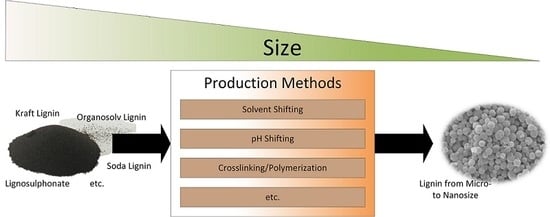
Lignin from Micro- to Nanosize: Production Methods
Abstract
:1. Introduction
2. Production of Nano-/Microsized Lignin Materials
2.1. Solvent Shifting
2.1.1. Solid Particles
2.1.2. Hollow Particles
2.2. pH Shifting
2.3. Crosslinking/Polymerization
2.3.1. Solid Structures
2.3.2. Hollow Structures
2.4 Other Formation Methods
2.4.1. Mechanical Treatment
2.4.2. Ice-Segregation-Induced Self-Assembly
2.4.3. Template-Based Synthesis
2.4.4. Aerosol Processing
2.4.5. Electrospinning
2.4.6. CO2 Antisolvent
3. Comparison
4. Conclusions and Outlook
Acknowledgments
Author Contributions
Conflicts of Interest
Abbreviations
| ACL | Acetylated lignin |
| AL | Alkali lignin |
| APTES | (3-aminopropyl)-triethoxysilane |
| CL | Cationic lignin |
| DHP | Dehydrogenation polymer |
| DLS | Dynamic Light Scattering |
| DMF | Dimethylformamide |
| DMSO | Dimethyl sulfoxide |
| EG | Ethylene glycol |
| EHL | Enzymatic hydrolysis lignin |
| ISISA | Ice- segregation-induced self-assembly |
| KL | Kraft lignin |
| MMT | Montmorillonite |
| NP | Nanoparticle |
| OS | Organosolv lignin |
| PDAC | Poly(diallyldimethylammonium chloride) |
| PEGDEG | Poly(ethylene glycol) diglycidyl ether |
| PEO | Poly(ethylene oxide) |
| PL | Pyrolytic lignin |
| RAFT | Reversible addition-fragmentation chain transfer |
| SEM | Scanning electron microscopy |
| SLI | Scattered light intensity |
| TEM | Transmission electron microscopy |
| THF | Tetrahydrofuran |
| XRD | X-ray diffraction |
References
- Kamm, B.; Kamm, M. Principles of biorefineries. Appl. Microbiol. Biotechnol. 2004, 64, 137–145. [Google Scholar] [CrossRef] [PubMed]
- Sánchez, Ó.J.; Cardona, C.A. Trends in biotechnological production of fuel ethanol from different feedstocks. Bioresour. Technol. 2008, 99, 5270–5295. [Google Scholar] [CrossRef] [PubMed]
- Boerjan, W.; Ralph, J.; Baucher, M. Lignin Biosynthesis. Annu. Rev. Plant Biol. 2003, 54, 519–546. [Google Scholar] [CrossRef] [PubMed]
- Sannigrahi, P.; Ragauskas, A.J. Characterization of fermentation residues from the production of bio-ethanol from lignocellulosic feedstocks. J. Biobased Mater. Biol. 2011, 5, 514–519. [Google Scholar] [CrossRef]
- Vishtal, A.; Kraslawski, A. Challenges in industrial applications of technical lignins. BioResources 2011, 6, 3547–3568. [Google Scholar]
- Doherty, W.O.S.; Mousavioun, P.; Fellows, C.M. Value-adding to cellulosic ethanol: Lignin polymers. Ind. Crops Prod. 2011, 33, 259–276. [Google Scholar] [CrossRef]
- FitzPatrick, M.; Champagne, P.; Cunningham, M.F.; Whitney, R.A. A biorefinery processing perspective: Treatment of lignocellulosic materials for the production of value-added products. Bioresour. Technol. 2010, 101, 8915–8922. [Google Scholar] [CrossRef] [PubMed]
- Suhas; Carrott, P.J.M.; Ribeiro Carrott, M.M.L. Lignin–from natural adsorbent to activated carbon: A review. Bioresour. Technol. 2007, 98, 2301–2312. [Google Scholar] [CrossRef] [PubMed]
- Hu, T.Q. (Ed.) Chemical Modification, Properties, and Usage of Lignin; Springer: Boston, MA, USA, 2002. [Google Scholar]
- Langholtz, M.; Downing, M.; Graham, R.; Baker, F.; Compere, A.; Griffith, W.; Boeman, R.; Keller, M. Lignin-derived carbon fiber as a co-product of refining cellulosic biomass. SAE Int. J. Mater. Manuf. 2014, 7, 115–121. [Google Scholar] [CrossRef]
- Calvo-Flores, F.G.; Dobado, J.A. Lignin as renewable raw material. ChemSusChem 2010, 3, 1227–1235. [Google Scholar] [CrossRef] [PubMed]
- Gellerstedt, G.; Henriksson, G. Lignins: Major Sources, Structure and Properties. In Monomers, Polymers and Composites from Renewable Resources; Elsevier: Amsterdam, the Netherlands, 2008; pp. 201–224. [Google Scholar]
- Chandra, R.P.; Bura, R.; Mabee, W.E.; Berlin, A.; Pan, X.; Saddler, J.N. Substrate Pretreatment: The Key to Effective Enzymatic Hydrolysis of Lignocellulosics? In Biofuels; Springer: Berlin/Heidelberg, Gemany, 2007; pp. 67–93. [Google Scholar]
- Li, C.; Zhao, X.; Wang, A.; Huber, G.W.; Zhang, T. Catalytic transformation of lignin for the production of chemicals and fuels. Chem. Rev. 2015, 115, 11559–11624. [Google Scholar] [CrossRef] [PubMed]
- Laurichesse, S.; Avérous, L. Chemical modification of lignins: Towards biobased polymers. Prog. Polym. Sci. 2014, 39, 1266–1290. [Google Scholar] [CrossRef]
