. Curr. Pharm. Des. 2014, 20, 5348–5353. [Google Scholar] [CrossRef] [PubMed]
- De la Parra, C.; Castillo-Pichardo, L.; Cruz-Collazo, A.; Cubano, L.; Redis, R.; Calin, G.A.; Dharmawardhane, S. Soy isoflavone genistein-mediated downregulation of miR-155 contributes to the anticancer effects of genistein. Nutr. Cancer 2016, 68, 154–164. [Google Scholar] [CrossRef] [PubMed]
- Zaman, M.S.; Shahryari, V.; Deng, G.; Thamminana, S.; Saini, S.; Majid, S.; Chang, I.; Hirata, H.; Ueno, K.; Yamamura, S.; et al. Up-regulation of microRNA-21 correlates with lower kidney cancer survival. PLoS ONE 2012, 7, e31060. [Google Scholar] [CrossRef]
- Chiyomaru, T.; Yamamura, S.; Fukuhara, S.; Hidaka, H.; Majid, S.; Saini, S.; Arora, S.; Deng, G.; Shahryari, V.; Chang, I.; et al. Genistein up-regulates tumor suppressor microRNA-574-3p in prostate cancer. PLoS ONE 2013, 8, e58929. [Google Scholar] [CrossRef] [PubMed]
- Hirata, H.; Hinoda, Y.; Shahryari, V.; Deng, G.; Tanaka, Y.; Tabatabai, Z.L.; Dahiya, R. Genistein downregulates onco-miR-1260b and upregulates sFRP1 and Smad4 via demethylation and histone modification in prostate cancer cells. Br. J. Cancer 2014, 110, 1645–1654. [Google Scholar] [CrossRef] [PubMed]
- Qin, J.; Chen, J.X.; Zhu, Z.; Teng, J.A. Genistein inhibits human colorectal cancer growth and suppresses miR-95, Akt and SGK1. Cell. Physiol. Biochem. 2015, 35, 2069–2077. [Google Scholar] [CrossRef] [PubMed]
- Ma, J.; Liu, H.; Cheng, L.; Zhang, J.; Shi, Y.; Zeng, F.; Miele, L.; Sarkar, F.H.; Xia, J.; Wang, Z. Genistein down-regulates miR-223 expression in pancreatic cancer cells. Curr. Drug. Targets 2013, 14, 1150–1160. [Google Scholar] [CrossRef] [PubMed]
- Sun, Q.; Cong, R.; Yan, H.; Gu, H.; Zeng, Y.; Liu, N.; Chen, J.; Wang, B. Genistein inhibits growth of human uveal melanoma cells and affects microRNA-27a and target gene expression. Oncol. Rep. 2009, 22, 563–567. [Google Scholar] [PubMed]
- Avci, C.B.; Susluer, S.Y.; Caglar, H.O.; Balci, T.; Aygunes, D.; Dodurga, Y.; Gunduz, C. Genistein-induced miR-23b expression inhibits the growth of breast cancer cells. Contemp. Oncol. 2015, 19, 32–35. [Google Scholar] [CrossRef] [PubMed]
- Wu, W.L.; Wang, W.Y.; Yao, W.Q.; Li, G.D. Suppressive effects of microRNA-16 on the proliferation, invasion and metastasis of hepatocellular carcinoma cells. Int. J. Mol. Med. 2015, 36, 1713–1719. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Bian, S.; Yang, C.S. Green tea polyphenol EGCG suppresses lung cancer cell growth through upregulating miR-210 expression caused by stabilizing HIF-1α. Carcinogenesis 2011, 32, 1881–1889. [Google Scholar] [CrossRef] [PubMed]
- Yamada, S.; Tsukamoto, S.; Huang, Y.; Makio, A.; Kumazoe, M.; Yamashita, S.; Tachibana, H. Epigallocatechin-3-O-gallate up-regulates microRNA-let-7b expression by activating 67-kDa laminin receptor signaling in melanoma cells. Sci. Rep. 2016, 6, 19225. [Google Scholar] [CrossRef] [PubMed]
- Jiang, L.; Tao, C.; He, A.; He, X. Overexpression of miR-126 sensitizes osteosarcoma cells to apoptosis induced by epigallocatechin-3-gallate. World J. Surg. Oncol. 2014, 12, 383. [Google Scholar] [CrossRef] [PubMed]
