FGF-2 Gene Polymorphism in Osteoporosis among Guangxi’s Zhuang Chinese
Abstract
:1. Introduction
2. Results
2.1. The Bone Mass Decreasing Trends of the Male and Female Groups with Age
2.2. FGF-2 Genotype and Allelic Gene Frequency Distribution
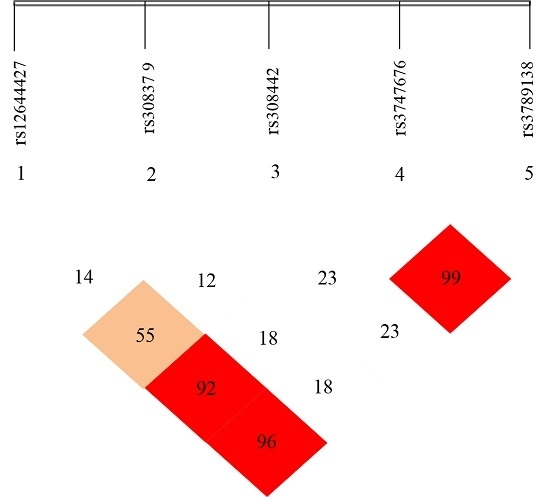
2.3. Linkage Disequilibrium (LD) Analyses
3. Discussion
3.1. Age-Related Changing Trends of Bone Mass
3.2. Distribution of FGF-2 Gene SNPs in Zhuang Osteoporosis Patients and Normal Bone Mass Individuals
3.3. Linkage Disequilibrium (LD) Analyses of the SNPs of FGF-2 Gene
4. Materials and Methods
4.1. Analyses of Bone Density
4.2. Analyses of FGF-2 Gene Polymorphism
4.3. Linkage Disequilibrium (LD) Analyses on the Five SNP Loci of FGF-2 Gene
4.4. Statistical Analyses
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Kanis, J.A.; McCloskey, E.V.; Johansson, H.; Oden, A.; Melton, L.J.; Khaltaev, N. A reference standard for the description of osteoporosis. Bone 2008, 42, 467–475. [Google Scholar] [CrossRef] [PubMed]
- Boudin, E.; Fijalkowski, I.; Hendrickx, G.; Van Hul, W. Genetic control of bone mass. Mol. Cell. Endocrinol. 2016, 432, 3–13. [Google Scholar] [CrossRef] [PubMed]
- Sigurdsson, G.; Halldorsson, B.V.; Styrkarsdottir, U.; Kristjansson, K.; Stefansson, K. Impact of genetics on low bone mass in adults. J. Bone Miner. Res. 2008, 23, 1584–1590. [Google Scholar] [CrossRef] [PubMed]
- Yamashita, T.; Takahashi, N.; Udagawa, N. New roles of osteoblasts involved in osteoclast differentiation. World J. Orthop. 2012, 3, 175–181. [Google Scholar] [CrossRef] [PubMed]
- Glass, D.A.; Bialek, P.; Ahn, J.D.; Starbuck, M.; Patel, M.S.; Clevers, H.; Karsenty, G. Canonical Wnt signaling in differentiated osteoblasts controls osteoclast differentiation. Dev. Cell 2005, 8, 751–764. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Buki, K.; Vääräniemi, J.; Gu, G.; Väänänen, H.K. The critical role of IL-34 in osteoclastogenesis. PLoS ONE 2011, 6, e18689. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Bao, Q.; Chen, S.; Liu, H.; Feng, J.; Qin, H.; Li, A.; Liu, D.; Shen, Y.; Zhao, Y.; et al. Different bone remodeling levels of trabecular and cortical bone in response to changes in Wnt/β-catenin signaling in mice. J. Orthop. Res. 2016. [Google Scholar] [CrossRef]
- Macsai, C.E.; Foster, B.K.; Xian, C.J. Roles of Wnt signalling in bone growth, remodelling, skeletal disorders and fracture repair. J. Cell. Physiol. 2008, 215, 578–587. [Google Scholar] [CrossRef] [PubMed]
- Salazar, V.S.; Gamer, L.W.; Rosen, V. BMP signalling in skeletal development, disease and repair. Nat. Rev. Endocrinol. 2016, 12, 203–221. [Google Scholar] [CrossRef] [PubMed]
