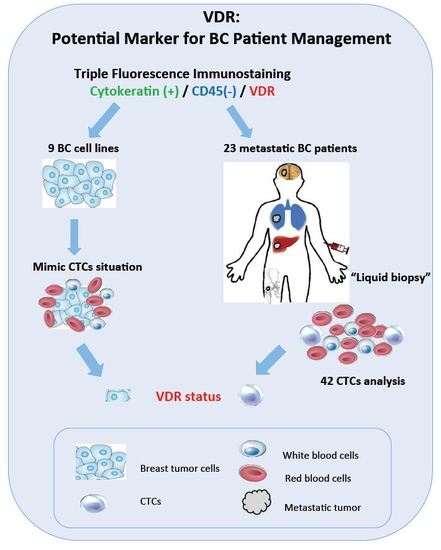
Fluorescence Analysis of Vitamin D Receptor Status of Circulating Tumor Cells (CTCS) in Breast Cancer: From Cell Models to Metastatic Patients
Abstract
:1. Introduction
2. Results
2.1. Fluorescence Labeling of VDR, CK, and CD45 on MCF-7 Cells Mixed with Peripheral Blood Mononuclear Cells (PBMCs)
2.2. Fluorescence Labeling of VDR and CK with Parallel 4′-6-Diamidino-2-Phenylindole (DAPI) Staining in MCF-7 Cells and in a Panel of Eight other BC Cell Lines and One Endometrial Cancer Cell Line
2.3. Patient Cohort
2.4. CTCs Screening and Enumeration
2.5. VDR Status Determination in CTCs
2.6. Correlation between CTC Presence, VDR Status and the Primary Tumor Characteristics
2.7. Specific CK Positive Cell Subtypes
3. Discussion
4. Materials and Methods
4.1. Cell Culture and Cytospin Preparation
4.2. Triple Fluorescence Labeling of CK, VDR, and CD45 with Parallel Phase Analysis
4.3. Fluorescence Labeling of VDR and Cytokeratin (CK) with Parallel 4′-6-Diamidino-2-Phenylindole (DAPI) Analysis
4.4. Patient Cohort
4.5. Blood Sampling, Ficoll and Cytospin Preparation
4.6. CTC Analysis by Triple Fluorescence
4.7. Statistical Analysis
5. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
Abbreviations
| BC | Breast cancer |
| CTC | Circulating tumor cell |
| CK | Cytokeratin |
| EMT | Epithelial–mesenchymal transition |
| ER | Estrogen receptor |
| FDA | Food and drug administration |
| FISH | Fluorescence in situ hybridization |
| HER2 | Human epidermal growth factor receptor 2 |
| HR | Hormone receptor |
| NR | Nuclear receptor |
| OS | Overall survival |
| PBC | Peripheral blood cell |
| PBMC | Peripheral blood mononuclear cell |
| PFS | Progression free survival |
| PR | Progesterone receptor |
| RAR | Retinoic acid receptor |
| RXR | Retinoid X receptor |
| THR | Thyroid hormone receptor |
| VDRE | Vitamin D response element |
| VDR | Vitamin D receptor |
| WBC | White blood cell |
References
- Lundqvist, J.; Yde, C.W.; Lykkesfeldt, A.E. 1α, 25-dihydroxyvitamin D3 inhibits cell growth and NF-κB signaling in tamoxifen-resistant breast cancer cells. Steroids 2014, 85, 30–35. [Google Scholar] [CrossRef] [PubMed]
- Montemurro, F.; Di Cosimo, S.; Arpino, G. Human epidermal growth factor receptor 2 (HER2)-positive and hormone receptor-positive breast cancer: New insights into molecular interactions and clinical implications. Ann. Oncl. 2013, 24, 2715–2724. [Google Scholar] [CrossRef] [PubMed]
- Jeselsohn, R.; Buchwalter, G.; De Angelis, C.; Brown, M.; Schiff, R. ESR1 mutations—A mechanism for acquired endocrine resistance in breast cancer. Nat. Rev. Clin. Oncol. 2015, 12, 573–583. [Google Scholar] [CrossRef] [PubMed]
- Hart, C.D.; Migliaccio, I.; Malorni, L.; Guarducci, C.; Biganzoli, L.; Di Leo, A. Challenges in the management of advanced, ER-positive, HER2-negative breast cancer. Nat. Rev. Clin. Oncol. 2015, 12, 541–552. [Google Scholar] [CrossRef] [PubMed]
- Welsh, J.; Wietzke, J.A.; Zinser, G.M.; Byrne, B.; Smith, K.; Narvaez, C.J. Vitamin D-3 receptor as a target for breast cancer prevention. J. Nutr. 2003, 133, 2425S–2433S. [Google Scholar] [PubMed]
- Chen, P.; Hu, P.; Xie, D.; Qin, Y.; Wang, F.; Wang, H. Meta-analysis of vitamin D, calcium and the prevention of breast cancer. Breast Cancer Res. Treat. 2010, 121, 469–477. [Google Scholar] [CrossRef] [PubMed]