- Naso, L.G.; Lezama, L.; Rojo, T.; Etcheverry, S.B.; Valcarcel, M.; Roura, M.; Salado, C.; Ferrer, E.G.; Williams, P.A. Biological evaluation of morin and its new oxovanadium(IV) complex as antioxidant and specific anti-cancer agents. Chem. Biol. Interact. 2013, 206, 289–301. [Google Scholar] [CrossRef] [PubMed]
- Kumar, V.K.; Vennila, S.; Nalini, N. Modifying effects of morin on the development of aberrant crypt foci and bacterial enzymes in experimental colon cancer. Food Chem. Toxicol. 2009, 47, 309–315. [Google Scholar] [CrossRef] [PubMed]
- Jung, J.S.; Choi, M.J.; Lee, Y.Y.; Moon, B.I.; Park, J.S.; Kim, H.S. Suppression of lipopolysaccharide-induced neuroinflammation by morin via MAPK, PI3K/Akt, and PKA/HO-1 signaling pathway modulation. J. Agric. Food Chem. 2017, 18, 373–382. [Google Scholar] [CrossRef] [PubMed]
- Hussain, J.; Ali, L.; Khan, A.L.; Rehman, N.U.; Jabeen, F.; Kim, J.S.; Al-Harrasi, A. Isolation and bioactivities of the flavonoids morin and morin-3-O-β-d-glucopyranoside from Acridocarpus orientalis—A wild Arabian medicinal plant. Molecules 2014, 19, 17763–17772. [Google Scholar] [CrossRef] [PubMed]
- Stagos, D.; Amoutzias, G.D.; Matakos, A.; Spyrou, A.; Tsatsakis, A.M.; Kouretas, D. Chemoprevention of liver cancer by plant polyphenols. Food Chem. Toxicol. 2012, 50, 2155–2170. [Google Scholar] [CrossRef] [PubMed]
- Lall, R.K.; Syed, D.N.; Adhami, V.M.; Khan, M.I.; Mukhtar, H. Dietary polyphenols in prevention and treatment of prostate cancer. Int. J. Mol. Sci. 2015, 16, 3350–3376. [Google Scholar] [CrossRef] [PubMed]
- Tolba, M.F.; Azab, S.S.; Khalifa, A.E.; Abdel-Rahman, S.Z.; Abdel-Naim, A.B. Caffeic acid phenethyl ester, a promising component of propolis with a plethora of biological activities: A review on its anti-inflammatory, neuroprotective, hepatoprotective, and cardioprotective effects. IUBMB Life 2013, 65, 699–709. [Google Scholar] [CrossRef] [PubMed]
- Milenkovic, D.; Deval, C.; Gouranton, E.; Landrier, J.F.; Scalbert, A.; Morand, C.; Mazur, A. Modulation of miRNA expression by dietary polyphenols in apoE deficient mice: A new mechanism of the action of polyphenols. PLoS ONE 2012, 7, e29837. [Google Scholar] [CrossRef] [PubMed]
- Taylor, C.K.; Levy, R.M.; Elliott, J.C.; Burnett, B.P. The effect of genistein aglycone on cancer and cancer risk: A review of in vitro, preclinical, and clinical studies. Nutr. Rev. 2009, 67, 398–415. [Google Scholar] [CrossRef] [PubMed]
- Chiyomaru, T.; Yamamura, S.; Fukuhara, S.; Yoshino, H.; Kinoshita, T.; Majid, S.; Saini, S.; Chang, I.; Tanaka, Y.; Enokida, H.; et al. Genistein inhibits prostate cancer cell growth by targeting miR-34a and oncogenic HOTAIR. PLoS ONE 2013, 8, e70372. [Google Scholar] [CrossRef] [PubMed]
- Phuah, N.H.; Nagoor, N.H. Regulation of microRNAs by natural agents: New strategies in cancer therapies. BioMed Res. Int. 2014, 2014, 804510. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Zaman, M.S.; Deng, G.; Majid, S.; Saini, S.; Liu, J.; Tanaka, Y.; Dahiya, R. MicroRNAs 221/222 and genistein-mediated regulation of ARHI tumor suppressor gene in prostate cancer. Cancer Prev. Res. 2011, 4, 76–86. [Google Scholar] [CrossRef] [PubMed]