- Akl, M.R.; Nagpal, P.; Ayoub, N.M.; Tai, B.; Prabhu, S.A.; Capac, C.M.; Gliksman, M.; Goy, A.; Suh, K.S. Molecular and clinical significance of fibroblast growth factor 2 (FGF2/bFGF) in malignancies of solid and hematological cancers for personalized therapies. Oncotarget 2016, 1949–2553. [Google Scholar]
- Du, X.; Xie, Y.; Xian, C.J.; Chen, L. Role of FGFs/FGFRs in skeletal development and bone regeneration. J. Cell. Physiol. 2012, 227, 3731–3743. [Google Scholar] [CrossRef] [PubMed]
- Lei, L.; Wang, S.; Wu, H.; Ju, W.; Peng, J.; Qahtan, A.S.A.; Chen, C.; Lu, Y.; Peng, J.; Zhang, X.; et al. Optimization of release pattern of FGF-2 and BMP-2 for osteogenic differentiation of low-population density hMSCs. J. Biomed. Mater. Res. A 2015, 103, 252–261. [Google Scholar] [CrossRef] [PubMed]
- Naganawa, T.; Xiao, L.; Abogunde, E.; Sobue, T.; Kalajzic, I.; Sabbieti, M.; Agas, D.; Hurley, M.M. In Vivo and in vitro comparison of the effects of FGF-2 null and haplo-insufficiency on bone formation in mice. Biochem. Biophys. Res. Commun. 2006, 339, 490–498. [Google Scholar] [CrossRef] [PubMed]
- Nagayasu-Tanaka, T.; Nozaki, T.; Miki, K.; Sawada, K.; Kitamura, M.; Murakami, S. FGF-2 promotes initial osseointegration and enhances stability of implants with low primary stability. Clin. Oral Implants Res. 2017, 28, 291–297. [Google Scholar] [CrossRef] [PubMed]
- Vincent, T.; Hermansson, M.; Bolton, M.; Wait, R.; Saklatvala, J. Basic FGF mediates an immediate response of articular cartilage to mechanical injury. Proc. Natl. Acad. Sci. USA 2002, 99, 8259–8264. [Google Scholar] [CrossRef] [PubMed]
- Hao, R.H.; Guo, Y.; Dong, S.S.; Weng, G.Z.; Yan, H.; Zhu, D.L.; Chen, F.X.; Chen, J.B.; Yang, T.L. Associations of Plasma FGF2 Levels and Polymorphisms in the FGF2 Gene with Obesity Phenotypes in Han Chinese Population. Sci. Rep. 2016, 6, 19868. [Google Scholar] [CrossRef] [PubMed]
- Lashkari, B.; Yang, L.; Mandelis, A. The application of backscattered ultrasound and photoacoustic signals for assessment of bone collagen and mineral contents. Quant. Imaging Med. Surg. 2015, 5, 46–56. [Google Scholar]
- Fantauzzi, A.; Floridia, M.; Ceci, F.; Cacciatore, F.; Vullo, V.; Mezzaroma, I. Usefulness of calcaneal quantitative ultrasound stiffness for the evaluation of bone health in HIV-1-infected subjects: Comparison with dual X-ray absorptiometry. HIV AIDS (Auckl.) 2016, 8, 109–117. [Google Scholar] [PubMed]
- Moayyeri, A.; Adams, J.E.; Adler, R.A.; Krieg, M.A.; Hans, D.; Compston, J.; Lewiecki, E.M. Quantitative ultrasound of the heel and fracture risk assessment: An updated meta-analysis. Osteoporos. Int. 2012, 23, 143–153. [Google Scholar] [CrossRef] [PubMed]