- Ditsch, N.; Toth, B.; Mayr, D.; Lenhard, M.; Gallwas, J.; Weissenbacher, T.; Dannecker, C.; Friese, K.; Jeschke, U. The association between vitamin D receptor expression and prolonged overall survival in breast cancer. J. Histochem. Cytochem. 2012, 60, 121–129. [Google Scholar] [CrossRef] [PubMed]
- Mun, M.J.; Kim, T.H.; Hwang, J.Y.; Jang, W.C. Vitamin D receptor gene polymorphisms and the risk for female reproductive cancers: A meta-analysis. Maturitas 2015, 81, 256–265. [Google Scholar] [CrossRef] [PubMed]
- Welsh, J. Targets of vitamin D receptor signaling in the mammary gland. J. Bone Miner. Res. 2007, 22, V86–V90. [Google Scholar] [CrossRef] [PubMed]
- Narvaez, C.J.; Matthews, D.; LaPorta, E.; Simmons, K.M.; Beaudin, S.; Welsh, J. The impact of vitamin D in breast cancer: Genomics, pathways, metabolism. Front. Physiol. 2014, 5, 213. [Google Scholar] [CrossRef] [PubMed]
- Yen, P.M. Classical nuclear hormone receptor activity as a mediator of complex biological responses: A look at health and disease. Best Pract. Res. Clin. Gastroenterol. 2015, 29, 517–528. [Google Scholar] [CrossRef] [PubMed]
- Feldman, D.; Krishnan, A.V.; Swami, S.; Giovannucci, E.; Feldman, B.J. The role of vitamin D in reducing cancer risk and progression. Nat. Rev. Cancer 2014, 14, 342–357. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Harbeck, N.; Jeschke, U.; Doisneau-Sixou, S. Influence of vitamin D signaling on hormone receptor status and HER2 expression in breast cancer. J. Cancer Res. Clin. Oncol. 2016, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Beije, N.; Jager, A.; Sleijfer, S. Circulating tumor cell enumeration by the Cellsearch system: The clinician’s guide to breast cancer treatment? Cancer Treat. Rev. 2015, 41, 144–150. [Google Scholar] [CrossRef] [PubMed]
- Bock, C.; Rack, B.; Kuhn, C.; Hofmann, S.; Finkenzeller, C.; Jager, B.; Jeschke, U.; Doisneau-Sixou, S.F. Heterogeneity of ERα and ErbB2 status in cell lines and circulating tumor cells of metastatic breast cancer patients. Trans. Oncol. 2012, 5, 475–485. [Google Scholar] [CrossRef]
- Wallwiener, M.; Hartkopf, A.D.; Baccelli, I.; Riethdorf, S.; Schott, S.; Pantel, K.; Marme, F.; Sohn, C.; Trumpp, A.; Rack, B.; et al. The prognostic impact of circulating tumor cells in subtypes of metastatic breast cancer. Breast Cancer Res. Treat. 2013, 137, 503–510. [Google Scholar] [CrossRef] [PubMed]
- Rack, B.; Schindlbeck, C.; Juckstock, J.; Andergassen, U.; Hepp, P.; Zwingers, T.; Friedl, T.W.; Lorenz, R.; Tesch, H.; Fasching, P.A.; et al. Circulating tumor cells predict survival in early average-to-high risk breast cancer patients. J. Natl. Cancer Inst. 2014, 106. [Google Scholar] [CrossRef] [PubMed]
- Hall, C.; Karhade, M.; Laubacher, B.; Anderson, A.; Kuerer, H.; DeSynder, S.; Lucci, A. Circulating tumor cells after neoadjuvant chemotherapy in stage I-III triple-negative breast cancer. Ann. Surg. Oncol. 2015, 22, 552–558. [Google Scholar] [CrossRef] [PubMed]
- Banys-Paluchowski, M.; Krawczyk, N.; Meier-Stiegen, F.; Fehm, T. Circulating tumor cells in breast cancer-current status and perspectives. Crit. Rev. Oncol. Hematol. 2016, 97, 22–29. [Google Scholar] [CrossRef] [PubMed]
- Alix-Panabieres, C.; Pantel, K. Circulating tumor cells: Liquid biopsy of cancer. Clin. Chem. 2013, 59, 110–118. [Google Scholar] [CrossRef] [PubMed]