- Frangville, C.; Rutkevičius, M.; Richter, A.P.; Velev, O.D.; Stoyanov, S.D.; Paunov, V.N. Fabrication of environmentally biodegradable lignin nanoparticles. ChemPhysChem 2012, 13, 4235–4243. [Google Scholar] [CrossRef] [PubMed]
- Qian, Y.; Deng, Y.; Qiu, X.; Li, H.; Yang, D. Formation of uniform colloidal spheres from lignin, a renewable resource recovered from pulping spent liquor. Green Chem. 2014, 16, 2156. [Google Scholar] [CrossRef]
- Xu, T.; Zhang, N.; Nichols, H.L.; Shi, D.; Wen, X. Modification of nanostructured materials for biomedical applications. Mater. Sci. Eng. C 2007, 27, 579–594. [Google Scholar] [CrossRef]
- Hussain, F. Review article: Polymer-matrix nanocomposites, processing, manufacturing, and application: An overview. J. Compos. Mater. 2006, 40, 1511–1575. [Google Scholar] [CrossRef]
- Tortora, M.; Cavalieri, F.; Mosesso, P.; Ciaffardini, F.; Melone, F.; Crestini, C. Ultrasound driven assembly of lignin into microcapsules for storage and delivery of hydrophobic molecules. Biomacromolecules 2014, 15, 1634–1643. [Google Scholar] [CrossRef] [PubMed]
- Gîlcă, I.-A.; Popa, V.I. Study on biocidal properties of some nanoparticles based on epoxy lignin. Cellul. Chem. Technol. 2013, 47, 3–4. [Google Scholar]
- Lu, Q.; Zhu, M.; Zu, Y.; Liu, W.; Yang, L.; Zhang, Y.; Zhao, X.; Zhang, X.; Zhang, X.; Li, W. Comparative antioxidant activity of nanoscale lignin prepared by a supercritical antisolvent (SAS) process with non-nanoscale lignin. Food Chem. 2012, 135, 63–67. [Google Scholar] [CrossRef]
- Yang, W.; Kenny, J.M.; Puglia, D. Structure and properties of biodegradable wheat gluten bionanocomposites containing lignin nanoparticles. Ind. Crops Prod. 2015, 74, 348–356. [Google Scholar] [CrossRef]
- Milczarek, G.; Inganas, O. Renewable cathode materials from biopolymer/conjugated polymer interpenetrating networks. Science 2012, 335, 1468–1471. [Google Scholar] [CrossRef] [PubMed]
- Thostenson, E.; Li, C.; Chou, T. Nanocomposites in context. Compos. Sci. Technol. 2005, 65, 491–516. [Google Scholar] [CrossRef]
- Wurm, F.R.; Weiss, C.K. Nanoparticles from renewable polymers. Front. Chem. 2014, 2, 49. [Google Scholar] [CrossRef] [PubMed]
- Duval, A.; Lawoko, M. A review on lignin-based polymeric, micro- and nano-structured materials. React. Funct. Polym. 2014, 85, 78–96. [Google Scholar] [CrossRef]
- Zhao, W.; Simmons, B.; Singh, S.; Ragauskas, A.; Cheng, G. From lignin association to nano-/micro-particle preparation: extracting higher value of lignin. Green Chem. 2016, 18, 5693–5700. [Google Scholar] [CrossRef]
- Feldman, D. Lignin nanocomposites. J. Macromol. Sci. A 2016, 53, 382–387. [Google Scholar] [CrossRef]
- Jelinek, R. Nanoparticles; Walter de Gruyter GmbH: Berlin, Germany, 2015. [Google Scholar]
- Ganachaud, F.; Katz, J.L. Nanoparticles and nanocapsules created using the ouzo effect: Spontaneous emulsification as an alternative to ultrasonic and high-shear devices. ChemPhysChem 2005, 6, 209–216. [Google Scholar] [CrossRef] [PubMed]
- Aubry, J.; Ganachaud, F.; Cohen Addad, J.-P.; Cabane, B. Nanoprecipitation of polymethylmethacrylate by solvent shifting:1. Boundaries. Langmuir 2009, 25, 1970–1979. [Google Scholar] [CrossRef] [PubMed]
- Brick, M.C.; Palmer, H.J.; Whitesides, T.H. Formation of colloidal dispersions of organic materials in aqueous media by solvent shifting. Langmuir 2003, 19, 6367–6380. [Google Scholar] [CrossRef]
- Lievonen, M.; Valle-Delgado, J.J.; Mattinen, M.-L.; Hult, E.-L.; Lintinen, K.; Kostiainen, M.A.; Paananen, A.; Szilvay, G.R.; Setälä, H.; Österberg, M. A simple process for lignin nanoparticle preparation. Green Chem. 2016, 18, 1416–1422. [Google Scholar] [CrossRef]
- Yiamsawas, D.; Baier, G.; Thines, E.; Landfester, K.; Wurm, F.R. Biodegradable lignin nanocontainers. RSC Adv. 2014, 4, 11661. [Google Scholar] [CrossRef]
- Gilca, I.A.; Popa, V.I.; Crestini, C. Obtaining lignin nanoparticles by sonication. Ultrason. Sonochem. 2015, 23, 369–375. [Google Scholar] [CrossRef] [PubMed]
- Figueiredo, P.; Lintinen, K.; Kiriazis, A.; Hynninen, V.; Liu, Z.; Bauleth-Ramos, T.; Rahikkala, A.; Correia, A.; Kohout, T.; Sarmento, B.; et al. In vitro evaluation of biodegradable lignin-based nanoparticles for drug delivery and enhanced antiproliferation effect in cancer cells. Biomaterials 2017, 121, 97–108. [Google Scholar] [CrossRef] [PubMed]