- Chiyomaru, T.; Yamamura, S.; Zaman, M.S.; Majid, S.; Deng, G.; Shahryari, V.; Saini, S.; Hirata, H.; Ueno, K.; Chang, I.; et al. Genistein suppresses prostate cancer growth through inhibition of oncogenic microRNA-151. PLoS ONE 2012, 7, e43812. [Google Scholar] [CrossRef] [PubMed]
- Zaman, M.S.; Thamminana, S.; Shahryari, V.; Chiyomaru, T.; Deng, G.; Saini, S.; Majid, S.; Fukuhara, S.; Chang, I.; Arora, S.; et al. Inhibition of PTEN gene expression by oncogenic miR-23b-3p in renal cancer. PLoS ONE 2012, 7, e50203. [Google Scholar] [CrossRef] [PubMed]
- Hirata, H.; Ueno, K.; Nakajima, K.; Tabatabai, Z.L.; Hinoda, Y.; Ishii, N.; Dahiya, R. Genistein downregulates onco-miR-1260b and inhibits Wnt-signalling in renal cancer cells. Br. J. Cancer 2013, 108, 2070–2078. [Google Scholar] [CrossRef] [PubMed]
- Azevedo, C.; Correia-Branco, A.; Araujo, J.R.; Guimaraes, J.T.; Keating, E.; Martel, F. The chemopreventive effect of the dietary compound kaempferol on the MCF-7 human breast cancer cell line is dependent on inhibition of glucose cellular uptake. Nutr. Cancer 2015, 67, 504–513. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Zhao, Y.; Yang, D.; Yu, Y.; Guo, H.; Zhao, Z.; Zhang, B.; Yin, X. Inhibitory effects of kaempferol on the invasion of human breast carcinoma cells by downregulating the expression and activity of matrix metalloproteinase-9. Biochem. Cell Biol. 2015, 93, 16–27. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.F.; Yang, J.S.; Tsai, F.J.; Chiang, N.N.; Lu, C.C.; Huang, Y.S.; Chen, C.; Chen, F.A. Kaempferol induces ATM/p53-mediated death receptor and mitochondrial apoptosis in human umbilical vein endothelial cells. Int. J. Oncol. 2016, 48, 2007–2014. [Google Scholar] [CrossRef] [PubMed]
- Braicu, C.; Catana, C.; Calin, G.A.; Berindan-Neagoe, I. NCRNA combined therapy as future treatment option for cancer. Curr. Pharm. Des. 2014, 20, 6565–6574. [Google Scholar] [CrossRef] [PubMed]
- Gibb, E.A.; Brown, C.J.; Lam, W.L. The functional role of long non-coding RNA in human carcinomas. Mol. Cancer 2011, 10, 38. [Google Scholar] [CrossRef] [PubMed]
- Prensner, J.R.; Chinnaiyan, A.M. The emergence of lncRNAs in cancer biology. Cancer Discov. 2011, 1, 391–407. [Google Scholar] [CrossRef] [PubMed]
- Liu, G.; Zheng, X.; Xu, Y.; Lu, J.; Chen, J.; Huang, X. Long non-coding RNAs expression profile in HepG2 cells reveals the potential role of long non-coding RNAs in the cholesterol metabolism. Chin. Med. J. 2015, 128, 91–97. [Google Scholar] [PubMed]
- Jiang, P.; Wu, X.; Wang, X.; Huang, W.; Feng, Q. NEAT1 upregulates EGCG-induced CTR1 to enhance cisplatin sensitivity in lung cancer cells. Oncotarget 2016, 7, 43337–43351. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Hou, R.; Zhang, X.; Ye, Y.; Wang, Y.; Tian, J. Calycosin suppresses breast cancer cell growth via ERβ-dependent regulation of IGF-1R, p38 MAPK and PI3K/Akt pathways. PLoS ONE 2014, 9, e91245. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Lin, C.; Yong, W.; Ye, Y.; Huang, Z. Calycosin and genistein induce apoptosis by inactivation of HOTAIR/p-Akt signaling pathway in human breast cancer mcf-7 cells. Cell. Physiol. Biochem. 2015, 35, 722–728. [Google Scholar] [CrossRef] [PubMed]