- McInnes, L.M.; Jacobson, N.; Redfern, A.; Dowling, A.; Thompson, E.W.; Saunders, C.M. Clinical implications of circulating tumor cells of breast cancer patients: Role of epithelial-mesenchymal plasticity. Front. Oncol. 2015, 5, 42. [Google Scholar] [CrossRef] [PubMed]
- Onstenk, W.; Gratama, J.W.; Foekens, J.A.; Sleijfer, S. Towards a personalized breast cancer treatment approach guided by circulating tumor cell (CTC) characteristics. Cancer Treat. Rev. 2013, 39, 691–700. [Google Scholar] [CrossRef] [PubMed]
- Wan, L.; Pantel, K.; Kang, Y. Tumor metastasis: Moving new biological insights into the clinic. Nat. Med. 2013, 19, 1450–1464. [Google Scholar] [CrossRef] [PubMed]
- Elstner, E.; Linker-Israeli, M.; Said, J.; Umiel, T.; de Vos, S.; Shintaku, I.P.; Heber, D.; Binderup, L.; Uskokovic, M.; Koeffler, H.P. 20-epi-vitamin D3 analogues: A novel class of potent inhibitors of proliferation and inducers of differentiation of human breast cancer cell lines. Cancer Res. 1995, 55, 2822–2830. [Google Scholar] [PubMed]
- Lopes, N.; Sousa, B.; Martins, D.; Gomes, M.; Vieira, D.; Veronese, L.A.; Milanezi, F.; Paredes, J.; Costa, J.L.; Schmitt, F. Alterations in vitamin D signalling and metabolic pathways in breast cancer progression: A study of VDR, CYP27B1 and CYP24A1 expression in benign and malignant breast lesions. BMC Cancer 2010, 10, 483. [Google Scholar] [CrossRef] [PubMed]
- Bock, C.; Rack, B.; Huober, J.; Andergassen, U.; Jeschke, U.; Doisneau-Sixou, S. Distinct expression of cytokeratin, n-cadherin and cd133 in circulating tumor cells of metastatic breast cancer patients. Future Oncol. 2014, 10, 1751–1765. [Google Scholar] [CrossRef] [PubMed]
- Joosse, S.A.; Gorges, T.M.; Pantel, K. Biology, detection, and clinical implications of circulating tumor cells. EMBO Mol. Med. 2015, 7, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Shao, M.M.; Chan, S.K.; Yu, A.M.; Lam, C.C.; Tsang, J.Y.; Lui, P.C.; Law, B.K.; Tan, P.H.; Tse, G.M. Keratin expression in breast cancers. Virchows Arch. 2012, 461, 313–322. [Google Scholar] [CrossRef] [PubMed]
- You, F.; Roberts, L.A.; Kang, S.P.; Nunes, R.A.; Dias, C.; Iglehart, J.D.; Solomon, N.A.; Friedman, P.N.; Harris, L.N. Low-level expression of HER2 and CK19 in normal peripheral blood mononuclear cells: Relevance for detection of circulating tumor cells. J. Hematol. Oncol. 2008, 1, 2. [Google Scholar] [CrossRef] [PubMed]
- Khatami, F.; Aghayan, H.R.; Sanaei, M.; Heshmat, R.; Tavangar, S.M.; Larijani, B. The potential of circulating tumor cells in personalized management of breast cancer: A systematic review. Acta Med. Iran. 2017, 55, 175–193. [Google Scholar] [PubMed]
- Somlo, G.; Lau, S.K.; Frankel, P.; Hsieh, H.B.; Liu, X.; Yang, L.; Krivacic, R.; Bruce, R.H. Multiple biomarker expression on circulating tumor cells in comparison to tumor tissues from primary and metastatic sites in patients with locally advanced/inflammatory, and stage iv breast cancer, using a novel detection technology. Breast Cancer Res. Treat. 2011, 128, 155–163. [Google Scholar] [CrossRef] [PubMed]
- Hayashi, N.; Nakamura, S.; Tokuda, Y.; Shimoda, Y.; Yagata, H.; Yoshida, A.; Ota, H.; Hortobagyi, G.N.; Cristofanilli, M.; Ueno, N.T. Prognostic value of HER2-positive circulating tumor cells in patients with metastatic breast cancer. Int. J. Clin. Oncol. 2012, 17, 96–104. [Google Scholar] [CrossRef] [PubMed]