- Xiong, K.; Jin, C.; Liu, G.; Wu, G.; Chen, J.; Kong, Z. Preparation and characterization of lignin nanoparticles with controllable size by nanoprecipitation method. Chem. Ind. For. Prod. 2015, 35, 85–92. [Google Scholar]
- Zhenwu, K.; Kai, X.; Can, J. Method for Preparing Nano Lignin by Use of Liquid Phase Deposition Technique. C.N. Patent 104,497,322, 19 December 2014. [Google Scholar]
- Qian, Y.; Zhong, X.; Li, Y.; Qiu, X. Fabrication of uniform lignin colloidal spheres for developing natural broad-spectrum sunscreens with high sun protection factor. Ind. Crops Prod. 2017, 101, 54–60. [Google Scholar] [CrossRef]
- Yearla, S.R.; Padmasree, K. Preparation and characterisation of lignin nanoparticles: Evaluation of their potential as antioxidants and UV protectants. J. Exp. Nanosci. 2016, 11, 289–302. [Google Scholar] [CrossRef]
- Fukushima, R.S.; Hatfield, R.D. Extraction and isolation of lignin for utilization as a standard to determine lignin concentration using the acetyl bromide spectrophotometric method. J. Agric. Food Chem. 2001, 49, 3133–3139. [Google Scholar] [CrossRef] [PubMed]
- Richter, A.P.; Bharti, B.; Armstrong, H.B.; Brown, J.S.; Plemmons, D.; Paunov, V.N.; Stoyanov, S.D.; Velev, O.D. Synthesis and characterization of biodegradable lignin nanoparticles with tunable surface properties. Langmuir 2016, 32, 6468–6477. [Google Scholar] [CrossRef] [PubMed]
- Tian, D.; Hu, J.; Chandra, R.P.; Saddler, J.N.; Lu, C. valorizing recalcitrant cellulolytic enzyme lignin via lignin nanoparticles fabrication in an integrated biorefinery. ACS Sustain. Chem. Eng. 2017, 5, 2702–2710. [Google Scholar] [CrossRef]
- Hu, J.; Pribowo, A.; Saddler, J.N. Oxidative cleavage of some cellulosic substrates by auxiliary activity (AA) family 9 enzymes influences the adsorption/desorption of hydrolytic cellulase enzymes. Green Chem. 2016, 18, 6329–6336. [Google Scholar] [CrossRef]
- Shi, Q.; Zhang, P.; Li, Y.; Xia, H.; Wang, D.; Tao, X. Synthesis of open-mouthed, yolk–shell Au@AgPd nanoparticles with access to interior surfaces for enhanced electrocatalysis. Chem. Sci. 2015, 6, 4350–4357. [Google Scholar] [CrossRef]
- Li, X.; Zhou, L.; Wei, Y.; El-Toni, A.M.; Zhang, F.; Zhao, D. Anisotropic encapsulation-induced synthesis of asymmetric single-hole mesoporous nanocages. J. Am. Chem. Soc. 2015, 137, 5903–5906. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Ma, A.; Xu, Z.; Liu, X.; Chu, D.; Xu, H. Synthesis of Au and Pt hollow capsules with single holes via pickering emulsion strategy. J. Phys. Chem. C 2015, 119, 28055–28060. [Google Scholar] [CrossRef]
- Ding, S.; Lin, T.; Wang, Y.; Lü, X.; Huang, F. New facile synthesis of TiO2 hollow sphere with an opening hole and its enhanced rate performance in lithium-ion batteries. New J. Chem. 2013, 37, 784–789. [Google Scholar] [CrossRef]
- Hyuk Im, S.; Jeong, U.; Xia, Y. Polymer hollow particles with controllable holes in their surfaces. Nat. Mater. 2005, 4, 671–675. [Google Scholar] [CrossRef] [PubMed]
- Xiong, F.; Han, Y.; Wang, S.; Li, G.; Qin, T.; Chen, Y.; Chu, F. Preparation and formation mechanism of renewable lignin hollow nanospheres with a single hole by self-assembly. ACS Sustain. Chem. Eng. 2017, 5, 2273–2281. [Google Scholar] [CrossRef]
- Li, H.; Deng, Y.; Liang, J.; Dai, Y.; Li, B.; Ren, Y.; Qiu, X.; Li, C. Direct preparation of hollow nanospheres with kraft lignin: A facile strategy for effective utilization of biomass waste. BioResources 2016, 11, 3073–3083. [Google Scholar] [CrossRef]
- Li, H.; Deng, Y.; Wu, H.; Ren, Y.; Qiu, X.; Zheng, D.; Li, C. Self-assembly of kraft lignin into nanospheres in dioxane-water mixtures. Holzforschung 2016, 70, 725–731. [Google Scholar] [CrossRef]
- Li, H.; Deng, Y.; Liu, B.; Ren, Y.; Liang, J.; Qian, Y.; Qiu, X.; Li, C.; Zheng, D. Preparation of nanocapsules via the self-assembly of kraft lignin: A totally green process with renewable resources. ACS Sustain. Chem. Eng. 2016, 4, 1946–1953. [Google Scholar] [CrossRef]
- Gupta, A.K.; Mohanty, S.; Nayak, S.K. Synthesis, characterization and application of lignin nanoparticles (LNPs). Mater. Focus 2014, 3, 444–454. [Google Scholar] [CrossRef]