- Gupta, R.A.; Shah, N.; Wang, K.C.; Kim, J.; Horlings, H.M.; Wong, D.J.; Tsai, M.C.; Hung, T.; Argani, P.; Rinn, J.L.; et al. Long non-coding RNA HOTAIR reprograms chromatin state to promote cancer metastasis. Nature 2010, 464, 1071–1076. [Google Scholar] [CrossRef] [PubMed]
- Prensner, J.R.; Iyer, M.K.; Balbin, O.A.; Dhanasekaran, S.M.; Cao, Q.; Brenner, J.C.; Laxman, B.; Asangani, I.A.; Grasso, C.S.; Kominsky, H.D.; et al. Transcriptome sequencing across a prostate cancer cohort identifies PCAT-1, an unannotated lincRNA implicated in disease progression. Nat. Biotechnol. 2011, 29, 742–749. [Google Scholar] [CrossRef] [PubMed]
- Ciruelos Gil, E.M. Targeting the PI3K/AKT/mTOR pathway in estrogen receptor-positive breast cancer. Cancer Treat. Rev. 2014, 40, 862–871. [Google Scholar] [CrossRef] [PubMed]
- Anonymous. Cancer multidrug resistance. Nat. Biotechnol. 2000, 18, IT18–IT20. [Google Scholar]
- Wu, C.P.; Ohnuma, S.; Ambudkar, S.V. Discovering natural product modulators to overcome multidrug resistance in cancer chemotherapy. Curr. Pharm. Biotechnol. 2011, 12, 609–620. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues, T.; Reker, D.; Schneider, P.; Schneider, G. Counting on natural products for drug design. Nat. Chem. 2016, 8, 531–541. [Google Scholar] [CrossRef] [PubMed]
- Limtrakul, P.; Chearwae, W.; Shukla, S.; Phisalphong, C.; Ambudkar, S.V. Modulation of function of three ABC drug transporters, P-glycoprotein (ABCB1), mitoxantrone resistance protein (ABCG2) and multidrug resistance protein 1 (ABCC1) by tetrahydrocurcumin, a major metabolite of curcumin. Mol. Cell. Biochem. 2007, 296, 85–95. [Google Scholar] [CrossRef] [PubMed]
- Chearwae, W.; Wu, C.P.; Chu, H.Y.; Lee, T.R.; Ambudkar, S.V.; Limtrakul, P. Curcuminoids purified from turmeric powder modulate the function of human multidrug resistance protein 1 (ABCC1). Cancer Chemother. Pharmacol. 2006, 57, 376–388. [Google Scholar] [CrossRef] [PubMed]
- Chearwae, W.; Anuchapreeda, S.; Nandigama, K.; Ambudkar, S.V.; Limtrakul, P. Biochemical mechanism of modulation of human P-glycoprotein (ABCB1) by curcumin I, II, and III purified from Turmeric powder. Biochem. Pharmacol. 2004, 68, 2043–2052. [Google Scholar] [CrossRef] [PubMed]
- Boumendjel, A.; di Pietro, A.; Dumontet, C.; Barron, D. Recent advances in the discovery of flavonoids and analogs with high-affinity binding to P-glycoprotein responsible for cancer cell multidrug resistance. Med. Res. Rev. 2002, 22, 512–529. [Google Scholar] [CrossRef] [PubMed]
- Conseil, G.; Baubichon-Cortay, H.; Dayan, G.; Jault, J.M.; Barron, D.; di Pietro, A. Flavonoids: A class of modulators with bifunctional interactions at vicinal ATP- and steroid-binding sites on mouse P-glycoprotein. Proc. Natl. Acad. Sci. USA 1998, 95, 9831–9836. [Google Scholar] [CrossRef] [PubMed]