- Ligthart, S.T.; Bidard, F.C.; Decraene, C.; Bachelot, T.; Delaloge, S.; Brain, E.; Campone, M.; Viens, P.; Pierga, J.Y.; Terstappen, L.W. Unbiased quantitative assessment of HER-2 expression of circulating tumor cells in patients with metastatic and non-metastatic breast cancer. Ann. Oncol. 2013, 24, 1231–1238. [Google Scholar] [CrossRef] [PubMed]
- Nadal, R.; Fernandez, A.; Sanchez-Rovira, P.; Salido, M.; Rodriguez, M.; Garcia-Puche, J.L.; Macia, M.; Corominas, J.M.; Delgado-Rodriguez, M.; Gonzalez, L.; et al. Biomarkers characterization of circulating tumour cells in breast cancer patients. Breast Cancer Res. 2012, 14, R71. [Google Scholar] [CrossRef] [PubMed]
- Gasch, C.; Bauernhofer, T.; Pichler, M.; Langer-Freitag, S.; Reeh, M.; Seifert, A.M.; Mauermann, O.; Izbicki, J.R.; Pantel, K.; Riethdorf, S. Heterogeneity of epidermal growth factor receptor status and mutations of KRAS/PIK3CA in circulating tumor cells of patients with colorectal cancer. Clin. Chem. 2013, 59, 252–260. [Google Scholar] [CrossRef] [PubMed]
- Buras, R.R.; Schumaker, L.M.; Davoodi, F.; Brenner, R.V.; Shabahang, M.; Nauta, R.J.; Evans, S.R. Vitamin D receptors in breast cancer cells. Breast Cancer Res. Treat. 1994, 31, 191–202. [Google Scholar] [CrossRef] [PubMed]
- Pendas-Franco, N.; Gonzalez-Sancho, J.M.; Suarez, Y.; Aguilera, O.; Steinmeyer, A.; Gamallo, C.; Berciano, M.T.; Lafarga, M.; Munoz, A. Vitamin D regulates the phenotype of human breast cancer cells. Differentiation 2007, 75, 193–207. [Google Scholar] [CrossRef] [PubMed]
- Lopes, N.; Carvalho, J.; Duraes, C.; Sousa, B.; Gomes, M.; Costa, J.L.; Oliveira, C.; Paredes, J.; Schmitt, F. 1α,25-dihydroxyvitamin D3 induces de novo E-cadherin expression in triple-negative breast cancer cells by CDH1-promoter demethylation. Anticancer Res. 2012, 32, 249–257. [Google Scholar] [PubMed]
- Reimers, L.L.; Crew, K.D.; Bradshaw, P.T.; Santella, R.M.; Steck, S.E.; Sirosh, I.; Terry, M.B.; Hershman, D.L.; Shane, E.; Cremers, S.; et al. Vitamin D-related gene polymorphisms, plasma 25-hydroxyvitamin D, and breast cancer risk. Cancer Causes Control 2015, 26, 187–203. [Google Scholar] [CrossRef] [PubMed]
- Pulito, C.; Terrenato, I.; Di Benedetto, A.; Korita, E.; Goeman, F.; Sacconi, A.; Biagioni, F.; Blandino, G.; Strano, S.; Muti, P.; et al. Cdx2 polymorphism affects the activities of vitamin D receptor in human breast cancer cell lines and human breast carcinomas. PLoS ONE 2015, 10, e0124894. [Google Scholar] [CrossRef] [PubMed]
- Alimirah, F.; Peng, X.; Murillo, G.; Mehta, R.G. Functional significance of vitamin D receptor Foki polymorphism in human breast cancer cells. PLoS ONE 2011, 6, e16024. [Google Scholar] [CrossRef] [PubMed]
- Segaert, S.; Degreef, H.; Bouillon, R. Vitamin D receptor expression is linked to cell cycle control in normal human keratinocytes. Biochem. Biophys. Res. Commun. 2000, 279, 89–94. [Google Scholar] [CrossRef] [PubMed]
- Saccone, D.; Asani, F.; Bornman, L. Regulation of the vitamin D receptor gene by environment, genetics and epigenetics. Gene 2015, 561, 171–180. [Google Scholar] [CrossRef] [PubMed]
- Baeke, F.; Takiishi, T.; Korf, H.; Gysemans, C.; Mathieu, C. Vitamin D: Modulator of the immune system. Curr. Opin. Pharmacol. 2010, 10, 482–496. [Google Scholar] [CrossRef] [PubMed]
- Coleman, L.A.; Mishina, M.; Thompson, M.; Spencer, S.M.; Reber, A.J.; Davis, W.G.; Cheng, P.Y.; Belongia, E.A.; Talbot, H.K.; Sundaram, M.E.; et al. Age, serum 25-hydroxyvitamin D and vitamin D receptor (VDR) expression and function in peripheral blood mononuclear cells. Oncotarget 2016, 7, 35512–35521. [Google Scholar] [CrossRef] [PubMed]