- Bonjour, J.P.; Chevalley, T. Pubertal timing, bone acquisition, and risk of fracture throughout life. Endocr. Rev. 2014, 35, 820–847. [Google Scholar] [CrossRef] [PubMed]
- Adami, S.; Giannini, S.; Giorgino, R.; Isaia, G.C.; Maggi, S.; Sinigaglia, L.; Filipponi, P.; Crepaldi, G. Effect of age, weight and lifestyle factors on calcaneal quantitative ultrasound in premenopausal women: the ESOPO study. Calcif. Tissue Int. 2004, 74, 317–321. [Google Scholar] [CrossRef] [PubMed]
- Evans, A.L.; Paggiosi, M.A.; Eastell, R.; Walsh, J.S. Bone density, microstructure and strength in obese and normal weight men and women in younger and older adulthood. J. Bone Miner. Res. 2015, 30, 920–928. [Google Scholar] [CrossRef] [PubMed]
- Srivastava, M.; Deal, C. Osteoporosis in elderly: Prevention and treatment. Clin. Geriatr. Med. 2002, 18, 529–555. [Google Scholar] [CrossRef]
- Donner, D.G.; Elliott, G.E.; Beck, B.R.; Forwood, M.R.; Du Toit, E.F. The effects of visceral obesity and androgens on bone: Trenbolone protects against loss of femoral bone mineral density and structural strength in viscerally obese and testosterone-deficient male rats. Osteoporos. Int. 2016, 27, 1073–1082. [Google Scholar] [CrossRef] [PubMed]
- Murakami, M.; Nguyen, L.T.; Hatanaka, K.; Schachterle, W.; Chen, P.Y.; Zhuang, Z.W.; Black, B.L.; Simons, M. FGF-dependent regulation of VEGF receptor 2 expression in mice. J. Clin. Investig. 2011, 121, 2668–2678. [Google Scholar] [CrossRef] [PubMed]
- Brown, K.C.; Lau, J.K.; Dom, A.M.; Witte, T.R.; Luo, H.; Crabtree, C.M.; Shah, Y.H.; Shiflett, B.S.; Marcelo, A.J.; Proper, N.A.; et al. MG624, an α7-nAChR antagonist, inhibits angiogenesis via the Egr-1/FGF2 pathway. Angiogenesis 2012, 15, 99–114. [Google Scholar] [CrossRef] [PubMed]
- Lim, S.; Cho, H.; Lee, E.; Won, Y.; Kim, C.; Ahn, W.; Lee, E.; Son, Y. Osteogenic stimulation of human adipose-derived stem cells by pre-treatment with fibroblast growth factor 2. Cell Tissue Res. 2016, 364, 137–147. [Google Scholar] [CrossRef] [PubMed]
- Kalomoiris, S.; Cicchetto, A.C.; Lakatos, K.; Nolta, J.A.; Fierro, F.A. Fibroblast growth factor 2 regulates high mobility Group A2 Expression in Human Bone Marrow-Derived Mesenchymal Stem Cells. J. Cell. Biochem. 2016, 117, 2128–2137. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Liu, H.; Liu, X.; Qi, X. Effect of variation of FGF2 genotypes on the risk of osteosarcoma susceptibly: A case control study. Int. J. Clin. Exp. Med. 2015, 8, 6114–6118. [Google Scholar] [PubMed]
- Kang, S.; Li, S.Z.; Wang, N.; Zhou, R.M.; Wang, T.; Wang, D.J.; Li, X.F.; Bui, J.; Li, Y. Association between genetic polymorphisms in fibroblast growth factor (FGF)1 and FGF2 and risk of endometriosis and adenomyosis in Chinese women. Hum. Reprod. 2010, 25, 1806–1811. [Google Scholar] [CrossRef] [PubMed]