- Zhou, S.; Lim, L.Y.; Chowbay, B. Herbal modulation of P-glycoprotein. Drug Metab. Rev. 2004, 36, 57–104. [Google Scholar] [CrossRef] [PubMed]




| Natural Phytochemical | Dose | Preclinical Test | Target Mechanism | Target Gene | Reference |
|---|---|---|---|---|---|
| EGCG | 0–50 µM | Colon cancer (HT-29 and HCT-116), human embryonic kidney (HEK)-293T cell, Triple negative breast cancer cells (MDA-231) | Apoptosis activation and reduction of cell proliferation via targeting MAPK | Akt, ERK1/2, p38 | [56] |
| 0–200 µM | oral cancer (SSC5) | Reduce cell proliferation, activate apoptosis and autophagy | BAD, BAK, FAS, IGF1R, WNT11, ZEB1 CASP8, MYC, and TP53 | [48] | |
| 0–35 µM | Colorectal cancer cells (LoVo cells, SW480 cells, HT29 cells, and HCT-8 cells) and animal models | induced the apoptosis and affected the cell cycle via Notch signalling | HES1 and Notch2 | [56] | |
| 0–160 µM | Inflammatory breast cancer cells (UM-149 and SUM-190) | Inhibit tumoural stemm like comportment | VEGFD | [113] | |
| 0–25 µM | Triple negative breast cancer cells | Invasion and angiogenesis | VEGFA | [45] | |
| 0–50 µM | Breast cancer and nude mice | Cell proliferation and invasion | RS/MAPK/p-S6K1 | [55] | |
| 0–100 µM | Gastric cancer cells and nude mice | Cell proliferation, cell cycle, invasion and metastasis | Wnt/β-catenin | [114] | |
| Morin | 0–350 µM | human leukemic cells (U937 cells) | caspase-dependent apoptosis via intrinsic pathway | BAX, BCL-2, cytochrome c | [64] |
| 0–400 µM | human colon cancer cells (HCT-116) | ROS generation, extrinsic and intrinsic apoptosis | Bcl-2 and IAP family members, Fas and Akt | [64] | |
| 0–200 µM | Triple negative breast cancer cells, nude mice | Cell adhesion, EMT, invasion and inhibit lung metastasis | TNF-α, VCAM1 and N-cadherin | [67] | |
| 50 µM | Triple negative breast cancer cells, nude mice | EMT, invasion and metastasis | AKT and related targets, MMP-9 | [66] | |
| CAPE | 0–100 µM | Breast cancer (MCF-7) | Activate apoptosis and reduce cell proliferation | NFkB, Fas, p53, Bax and JNK | [79] |
| 0–50 µM | Oral cancer cells (TW2.6) | Suppress the proliferation, invasion and metastatic potential | Akt and NFkB | [115] | |
| 0–12 µM | Prostate cancer cells (PC-3) | suppresses the proliferation | 70S6K and Akt | [116] | |
| 0–50 µM | Prostate cancer cells (CRPC) | Cycle arrest and growth inhibition in CRPC cells | Skp2, p53, p21Cip1 and p27Kip1 | [117] | |
| Genistein | 0–100 µM | breast cancer cells (MCF-7) | cell proliferation and apoptosis via IGF1R-Akt-Bcl-2 and Bax-mediated pathways | IGF-1R, p-Akt, Bcl-2, and Bax | [108] |
| 10 µmol/L | breast cancer cells (MCF-7) | Cell cycle regulation | GLIPR1, CDC20, BUB1, MCM2 and CCNB1 | [109] | |
| 0–50 µM | Colorectal cancer models and orthotopic mouse models | cell invasion and migration, inhibit distant metastasis | MMP-2 and FLT4 | [110] | |
| 0–100 µM | colon cancer cells (HCT-116) | Activate mitochondrial apoptosis | Akt and Bax | [107] | |