- Zerwekh, J.E.; Yu, X.P.; Breslau, N.A.; Manolagas, S.; Pak, C.Y. Vitamin D receptor quantitation in human blood mononuclear cells in health and disease. Mol. Cell. Endocrinol. 1993, 96, 1–6. [Google Scholar] [CrossRef]
- Vukic, M.; Neme, A.; Seuter, S.; Saksa, N.; de Mello, V.D.; Nurmi, T.; Uusitupa, M.; Tuomainen, T.P.; Virtanen, J.K.; Carlberg, C. Relevance of vitamin D receptor target genes for monitoring the vitamin D responsiveness of primary human cells. PLoS ONE 2015, 10, e0124339. [Google Scholar] [CrossRef] [PubMed]
- Gorges, T.M.; Tinhofer, I.; Drosch, M.; Rose, L.; Zollner, T.M.; Krahn, T.; von Ahsen, O. Circulating tumour cells escape from EpCAM-based detection due to epithelial-to-mesenchymal transition. BMC Cancer 2012, 12, 178. [Google Scholar] [CrossRef] [PubMed]
- Hyun, K.A.; Lee, T.Y.; Jung, H.I. Negative enrichment of circulating tumor cells using a geometrically activated surface interaction chip. Anal. Chem. 2013, 85, 4439–4445. [Google Scholar] [CrossRef] [PubMed]
- Krag, D.N.; Kusminsky, R.; Manna, E.; Weaver, D.; Harlow, S.P.; Covelli, M.; Stanley, M.A.; McCahill, L.; Ittleman, F.; Leavitt, B.; et al. Cytokeratin-positive cells in the bone marrow of breast cancer patients and noncancer donors. Appl. Immunohistochem. Mol. Morphol. 2009, 17, 403–408. [Google Scholar] [CrossRef] [PubMed]
- Byrne, B.; Welsh, J. Identification of novel mediators of vitamin D signaling and 1,25(OH)2D3 resistance in mammary cells. J. Steroid. Biochem. Mol. Biol. 2007, 103, 703–707. [Google Scholar] [CrossRef] [PubMed]
- Wang, K.; Dong, M.; Sheng, W.; Liu, Q.; Yu, D.; Dong, Q.; Li, Q.; Wang, J. Expression of vitamin D receptor as a potential prognostic factor and therapeutic target in pancreatic cancer. Histopathology 2015, 67, 386–397. [Google Scholar] [CrossRef] [PubMed]
- Wen, Y.; Da, M.; Zhang, Y.; Peng, L.; Yao, J.; Duan, Y. Alterations in vitamin D signaling pathway in gastric cancer progression: A study of vitamin D receptor expression in human normal, premalignant, and malignant gastric tissue. Int. J. Clin. Exp. Pathol. 2015, 8, 13176–13184. [Google Scholar] [PubMed]
- Al-Azhri, J.; Zhang, Y.; Bshara, W.; Zirpoli, G.; McCann, S.E.; Khoury, T.; Morrison, C.D.; Edge, S.B.; Ambrosone, C.B.; Yao, S. Tumor expression of vitamin D receptor and breast cancer histopathological characteristics and prognosis. Clin. Cancer Res. 2017, 23, 97–103. [Google Scholar] [CrossRef] [PubMed]
- Murray, A.; Madden, S.F.; Synnott, N.C.; Klinger, R.; O’Connor, D.; O’Donovan, N.; Gallagher, W.; Crown, J.; Duffy, M.J. Vitamin D receptor as a target for breast cancer therapy. Endocr. Relat. Cancer 2017, 24, 181–195. [Google Scholar] [CrossRef] [PubMed]
- Swami, S.; Krishnan, A.V.; Feldman, D. 1α, 25-dihydroxyvitamin D3 down-regulates estrogen receptor abundance and suppresses estrogen actions in MCF-7 human breast cancer cells. Clin. Cancer Res. 2000, 6, 3371–3379. [Google Scholar] [PubMed]
- Krishnan, A.V.; Swami, S.; Feldman, D. Vitamin D and breast cancer: Inhibition of estrogen synthesis and signaling. J. Steroid Biochem. Mol. Biol. 2010, 121, 343–348. [Google Scholar] [CrossRef] [PubMed]