- Richter, A.P.; Brown, J.S.; Bharti, B.; Wang, A.; Gangwal, S.; Houck, K.; Cohen Hubal, E.A.; Paunov, V.N.; Stoyanov, S.D.; Velev, O.D. An environmentally benign antimicrobial nanoparticle based on a silver-infused lignin core. Nat. Nanotechnol. 2015, 10, 817–823. [Google Scholar] [CrossRef] [PubMed]
- Yang, W.; Owczarek, J.S.; Fortunati, E.; Kozanecki, M.; Mazzaglia, A.; Balestra, G.M.; Kenny, J.M.; Torre, L.; Puglia, D. Antioxidant and antibacterial lignin nanoparticles in polyvinyl alcohol/chitosan films for active packaging. Ind. Crops Prod. 2016, 94, 800–811. [Google Scholar] [CrossRef]
- Li, Z.; Ge, Y.; Duo, X. Method for Preparing Lignin Nanoparticles by Taking Papermaking Black Liquid as Raw Material. C.N. Patent 103,275,331, 4 September 2013. [Google Scholar]
- Ge, Y.; Wei, Q.; Li, Z. Preparation and evaluation of the free radical scavenging activities of nanoscale lignin biomaterials. BioResources 2014, 9, 6699–6706. [Google Scholar] [CrossRef]
- Yang, W.; Fortunati, E.; Dominici, F.; Kenny, J.M.; Puglia, D. Effect of processing conditions and lignin content on thermal, mechanical and degradative behavior of lignin nanoparticles/polylactic (acid) bionanocomposites prepared by melt extrusion and solvent casting. Eur. Polym. J. 2015, 71, 126–139. [Google Scholar] [CrossRef]
- Yang, W.; Dominici, F.; Fortunati, E.; Kenny, J.M.; Puglia, D. Effect of lignin nanoparticles and masterbatch procedures on the final properties of glycidyl methacrylate-g-poly (lactic acid) films before and after accelerated UV weathering. Ind. Crops Prod. 2015, 77, 833–844. [Google Scholar] [CrossRef]
- Gupta, A.K.; Mohanty, S.; Nayak, S.K. Influence of addition of vapor grown carbon fibers on mechanical, thermal and biodegradation properties of lignin nanoparticle filled bio-poly(trimethylene terephthalate) hybrid nanocomposites. RSC Adv. 2015, 5, 56028–56036. [Google Scholar] [CrossRef]
- Yang, W.; Fortunati, E.; Dominici, F.; Giovanale, G.; Mazzaglia, A.; Balestra, G.M.; Kenny, J.M.; Puglia, D. Synergic effect of cellulose and lignin nanostructures in PLA based systems for food antibacterial packaging. Eur. Polym. J. 2016, 79, 1–12. [Google Scholar] [CrossRef]
- Wege, H.A.; Kim, S.; Paunov, V.N.; Zhong, Q.; Velev, O.D. Long-term stabilization of foams and emulsions with in situ formed microparticles from hydrophobic cellulose. Langmuir 2008, 24, 9245–9253. [Google Scholar] [CrossRef] [PubMed]
- Bobleter, O. Hydrothermal degradation of polymers derived from plants. Prog. Polym. Sci. 1994, 19, 797–841. [Google Scholar] [CrossRef]
- Thanh, N.T.K.; Maclean, N.; Mahiddine, S. Mechanisms of nucleation and growth of nanoparticles in solution. Chem. Rev. 2014, 114, 7610–7630. [Google Scholar] [CrossRef] [PubMed]
- Ribeiro, C.; Lee, E.J.H.; Longo, E.; Leite, E.R. A Kinetic model to describe nanocrystal growth by the oriented attachment mechanism. ChemPhysChem 2005, 6, 690–696. [Google Scholar] [CrossRef] [PubMed]
- Viswanatha, R.; Sarma, D.D. Growth of Nanocrystals in Solution. In Nanomaterials Chemistry; Wiley-VCH Verlag GmbH & Co. KGaA: Weinheim, Germany, 2007; pp. 139–170. [Google Scholar]
- Wei, Z.; Yang, Y.; Yang, R.; Wang, C. Alkaline lignin extracted from furfural residues for pH-responsive Pickering emulsions and their recyclable polymerization. Green Chem. 2012, 14, 3230. [Google Scholar] [CrossRef]
- Nypelö, T.E.; Carrillo, C.A.; Rojas, O.J. Lignin supracolloids synthesized from (W/O) microemulsions: use in the interfacial stabilization of Pickering systems and organic carriers for silver metal. Soft Matter 2015, 11, 2046–2054. [Google Scholar] [CrossRef] [PubMed]
- Popa, V.I.; Cǎpraru, A.M.; Grama, S.; Mǎluţan, T. Nanoparticles based on modified lignins with biocide properties. Cellul. Chem. Technol. 2011, 45, 221–226. [Google Scholar]
- Schilling, P. Submicron Lignin-Based Binders for Water-Based Black Ink Formulations. Patent US5192361 A, 9 March 1993. [Google Scholar]
- Malutan, T.; Nicu, R.; Popa, V. Contribution to the study of hydroxymetylation reaction of alkali lignin. BioResources 2007, 3. [Google Scholar]
- Malutan, T.; Nicu, R.; Popa, V.I. Lignin modification by epoxidation. BioResources 2008, 3, 1371–1376. [Google Scholar]
- Mitchell, D.S. Submicron Lignin Dispersions. U.S. Patent 4,957,557, 18 September 1990. [Google Scholar]