| 0.5–10 μmol/L | Prostate cancer cells LAPC-4 and PC-3 | Cell proliferation and hormonal receptor | ER-β | [106] | |
| Kaempferol | 25 μM | Breast cancer cells (MCF-7) | Modulated EMT, inhibit migration, and invasion | ER | [95] |
| 0–100 µM | Bladder cancer | Inhibit cell proliferation | c-Met/p38 | [94] | |
| 0–50 µM | Lung cancer cells (A549) | Modulated EMT, inhibit migration, and invasion | TGFβ1, SMAD3, Akt1 | [96] | |
| 0–100 µM | Oral cancer cells (SCC4) | anti-metastatic effect | MMP-2 and TIMP-2, c-JUN, and ERK1/2 | [111] |
| Phytochemicals | miRNA Transcripts | Expression in Cancer | miRNA Target Gene | Role | References |
|---|---|---|---|---|---|
| Epigallocatechin-3-Gallate (EGCG) | miR-16 | Hepatocellular carcinoma/↓ | Bcl-2 | Apoptosis induction | [150,166] |
| miRNA-330 | Breast cancer/↑ | AR | antagonizes androgen receptor function | [57] | |
| miR-21 | Breast cancer/↓ | AR | antagonizes androgen receptor function | [57] | |
| miR-98-5p | Lung cancer/↑ | - | Enhance the effect of ciplatin and determines the upregulation of p53 gene | [148] | |
| miR-30b, miR-453, miR-520-e, miR-629, miR-608 | Hepatocellular carcinoma/↑ | - | Regulation of inflammation, insulin secretion, glycolysis/gluconeogenesis pathways | [147] | |
| miR-210 | Lung cancer/↓ | HRE | Disable cell proliferation and suppress cell growth | [167] | |
| miR-let7b | Melanoma/↓ | 67LR | Inhibits melanoma cells growth via inhibition of HMGA2 | [168] | |
| miR-126 | Osteosarcoma/↓ | - | Induction of apoptosis and inhibition of cell proliferation | [169] | |
| Morin | No direct studies focused on miRNA expression levels in cancer | Oral tumours, breast, colon and other cancer types/- | - | Anticancer activity via suppression of cell growth and invasion; determines increased sensitivity to chemotherapeutic agents | [66,170,171,172,173] |
| Caffeic acid phenethyl ester (CAPE) | No direct studies focused on miRNA expression levels in cancer | Lung, prostate and liver cancer/- | - | Anticancer activity through modulation of inflammatory and oxidative stress parameters. Neuroprotective, cardioprotective and hepatoprotective functions | [174,175,176,177] |
| Genistein | miR-27a | Ovarian cancer/↑ | Sprouty2 | Oncogenic miRNA, promoting tumour growth and migration | [156,178] |
| miR-27a | Pancreatic cancer/↑ | - | inhibition of miR-27a suppressed cell growth and induced apoptosis as well as inhibited invasion | [157] | |
| miR-574-3p | Prostate cancer/↓ | RAC1, EGFR, EP300 | Tumour suppressor miRNA, inhibiting cell proliferation, migration and invasion | [160] | |
| miR-155 | Breast cancer/↑ | OXO3, PTEN, casein kinase, and p27 | Oncogenic miRNA, promoting tumour growth and migration | [158] | |
| miR-34a | Prostate cancer/↓ | HOX | Tumour suppressor miRNA; apoptosis, low invasiveness, decreased cell proliferation | [179] | |
| miR-1296 | Prostate cancer/↓ | MCM | Inhibits MCM gene family (oncogenes) which was associated with prostate cancer progression | [180] | |
| miR-221, miR-222 | Prostate cancer/↓ | ARH1 | Regulates the expression of ARH1 gene, determining decreased proliferation and invasiveness | [181] | |