- Slattery, M.L.; John, E.M.; Stern, M.C.; Herrick, J.; Lundgreen, A.; Giuliano, A.R.; Hines, L.; Baumgartner, K.B.; Torres-Mejia, G.; Wolff, R.K. Associations with growth factor genes (FGF1, FGF2, PDGFB, FGFR2, NRG2, EGF, ERBB2) with breast cancer risk and survival: The Breast Cancer Health Disparities Study. Breast Cancer Res. Treat. 2013, 140, 587–601. [Google Scholar] [CrossRef] [PubMed]
- Brión, M.; Sanchez-Salorio, M.; Cortón, M.; de la Fuente, M.; Pazos, B.; Othman, M.; Swaroop, A.; Abecasis, G.; Sobrino, B.; Carracedo, A.; et al. Genetic association study of age-related macular degeneration in the Spanish population. Acta Ophthalmol. 2011, l89, e12–e22. [Google Scholar] [CrossRef] [PubMed]
- Dong, S.S.; Yang, T.L.; Yan, H.; Rong, Z.Q.; Chen, J.B.; Hao, R.H.; Chen, X.F.; Guo, Y. Association analyses of FGFR2 gene polymorphisms with femoral neck bone mineral density in Chinese Han population. Mol. Genet. Genomics 2015, 290, 485–491. [Google Scholar] [CrossRef] [PubMed]
- Guryev, V.; Smits, B.M.; van de Belt, J.; Verheul, M.; Hubner, N.; Cuppen, E. Haplotype block structure is conserved across mammals. PLoS Genet. 2006, 2, e121. [Google Scholar] [CrossRef] [PubMed]
- Slatkin, M. Linkage disequilibrium—Understanding the evolutionary past and mapping the medical future. Nat. Rev. Genet. 2008, 9, 477–485. [Google Scholar] [CrossRef] [PubMed]
- Guo, S.W. Linkage disequilibrium measures for fine-scale mapping: A comparison. Hum. Hered. 1997, 47, 301–314. [Google Scholar] [CrossRef] [PubMed]
- Miller, S.A.; Dykes, D.D.; Polesky, H.F.R.N. A simple salting out procedure for extracting DNA from human nucleated cells. Nucleic Acids Res. 1988, 16, 1215–1219. [Google Scholar] [CrossRef] [PubMed]





| Age Groups | BUA (g/cm2) | |||
|---|---|---|---|---|
| n | Males | n | Females | |
| 45 | 26 | 60.03 ± 5.99 | 14 | 59.62 ± 5.47 |
| 50 | 66 | 62.74 ± 4.59 | 78 | 61.63 ± 5.44 |
| 55 | 59 | 61.81 ± 5.52 | 83 | 58.02 ± 4.07 ** |
| 60 | 81 | 60.67 ± 4.66 | 69 | 56.62 ± 4.77 ** |
| 65 | 59 | 59.94 ± 6.01 | 67 | 54.69 ± 4.16 ** |
| 70 | 10 | 58.79 ± 5.17 | 11 | 52.58 ± 2.03 ** |
| Total | 301 | 61.09 ± 5.32 | 322 | 57.79 ± 5.29 ** |
| Age Groups | Normal (n, %) | Osteopenia (n, %) | Osteoporosis (n, %) | |||
|---|---|---|---|---|---|---|
| Male | Female | Male | Female | Male | Female | |
| 45 | 12, 46.15% | 6, 42.86% | 10, 38.46% | 5, 35.71% | 4, 15.38% | 3, 21.43% |
| 50 | 43, 65.15% | 39, 50% | 19, 28.79% | 33, 42.31% | 4, 6.06% | 6, 7.69% |
| 55 | 30, 50.85% | 18, 21.69% | 24, 40.68% | 44, 53.01% | 5, 8.47% | 21, 25.3% |