- Chiefari, J.; Chong, Y.K.B.; Ercole, F.; Krstina, J.; Jeffery, J.; Le, T.P.T.; Mayadunne, R.T.A.; Meijs, G.F.; Moad, C.L.; Moad, G.; et al. Living free-radical polymerization by reversible addition−Fragmentation chain transfer: The RAFT process. Macromolecules 1998, 31, 5559–5562. [Google Scholar] [CrossRef]
- Moad, G.; Rizzardo, E.; Thang, S.H. Radical addition–fragmentation chemistry in polymer synthesis. Polymer 2008, 49, 1079–1131. [Google Scholar] [CrossRef]
- Gupta, C.; Washburn, N.R. Polymer-grafted lignin surfactants prepared via reversible addition–fragmentation chain-transfer polymerization. Langmuir 2014, 30, 9303–9312. [Google Scholar] [CrossRef] [PubMed]
- Silmore, K.S.; Gupta, C.; Washburn, N.R. Tunable Pickering emulsions with polymer-grafted lignin nanoparticles (PGLNs). J. Colloid Interface Sci. 2016, 466, 91–100. [Google Scholar] [CrossRef] [PubMed]
- Antonietti, M.; Landfester, K. Polyreactions in miniemulsions. Prog. Polym. Sci. 2002, 27, 689–757. [Google Scholar] [CrossRef]
- Pavel, F.M. Microemulsion polymerization. J. Dispers. Sci. Technol. 2004, 25, 1–16. [Google Scholar] [CrossRef]
- Cavalieri, F.; Zhou, M.; Tortora, M.; Ashokkumar, M. Ultrasound-assisted preparation of nanopolymeric and micropolymeric materials for the encapsulation of bioactive agents. In Cavitation; Pan Stanford Publishing: Singapore, 2014; pp. 227–261. [Google Scholar]
- Svitelska, G.V.; Gallios, G.P.; Zouboulis, A.I. Sonochemical decomposition of natural polyphenolic compound (condensed tannin). Chemosphere 2004, 56, 981–987. [Google Scholar] [CrossRef] [PubMed]
- Nishida, M.; Uraki, Y.; Sano, Y. Lignin gel with unique swelling property. Bioresour. Technol. 2003, 88, 81–83. [Google Scholar] [CrossRef]
- Chen, Z.; Gao, L. Synthesis and magnetic properties of CoFe2O4 nanoparticles by using PEG as surfactant additive. Mater. Sci. Eng. B 2007, 141, 82–86. [Google Scholar] [CrossRef]
- Chen, N.; Dempere, L.A.; Tong, Z. Synthesis of pH-responsive lignin-based nanocapsules for controlled release of hydrophobic molecules. ACS Sustain. Chem. Eng. 2016, 4, 5204–5211. [Google Scholar] [CrossRef]
- Zhong, X.; Qian, Y.; Huang, J.; Yang, D.; Deng, Y.; Qiu, X. Fabrication of lignosulfonate vesicular reverse micelles to immobilize horseradish peroxidase. Ind. Eng. Chem. Res. 2016, 55, 2731–2737. [Google Scholar] [CrossRef]
- Müller, R.H.; Peters, K. Nanosuspensions for the formulation of poorly soluble drugs. Int. J. Pharm. 1998, 160, 229–237. [Google Scholar] [CrossRef]
- Müller, R.H.; Peters, K.; Becker, R.; Kruss, B. Nanosuspensions for the iv administration of poorly soluble drugs-stability during sterilization and long-term storage. Proc. Int. Symp. Control Rel. Bioact. Mater. 1995, 22, 574–575. [Google Scholar]
- Merisko-Liversidge, E.; Sarpotdar, P.; Bruno, J.; Hajj, S.; Wei, L.; Peltier, N.; Rake, J.; Shaw, J.M.; Pugh, S.; Polin, L.; et al. Formulation and antitumor activity evaluation of nanocrystalline suspensions of poorly soluble anticancer drugs. Pharm. Res. 1996, 13, 272–278. [Google Scholar] [CrossRef] [PubMed]
- Malcolmson, R.J.; Embleton, J.K. Dry powder formulations for pulmonary delivery. Pharm. Sci. Technol. Today 1998, 1, 394–398. [Google Scholar] [CrossRef]
- Nair, S.S.; Sharma, S.; Pu, Y.; Sun, Q.; Pan, S.; Zhu, J.Y.; Deng, Y.; Ragauskas, A.J. High shear homogenization of lignin to nanolignin and thermal stability of nanolignin-polyvinyl alcohol blends. ChemSusChem 2014, 7, 3513–3520. [Google Scholar] [CrossRef] [PubMed]
- Zimniewska, M.; Kozłowski, R.; Batog, J. Nanolignin modified linen fabric as a multifunctional product. Mol. Cryst. Liq. Cryst. 2008, 484, 43–409. [Google Scholar] [CrossRef]
- Shawn, M.D.; Cicotte, K.N.; Wheeler, D.R.; Benko, D.A. Lignin Nanoparticle Synthesis. U.S. patent 9,102,801, 11 August 2015. [Google Scholar]
- Zhiming, L.; Guochao, L.; Haiying, W. Preparation Method of Nano Lignin with Controllable Particle Size. CN 103,145,999, 12 June 2013. [Google Scholar]
- Gutiérrez, M.C.; Ferrer, M.L.; Del Monte, F. Ice-templated materials: Sophisticated structures exhibiting enhanced functionalities obtained after unidirectional freezing and ice-segregation- induced self-assembly. Chem. Mater. 2008, 20, 634–648. [Google Scholar] [CrossRef]
- Spender, J.; Demers, A.L.; Xie, X.; Cline, A.E.; Earle, M.A.; Ellis, L.D.; Neivandt, D.J. Method for production of polymer and carbon nanofibers from water-soluble polymers. Nano Lett. 2012, 12, 3857–3860. [Google Scholar] [CrossRef] [PubMed]