| miR-151 | Prostate cancer/↑ | N4BP1, ASZ1, IL1RAPL1, SRY, ARHGDIA | Inhibition of miR-151 was associated with decreased cell migration and invasion, but not proliferation | [182] | |
| miR-23b-3p | Renal cancer/↑ | PTEN | Induction of apoptosis in the moment of downregulation | [183] | |
| miR-1260b | Renal cancer/↑ | sFRP1, Dkk2, Smad4 | Increased apoptosis and decreased cell proliferation and migration | [184] | |
| Kaempferol | miR-200 | Lung cancer/↓ | ZEB1, ZEB2 | Inhibitory activity regarding the epithelial-to-mesenchymal transition and migration | [96] |
| No other direct studies focused on miRNA expression levels in cancer | Bladder, pancreatic, breast, gastric and prostate cancer/- | - | Inhibitory effects on numerous cancer types, affecting a wide range of genes/pathways: matrix metalloproteinase-9, PTEN, ABCG2, p53, NF-κB, AhR and Nrf2 | [40,185,186,187] |
| Phytochemicals | ncRNA Transcript | Expression in Cancer | Expression after Natural Treatment | Target Coding or Non-Coding Gene | Role | References |
|---|---|---|---|---|---|---|
| EGCG | NEAT1 | Lung cancer/↓ | ↑ | sponging mir-98 | EGCG induced CTR1 and enhanced lung cancer cell sensitivity oxaliplatin via hsa-mir-98-5p and NEAT1 | [192] |
| Genistein | HOTAIR | Prostate cancer/↑ | ↓ | ABL2 SNAIL, LAMB3, LAMC2, MMP9 and VEGF | Oncogenic role; regulates invasion and metastasis | [179,195,196] |
| HOTAIR | Breast cancer/↑ | ↓ | HOXD, ABL2, SNAIL and LAMB3 | Oncogenic role; regulates invasion and metastasis | [193,197] | |
| HOTAIR | Breast cancer/↑ | ↓ | p-Akt | Oncogenic role; Inhibit proliferation and activate apoptosis | [194] |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Budisan, L.; Gulei, D.; Zanoaga, O.M.; Irimie, A.I.; Chira, S.; Braicu, C.; Gherman, C.D.; Berindan-Neagoe, I. Dietary Intervention by Phytochemicals and Their Role in Modulating Coding and Non-Coding Genes in Cancer. Int. J. Mol. Sci. 2017, 18, 1178. https://doi.org/10.3390/ijms18061178
Budisan L, Gulei D, Zanoaga OM, Irimie AI, Chira S, Braicu C, Gherman CD, Berindan-Neagoe I. Dietary Intervention by Phytochemicals and Their Role in Modulating Coding and Non-Coding Genes in Cancer. International Journal of Molecular Sciences. 2017; 18(6):1178. https://doi.org/10.3390/ijms18061178
Chicago/Turabian StyleBudisan, Liviuta, Diana Gulei, Oana Mihaela Zanoaga, Alexandra Iulia Irimie, Sergiu Chira, Cornelia Braicu, Claudia Diana Gherman, and Ioana Berindan-Neagoe. 2017. "Dietary Intervention by Phytochemicals and Their Role in Modulating Coding and Non-Coding Genes in Cancer" International Journal of Molecular Sciences 18, no. 6: 1178. https://doi.org/10.3390/ijms18061178
APA StyleBudisan, L., Gulei, D., Zanoaga, O. M., Irimie, A. I., Chira, S., Braicu, C., Gherman, C. D., & Berindan-Neagoe, I. (2017). Dietary Intervention by Phytochemicals and Their Role in Modulating Coding and Non-Coding Genes in Cancer. International Journal of Molecular Sciences, 18(6), 1178. https://doi.org/10.3390/ijms18061178