- Krishnan, A.V.; Swami, S.; Peng, L.; Wang, J.; Moreno, J.; Feldman, D. Tissue-selective regulation of aromatase expression by calcitriol: Implications for breast cancer therapy. Endocrinology 2010, 151, 32–42. [Google Scholar] [CrossRef] [PubMed]
- Santos-Martinez, N.; Diaz, L.; Ordaz-Rosado, D.; Garcia-Quiroz, J.; Barrera, D.; Avila, E.; Halhali, A.; Medina-Franco, H.; Ibarra-Sanchez, M.J.; Esparza-Lopez, J.; et al. Calcitriol restores antiestrogen responsiveness in estrogen receptor negative breast cancer cells: A potential new therapeutic approach. BMC Cancer 2014, 14, 230. [Google Scholar] [CrossRef] [PubMed]
- Duffy, M.J.; Murray, A.; Synnott, N.C.; O’Donovan, N.; Crown, J. Vitamin D analogues: Potential use in cancer treatment. Crit. Rev. Oncol. Hematol. 2017, 112, 190–197. [Google Scholar] [CrossRef] [PubMed]
- Yu, M.; Stott, S.; Toner, M.; Maheswaran, S.; Haber, D.A. Circulating tumor cells: Approaches to isolation and characterization. J. Cell Biol. 2011, 192, 373–382. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, M.M.; Ramani, V.C.; Jeffrey, S.S. Circulating tumor cell technologies. Mol. Oncol. 2016, 10, 374–394. [Google Scholar] [CrossRef] [PubMed]
- Gong, J.; Jaiswal, R.; Mathys, J.M.; Combes, V.; Grau, G.E.; Bebawy, M. Microparticles and their emerging role in cancer multidrug resistance. Cancer Treat. Rev. 2012, 38, 226–234. [Google Scholar] [CrossRef] [PubMed]
- Vishnoi, M.; Peddibhotla, S.; Yin, W.; Antonio, T.S.; George, G.C.; Hong, D.S.; Marchetti, D. The isolation and characterization of ctc subsets related to breast cancer dormancy. Sci. Rep. 2015, 5, 17533. [Google Scholar] [CrossRef] [PubMed]
- Meng, S.; Tripathy, D.; Frenkel, E.P.; Shete, S.; Naftalis, E.Z.; Huth, J.F.; Beitsch, P.D.; Leitch, M.; Hoover, S.; Euhus, D.; et al. Circulating tumor cells in patients with breast cancer dormancy. Clin. Cancer Res. 2004, 10, 8152–8162. [Google Scholar] [CrossRef] [PubMed]
- Marrinucci, D.; Bethel, K.; Bruce, R.H.; Curry, D.N.; Hsieh, B.; Humphrey, M.; Krivacic, R.T.; Kroener, J.; Kroener, L.; Ladanyi, A.; et al. Case study of the morphologic variation of circulating tumor cells. Hum. Pathol. 2007, 38, 514–519. [Google Scholar] [CrossRef] [PubMed]
- Aktas, B.; Tewes, M.; Fehm, T.; Hauch, S.; Kimmig, R.; Kasimir-Bauer, S. Stem cell and epithelial-mesenchymal transition markers are frequently overexpressed in circulating tumor cells of metastatic breast cancer patients. Breast Cancer Res. 2009, 11, R46. [Google Scholar] [CrossRef] [PubMed]
- Balic, M.; Lin, H.; Young, L.; Hawes, D.; Giuliano, A.; McNamara, G.; Datar, R.H.; Cote, R.J. Most early disseminated cancer cells detected in bone marrow of breast cancer patients have a putative breast cancer stem cell phenotype. Clin. Cancer Res. 2006, 12, 5615–5621. [Google Scholar] [CrossRef] [PubMed]
- Alix-Panabieres, C.; Pantel, K. Challenges in circulating tumour cell research. Nat. Rev. Cancer 2014, 14, 623–631. [Google Scholar] [CrossRef] [PubMed]
- Hyun, K.A.; Kim, J.; Gwak, H.; Jung, H.I. Isolation and enrichment of circulating biomarkers for cancer screening, detection, and diagnostics. Analyst 2016, 141, 382–392. [Google Scholar] [CrossRef] [PubMed]
- Lin, H.K.; Zheng, S.; Williams, A.J.; Balic, M.; Groshen, S.; Scher, H.I.; Fleisher, M.; Stadler, W.; Datar, R.H.; Tai, Y.C.; et al. Portable filter-based microdevice for detection and characterization of circulating tumor cells. Clin. Cancer Res. 2010, 16, 5011–5018. [Google Scholar] [CrossRef] [PubMed]