- Mishra, P.K.; Wimmer, R. Aerosol assisted self-assembly as a route to synthesize solid and hollow spherical lignin colloids and its utilization in layer by layer deposition. Ultrason. Sonochem. 2017, 35, 45–50. [Google Scholar] [CrossRef] [PubMed]
- Huczko, A. Template-based synthesis of nanomaterials. Appl. Phys. A Mater. Sci. Process. 2000, 70, 365–376. [Google Scholar] [CrossRef]
- Caicedo, H.M.; Dempere, L.A.; Vermerris, W. Template-mediated synthesis and bio-functionalization of flexible lignin-based nanotubes and nanowires. Nanotechnology 2012, 23, 105605. [Google Scholar] [CrossRef] [PubMed]
- Ten, E.; Ling, C.; Wang, Y.; Srivastava, A.; Dempere, L.A.; Vermerris, W. Lignin nanotubes as vehicles for gene delivery into human cells. Biomacromolecules 2014, 15, 327–338. [Google Scholar] [CrossRef] [PubMed]
- Vermerris, W.; Thompson, K.J.; Mcintyre, L.M. The maize Brown midrib1 locus affects cell wall composition and plant development in a dose-dependent manner. Heredity 2002, 88, 450–457. [Google Scholar] [CrossRef] [PubMed]
- Saballos, A.; Vermerris, W.; Rivera, L.; Ejeta, G. Allelic association, chemical characterization and saccharification properties of brown midrib mutants of sorghum (Sorghum bicolor (L.) Moench). BioEnergy Res. 2008, 1, 193–204. [Google Scholar] [CrossRef]
- Hatfield, R.D.; Jung, H.G.; Ralph, J.; Buxton, D.R.; Weimer, P.J. A comparison of the insoluble residues produced by the Klason lignin and acid detergent lignin procedures. J. Sci. Food Agric. 1994, 65, 51–58. [Google Scholar] [CrossRef]
- Bruce, R.J.; West, C.A. Elicitation of lignin biosynthesis and isoperoxidase activity by pectic fragments in suspension cultures of castor bean. PLANT Physiol. 1989, 91, 889–897. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Gellerstedt, G.; Toven, K. Steam explosion lignins; their extraction, structure and potential as feedstock for biodiesel and chemicals. Bioresour. Technol. 2009, 100, 2556–2561. [Google Scholar] [CrossRef] [PubMed]
- Jiang, C.; He, H.; Yu, P.; Wang, D.K.; Zhou, L.; Jia, D.M. Plane-interface-induced lignin-based nanosheets and its reinforcing effect on styrene-butadiene rubber. Express Polym. Lett. 2014, 8, 619–634. [Google Scholar] [CrossRef]
- Jiang, C.; He, H.; Jiang, H.; Ma, L.; Jia, D.M. Nano-lignin filled natural rubber composites: Preparation and characterization. Express Polym. Lett. 2013, 7, 480–493. [Google Scholar] [CrossRef]
- Pulkkinen, E.; Mäkelä, A.; Mikkonen, H. Preparation and Testing of Cationic Flocculants from Kraft Lignin. In Lignin: Properties and Materials; American Chemical Society: Washington, DC, USA, 1989; pp. 284–293. [Google Scholar]
- Laszlo, J.A. Solubility and dye-binding properties of quaternized and peroxidase-polymerized kraft lignin. Environ. Technol. 1999, 20, 607–615. [Google Scholar] [CrossRef]
- Eerikäinen, H.; Watanabe, W.; Kauppinen, E.I.; Ahonen, P.P. Aerosol flow reactor method for synthesis of drug nanoparticles. Eur. J. Pharm. Biopharm. 2003, 55, 357–360. [Google Scholar] [CrossRef]
- Buesser, B.; Pratsinis, S.E. Design of nanomaterial synthesis by aerosol processes. Annu. Rev. Chem. Biomol. Eng. 2012, 3, 103–127. [Google Scholar] [CrossRef] [PubMed]
- Pratsinis, S.E.; Mastrangelo, S.V.R. Material synthesis in aerosol reactors. Chem. Eng. Prog. 1989, 85, 62–66. [Google Scholar]
- Ago, M.; Huan, S.; Borghei, M.; Raula, J.; Kauppinen, E.I.; Rojas, O.J. High-throughput synthesis of lignin particles (∼30 nm to ∼2 μm) via aerosol flow reactor: size fractionation and utilization in pickering emulsions. ACS Appl. Mater. Interfaces 2016, 8, 23302–23310. [Google Scholar] [CrossRef] [PubMed]
- Hillamo, R.E.; Kauppinen, E.I. On the performance of the berner low pressure impactor. Aerosol Sci. Technol. 1991, 14, 33–47. [Google Scholar] [CrossRef]
- Broadhead, J.; Edmond Rouan, S.; Rhodes, C. The spray drying of pharmaceuticals. Drug Dev. Ind. Pharm. 1992, 18, 1169–1206. [Google Scholar] [CrossRef]
- Rutledge, G.C.; Fridrikh, S.V. Formation of fibers by electrospinning. Adv. Drug Deliv. Rev. 2007, 59, 1384–1391. [Google Scholar] [CrossRef] [PubMed]
- Reneker, D.H.; Yarin, A.L. Electrospinning jets and polymer nanofibers. Polymer 2008, 49, 2387–2425. [Google Scholar] [CrossRef]
- Greiner, A.; Wendorff, J.H. Electrospinning: A fascinating method for the preparation of ultrathin fibers. Angew. Chem. Int. Ed. 2007, 46, 5670–5703. [Google Scholar] [CrossRef] [PubMed]
- Dallmeyer, I.; Ko, F.; Kadla, J.F. Electrospinning of technical lignins for the production of fibrous Networks. J. Wood Chem. Technol. 2010, 30, 315–329. [Google Scholar] [CrossRef]
- Klossner, R.R.; Queen, H.A.; Coughlin, A.J.; Krause, W.E. Correlation of chitosan’s rheological properties and its ability to electrospin. Biomacromolecules 2008, 9, 2947–2953. [Google Scholar] [CrossRef] [PubMed]
- Nie, H.; He, A.; Wu, W.; Zheng, J.; Xu, S.; Li, J.; Han, C.C. Effect of poly(ethylene oxide) with different molecular weights on the electrospinnability of sodium alginate. Polymer 2009, 50, 4926–4934. [Google Scholar] [CrossRef]
- Varesano, A.; Aluigi, A.; Vineis, C.; Tonin, C. Study on the shear viscosity behavior of keratin/PEO blends for nanofibre electrospinning. J. Polym. Sci. B Polym. Phys. 2008, 46, 1193–1201. [Google Scholar] [CrossRef]
- Ruiz-Rosas, R.; Bedia, J.; Lallave, M.; Loscertales, I.G.; Barrero, A.; Rodríguez-Mirasol, J.; Cordero, T. The production of submicron diameter carbon fibers by the electrospinning of lignin. Carbon 2010, 48, 696–705. [Google Scholar] [CrossRef]
- Larsen, G.; Velarde-Ortiz, R.; Minchow, K.; Barrero, A.; Loscertales, I.G. A method for making inorganic and hybrid (organic/inorganic) fibers and vesicles with diameters in the submicrometer and micrometer range via sol-gel chemistry and electrically forced liquid jets. J. Am. Chem. Soc. 2003, 125, 1154–1155. [Google Scholar] [CrossRef] [PubMed]
- Díaz, J.E.; Barrero, A.; Márquez, M.; Loscertales, I.G. Controlled encapsulation of hydrophobic liquids in hydrophilic polymer nanofibers by co-electrospinning. Adv. Funct. Mater. 2006, 16, 2110–2116. [Google Scholar] [CrossRef]
- Loscertales, I.G.; Gañán-Calvo, A.M. Micro/nano encapsulation via electrified coaxial liquid jets. Science 2002, 295, 1695–1698. [Google Scholar] [CrossRef] [PubMed]
- Ago, M.; Jakes, J.E.; Rojas, O.J. Thermomechanical properties of lignin-based electrospun nanofibers and films reinforced with cellulose nanocrystals: A dynamic mechanical and nanoindentation study. ACS Appl. Mater. Interfaces 2013, 5, 11768–11776. [Google Scholar] [CrossRef] [PubMed]
- Ago, M.; Okajima, K.; Jakes, J.E.; Park, S.; Rojas, O.J. Lignin-Based electrospun nanofibers reinforced with cellulose nanocrystals. Biomacromolecules 2012, 13, 918–926. [Google Scholar] [CrossRef] [PubMed]
- Byrappa, K.; Ohara, S.; Adschiri, T. Nanoparticles synthesis using supercritical fluid technology—Towards biomedical applications. Adv. Drug Deliv. Rev. 2008, 60, 299–327. [Google Scholar] [CrossRef] [PubMed]
- Tomasko, D.L.; Li, H.B.; Liu, D.H.; Han, X.M.; Wingert, M.J.; Lee, L.J.; Koelling, K.W. A review of CO(2) applications in the processing of polymers. Ind. Eng. Chem. Res. 2003, 42, 6431–6456. [Google Scholar] [CrossRef]
- Mawson, S.; Kanakia, S.; Johnston, K.P. Coaxial nozzle for control of particle morphology in precipitation with a compressed fluid antisolvent. J. Appl. Polym. Sci. 1997, 64, 2105–2118. [Google Scholar] [CrossRef]
- Dixon, D.J.; Luna-Bárcenas, G.; Johnston, K.P. Microcellular microspheres and microballoons by precipitation with a vapour-liquid compressed fluid antisolvent. Polymer 1994, 35, 3998–4005. [Google Scholar] [CrossRef]
- Tu, L.S.; Dehghani, F.; Foster, N. Micronisation and microencapsulation of pharmaceuticals using a carbon dioxide antisolvent. Powder Technol. 2002, 126, 134–149. [Google Scholar] [CrossRef]
- Myint, A.A.; Lee, H.W.; Seo, B.; Son, W.-S.; Yoon, J.; Yoon, T.J.; Park, H.J.; Yu, J.; Yoon, J.; Lee, Y.-W. One pot synthesis of environmentally friendly lignin nanoparticles with compressed liquid carbon dioxide as an antisolvent. Green Chem. 2016, 18, 2129–2146. [Google Scholar] [CrossRef]
















| Raw Material | Isolation Method | Length (μm) | Diameter (nm) | Wall Thickness (nm) | Aspect Ratio |
|---|---|---|---|---|---|
| Sugar Cane Bagasse | Phosporic acid | 17.0 ± 2.5 | 200 ± 45 | 49.5 ± 2.9 | 85 |
| Sorghum | Klason | 15.2 ± 1.4 | 219.3 ± 71 | 75.2 ± 16 | 69 |
| Sorghum (BTx623-bmr6) | Klason | 14.3 ± 2.4 | 223.5 ± 32 | 59.7 ± 6.7 | 64 |