- Hong, B.; Zu, Y. Detecting circulating tumor cells: Current challenges and new trends. Theranostics 2013, 3, 377–394. [Google Scholar] [CrossRef] [PubMed]
- Fehm, T.; Muller, V.; Alix-Panabieres, C.; Pantel, K. Micrometastatic spread in breast cancer: Detection, molecular characterization and clinical relevance. Breast Cancer Res. 2008, 10, S1. [Google Scholar] [CrossRef] [PubMed]
- Ren, C.; Han, C.; Wang, D.; Zhao, X.; Jin, G.; Shen, H. Detection of circulating tumor cells: Clinical relevance of a novel metastatic tumor marker. Exp. Ther. Med. 2011, 2, 385–391. [Google Scholar] [CrossRef] [PubMed]
- Ksiazkiewicz, M.; Markiewicz, A.; Zaczek, A.J. Epithelial-mesenchymal transition: A hallmark in metastasis formation linking circulating tumor cells and cancer stem cells. Pathobiology 2012, 79, 195–208. [Google Scholar] [CrossRef] [PubMed]
- Fehm, T.; Hoffmann, O.; Aktas, B.; Becker, S.; Solomayer, E.F.; Wallwiener, D.; Kimmig, R.; Kasimir-Bauer, S. Detection and characterization of circulating tumor cells in blood of primary breast cancer patients by RT-PCR and comparison to status of bone marrow disseminated cells. Breast Cancer Res. 2009, 11, R59. [Google Scholar] [CrossRef] [PubMed]
- Brouns, I.; Van Nassauw, L.; Van Genechten, J.; Majewski, M.; Scheuermann, D.W.; Timmermans, J.P.; Adriaensen, D. Triple immunofluorescence staining with antibodies raised in the same species to study the complex innervation pattern of intrapulmonary chemoreceptors. J. Histochem. Cytochem. 2002, 50, 575–582. [Google Scholar] [CrossRef] [PubMed]
- Xu, L.; Mao, X.; Imrali, A.; Syed, F.; Mutsvangwa, K.; Berney, D.; Cathcart, P.; Hines, J.; Shamash, J.; Lu, Y.J. Optimization and evaluation of a novel size based circulating tumor cell isolation system. PLoS ONE 2015, 10, e0138032. [Google Scholar] [CrossRef] [PubMed]
- Fehm, T.; Braun, S.; Muller, V.; Janni, W.; Gebauer, G.; Marth, C.; Schindlbeck, C.; Wallwiener, D.; Borgen, E.; Naume, B.; et al. A concept for the standardized detection of disseminated tumor cells in bone marrow from patients with primary breast cancer and its clinical implementation. Cancer 2006, 107, 885–892. [Google Scholar] [CrossRef] [PubMed]
- Janni, W.; Hepp, F.; Strobl, B.; Rack, B.; Rjosk, D.; Kentenich, C.; Schindlbeck, C.; Hantschmann, P.; Pantel, K.; Sommer, H.; et al. Patterns of disease recurrence influenced by hematogenous tumor cell dissemination in patients with cervical carcinoma of the uterus. Cancer 2003, 97, 405–411. [Google Scholar] [CrossRef] [PubMed]





| Cell Line | ER | PR | HER2 | VDR Level |
|---|---|---|---|---|
| MDA-MB-231 | − | − | − | high |
| MCF-7 | + | + | − | high |
| Cama-1 | + | + | − | high |
| T47D | + | + | − | intermediate |
| HCC1937 | − | − | − | intermediate |
| ZR75 | + | − | − | intermediate |
| SK-Br-3 | − | − | + | intermediate |
| Ishikawa ERneg | − | − | − | intermediate |
| MDA-MB-468 | − | − | − | low |
| HCC3153 | − | − | − | low |
| Patient | Age * | Primary Tumor Status | Primary Diagnosis ~Metastasis (years) ** | Primary Diagnosis ~CTC Analysis (years) *** | CTCs | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| TNM | Grade | ERα | PR | HER2 | ||||||
| M1 CTC | 56 | pT2, pN2, M0 | G2 | + | + | − | 5 and 10 | 14 | >500 | |
| M2 | 62 | cT4, N3, M1 | G3 | − | − | + | 0 | 5 | - | |
| M4 | 69 | cT3, pNx, pM1 | G3 | − | − | + | 0 and 1 | 5 | - | |
| M6 CTC | 49 | ypTis, pN1, M0 | G3 | − | − | + | 2 | 7 | 1 | |
| M7 CTC | 75 | pT2, pN1, M0 | n.d. | + | − | − **** | 7 | 16 | 3 | |
| M8 CTC | 53 | pT1b, pN0, M0 | G2 | + | + | − | 8 | 16 | 5 | |
| M9 CTC | 63 | pT1c, pN0, M0 | G2 | + | + | − | 8 | 16 | 1 | |
| M10 CTC | 69 | Left | pT2, pN0, M0 | G2 | + | − | + | 5 and 6 | 10 | 1 |
| Right | pT1c, pNx, M0 | G2 | + | − | + | |||||
| M11 CTC | 66 | ypT2, pN3a, M0 | G3 | + | + | − | 2 and 3 | 9 | 1 | |
| M12 | 63 | ypT2, pN2, M0 | G3 | − | - | + | 3 | 11 | - | |
| M13 CTC | 77 | pT2, pN1, M0 | G2 | + | + | − | 2 and 6 | 11 | 1 | |
| M15 | 82 | cT3, cN1, cM1 | G2 | + | + | + | 0 | 5 | - | |
| M16 CTC | 69 | pT1c, pN0, M0 | n.d. | + | + | − | 10 and 12 | 16 | 16 | |
| M17 CTC | 64 | pT1c, pN0, M0 | n.d. | + | + | + | 6 | 13 | 1 | |
| M18 CTC | 70 | pT4b, pN1, pM1 | G2 | + | + | − | 0 | 4 | 2 | |
| M19 | 69 | pT2, N0, M0 | G2 | − | - | + | 0 | 4 | - | |
| M20 | 61 | pT3, pN3, M1 | n.d. | + | − | − | 0 and 1 | 5 | - | |
| M22 | 53 | pT2, pN0, M0 | G3 | − | − | − | 4 | 8 | - | |
| M25 CTC | 73 | Left | pT1c, pN0, M0 | G1 | + | + | − | 6 | 10 | 8 |
| Right | pT2, pN0, M0 | G2 | − | − | − | |||||
| M26 | 46 | ypT0, pN1a, M0 | G2 | + | + | − | 1 | 5 | - | |
| M27 CTC | 59 | Left | pT2, pN1, M1 | G2 | + | + | − | 3 | 7 | 1 |
| Right | pTis, pNx, M1 | n.d. | n.d. | n.d. | n.d. | |||||
| M28 CTC | 79 | ypT3, pN1a, M0 | G2 | + | + | − | 9 | 13 | 1 | |
| M29 | 67 | pT1c, pN0, M0 | G3 | − | − | − | 0, 2 and 5 | 15 | - | |
| Patients with CTCs | CTCs (n = 42 *) | Total | |||
|---|---|---|---|---|---|
| Tiny CK pos | Normal CK pos | ||||
| VDR | VDR | ||||
| Neg | Pos +~++ | Neg | Pos +~++ | ||
| M 1 | >500 | ||||
| M 6 | 1 | 1 | |||
| M 7 | 1 | 1 | 1 | 3 | |
| M 8 | 1 | 4 | 5 | ||
| M 9 | 1 | 1 | |||
| M 10 | 1 | 1 | |||
| M 11 | 1 | 1 | |||
| M 13 | 1 | 1 | |||
| M 16 | 3 | 12 | 1 | 16 | |
| M 17 | 1 | 1 | |||
| M 18 | 2 | 2 | |||
| M 25 | 1 | 4 | 3 | 8 | |
| M 27 | 1 | 1 | |||
| M 28 | 1 | 1 | |||
| Total | 12 | 12 | 11 | 7 | 42 * |
| % (n = 13) | 28.6 | 28.6 | 26.2 | 16.6 | 100 * |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, X.; Hofmann, S.; Rack, B.; Harbeck, N.; Jeschke, U.; Sixou, S. Fluorescence Analysis of Vitamin D Receptor Status of Circulating Tumor Cells (CTCS) in Breast Cancer: From Cell Models to Metastatic Patients. Int. J. Mol. Sci. 2017, 18, 1318. https://doi.org/10.3390/ijms18061318
Zhang X, Hofmann S, Rack B, Harbeck N, Jeschke U, Sixou S. Fluorescence Analysis of Vitamin D Receptor Status of Circulating Tumor Cells (CTCS) in Breast Cancer: From Cell Models to Metastatic Patients. International Journal of Molecular Sciences. 2017; 18(6):1318. https://doi.org/10.3390/ijms18061318
Chicago/Turabian StyleZhang, Xi, Simone Hofmann, Brigitte Rack, Nadia Harbeck, Udo Jeschke, and Sophie Sixou. 2017. "Fluorescence Analysis of Vitamin D Receptor Status of Circulating Tumor Cells (CTCS) in Breast Cancer: From Cell Models to Metastatic Patients" International Journal of Molecular Sciences 18, no. 6: 1318. https://doi.org/10.3390/ijms18061318
APA StyleZhang, X., Hofmann, S., Rack, B., Harbeck, N., Jeschke, U., & Sixou, S. (2017). Fluorescence Analysis of Vitamin D Receptor Status of Circulating Tumor Cells (CTCS) in Breast Cancer: From Cell Models to Metastatic Patients. International Journal of Molecular Sciences, 18(6), 1318. https://doi.org/10.3390/ijms18061318