| Pine | Klason | 15.9 ± 0.9 | 215.1 ± 33 | 45.2 ± 2.4 | 74 |
| Poplar | Klason | 16.2 ± 1.0 | 214.1 ± 39 | 51.6 ± 4.1 | 76 |
| Sorghum | Thioglycolic acid | 18.6 ± 1.6 | 219.1 ± 54 | 44.8 ± 4.3 | 85 |
| Sorghum (BTx623-bmr6) | Thioglycolic acid | 18.0 ± 1.2 | 203.7 ± 36 | 51.0 ± 3.6 | 89 |
| Pine | Thioglycolic acid | 17.4 ± 1.4 | 194.9 ± 20 | 47.8 ± 3.0 | 89 |
| Poplar | Thioglycolic acid | 17.9 ± 1.4 | 190.5 ± 99 | 58.1 ± 7.0 | 94 |
| Sorghum | NaOH | 14.7 ± 1.9 | 172.4 ± 78 | 67.9 ± 6.4 | 85 |
| Sorghum (BTx623-bmr6) | NaOH | 15.7 ± 1.5 | 180.5 ± 74 | 66.3 ± 7.1 | 87 |
| Pine | NaOH | 10.8 ± 0.7 | 172.3 ± 28 | 55.2 ± 3.8 | 62 |
| Poplar | NaOH | 10.9 ± 1.2 | 178.9 ± 62 | 69.2 ± 5.6 | 61 |
| T (K) | P (MPa) | Concentration Lignin Solution (wt %) | Flowrate Solution (kg/h) | Mole fraction CO2 | MPD (nm) | Yield (%) |
|---|---|---|---|---|---|---|
| 280.2 | 15.0 | 5.3 | 0.06 | 0.99 | 38.0 ± 7.2 | 77.3 |
| 288.2 | 15.0 | 5.3 | 0.06 | 0.99 | 51.0 ± 9.8 | 75.0 |
| 298.2 | 15.0 | 5.3 | 0.06 | 0.98 | 53.6 ± 11.2 | 70.0 |
| 280.2 | 7.5 | 5.3 | 0.06 | 0.94 | 38.0 ± 11.9 | 75.0 |
| 288.2 | 7.5 | 5.3 | 0.06 | 0.92 | 47.0 ± 12.8 | 67.5 |
| 298.2 | 7.5 | 5.3 | 0.06 | 0.90 | 74.0 ± 17.3 | 59.0 |
| 280.2 | 15.0 | 5.3 | 0.03 | 0.99 | 73.0 ± 17.5 | 51.0 |
| 280.2 | 15.0 | 10.6 | 0.06 | 0.99 | 54.5 ± 10.7 | 88.3 |
| Method | Source | Lignin Type 1 | Solvent 2 (L) | Antisolvent 2 (L) | Yield (%) | pH Stability | Ionic Strength Stability (mM NaCl) |
|---|---|---|---|---|---|---|---|
| Solvent shifting (solid particles) | [17] | AL | THF (1) | H2O (2.03) | - | <12 | - |
| [37] | KL | THF (0.1–1) | Dialysis with H2O | - | 7.4 | - | |
| [34] | KL | THF (0.1–1) | Dialysis with H2O | - | 4–12 | <500 | |
| [38] | OS | THF (0.01–0.1) | H2O (0.090–2) | - | - | - | |
| [43,56] | OS | Acetone (0.2) | H2O (1.8) | - | 3.5-8 | <70 | |
| [40] | EHL/OS | Acetone/H2O (0.1–10) | H2O (0.4–40) | - | - | - | |
| [41] | AL/Dioxane | Acetone/ H2O (0.1) | H2O (0.05) | 33–63 | - | - | |
| [44] | EHL | DMSO (0.333–0.488) | Dialysis with H2O | 41–90.9 | 4–10 | - | |
| Solvent shifting (hollow particles) | [51] | EHL | THF (0.5–2) | H2O (2–8) | - | 3.5–12 | - |
| [52] | KL | THF (0.2–2) | H2O (1.8–18) | - | - | - | |
| [54] | KL | Ethanol (0.4) | H2O (3.9) | - | - | - | |
| [53] | KL | Dioxane (0.3) | H2O (2.4) | - | - | - | |
| pH shifting (solid particles) | [43,56] | KL | EG (0.2) | 0.025M HNO3 (0.04–0.12) | - | 2–10.5 | <300 |
| [23] | EHL | EG (0.02) | 0.25M HCl (0.05) | ~10 | - | - | |
| [59] | AL | EG (0.025) | 0.1M HCl (0.001) 3 | - | - | - | |
| [16] | KL | EG (0.02–0.18) | 0.25M HCl (0.03) | - | 6–9 | - | |
| [16] | KL | H2O/NaOH (2) | 0.25M HNO3 (0.18) | - | instable | - | |
| [59] | AL | H2O/NaOH (0.011) | 0.25M HNO3 (to pH 1.9) | - | - | - | |
| Mechanical treatment | [92] | KL | H2O (0.2) | - | - | - | - |
| [36] | AL | H2O (1.4) | - | - | - | - | |
| Ice segregation | [98] | AL | DMSO (0.2–5) | Dialysis with H2O | - | 10.5 | - |
| [97] | AL | H2O/NaOH (3.3–10) | - | - | - | - | |
| Aerosol processing | [114] | AL | H2O (0.05–0.2) | - | >60 | - | - |
| KL/OS | DMF (0.05–0.2) | - | >60 | - | - | ||
| Electro-spinning | [124] | OS | Ethanol (0.0013) | - | - | - | - |
| [120] | KL/OS/PL/LS | DMF (0.001–0.01)H2O (0.001–0.01) | - | - | - | - | |
| [129] | KL | PVA/H2O (0.002) | - | - | - | - | |
| CO2 precipitation | [135] | KL | DMF (0.008–0.018) | CO2 (0.47–1.89 kg) | 51–88.3 | - | - |
| [22] | OS | Acetone (2) | CO2 (41 kg) | - | - | - |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Beisl, S.; Miltner, A.; Friedl, A. Lignin from Micro- to Nanosize: Production Methods. Int. J. Mol. Sci. 2017, 18, 1244. https://doi.org/10.3390/ijms18061244
Beisl S, Miltner A, Friedl A. Lignin from Micro- to Nanosize: Production Methods. International Journal of Molecular Sciences. 2017; 18(6):1244. https://doi.org/10.3390/ijms18061244
Chicago/Turabian StyleBeisl, Stefan, Angela Miltner, and Anton Friedl. 2017. "Lignin from Micro- to Nanosize: Production Methods" International Journal of Molecular Sciences 18, no. 6: 1244. https://doi.org/10.3390/ijms18061244
APA StyleBeisl, S., Miltner, A., & Friedl, A. (2017). Lignin from Micro- to Nanosize: Production Methods. International Journal of Molecular Sciences, 18(6), 1244. https://doi.org/10.3390/ijms18061244