| 60 | 41, 50.62% | 15, 21.74% | 28, 34.57% | 22, 31.88% | 12,14.81% | 32, 46.38% |
| 65 | 24, 40.68% | 5, 7.46% | 18, 30.51% | 19, 28.36% | 17, 28.81% | 43, 64.18% |
| ≥70 | 4, 40% | 0, 0% | 3, 30% | 2, 18.18% | 3, 30% | 9, 81.82% |
| Total | 154, 51.16% | 83, 25.78% | 102, 33.89% | 125, 38.82% | 45, 14.95% | 114, 35.4% |
| Normal vs.Osteoporosis | Normal vs.Osteopenia | |||||||
|---|---|---|---|---|---|---|---|---|
| Loci | Genotype | Normal | Osteopenia | Osteoporosis | OR (95% CI) | p | OR (95% CI) | p |
| rs12644427 | AA | 65 | 59 | 41 | 1.000 | 1.000 | ||
| GA | 111 | 110 | 81 | 0.864 (1.403–0.532) | 0.555 | 0.916 (1.422–0.590) | 0.696 | |
| GG | 61 | 58 | 37 | 1.040 (1.832–0.591) | 0.892 | 0.955 (1.579–0.577) | 0.857 | |
| Allelic gene | ||||||||
| A | 241 | 228 | 163 | 1.000 | 1.000 | |||
| G | 233 | 226 | 155 | 1.017 (1.351–0.765) | 0.909 | 0.975 (1.262–0.754) | 0.849 | |
| rs308379 | TT | 82 | 84 | 43 | 1.000 | 1.000 | ||
| TA | 109 | 101 | 82 | 0.697 (1.112–0.437) | 0.129 | 1.106 (1.661–0.736) | 0.629 | |
| AA | 46 | 42 | 34 | 0.709 (1.263–0.399) | 0.243 | 1.122 (1.882–0.669) | 0.663 | |
| Allelic gene | ||||||||
| T | 273 | 269 | 168 | 1.000 | 1.000 | |||
| A | 201 | 185 | 150 | 0.825 (1.097–0.62) | 0.186 | 1.071 (1.39–0.824) | 0.609 | |
| rs308442 | TT | 146 | 124 | 87 | 1.000 | |||
| TA | 50 | 84 | 91 | 1.652 (1.960–1.424) | 0.031 | 1.653 (1.968–1.441) | 0.033 | |
| AA | 21 | 12 | 8 | 1.565 (3.690–0.664) | 0.303 | 1.486 (3.142–0.703) | 0.297 | |
| Allelic gene | ||||||||
| T | 362 | 339 | 238 | 1.000 | 1.000 | |||
| A | 112 | 115 | 80 | 0.92 (1.281–0.662) | 0.623 | 0.912 (1.23–0.676) | 0.547 | |
| rs3747676 | TT | 69 | 70 | 43 | 1.000 | 1.000 | ||
| CT | 112 | 103 | 85 | 0.821 (1.319–0.511) | 0.415 | 1.103 (1.690–0.720) | 0.652 | |
| CC | 56 | 54 | 31 | 1.126 (2.012–0.630) | 0.689 | 1.052 (1.735–0.638) | 0.842 | |
| Allelic gene | ||||||||
| T | 250 | 243 | 171 | 1.000 | 1.000 | |||
| C | 224 | 211 | 147 | 1.042 (1.386–0.784) | 0.776 | 1.032 (1.336–0.797) | 0.811 | |
| rs3789138 | AA | 71 | 70 | 43 | 1.000 | 1.000 | ||
| GA | 116 | 104 | 87 | 0.808 (1.292–0.505) | 0.372 | 1.100 (1.679–0.720) | 0.660 | |
| GG | 50 | 53 | 29 | 1.044 (1.89–0.577) | 0.887 | 0.930 (1.546–0.560) | 0.780 | |
| Allelic gene | ||||||||
| A | 258 | 244 | 173 | 1.000 | 1.000 | |||
| G | 216 | 210 | 145 | 0.999 (1.329–0.751) | 0.994 | 0.973 (1.259–0.751) | 0.834 | |
| Loci | Genotype | Normal | Osteopenia | Osteoporosis | Total |
|---|---|---|---|---|---|
| rs12644427 | AA | 64.88 ± 3.84 | 58.11 ± 1.52 | 52.85 ± 1.53 | 59.47 ± 5.51 |
| GA | 65.39 ± 3.52 | 58.18 ± 1.55 | 52.67 ± 2.00 | 59.35 ± 5.69 | |
| GG | 64.83 ± 2.97 | 57.93 ± 1.4 | 52.51 ± 1.87 | 59.34 ± 5.36 | |
| p | 0.505 | 0.585 | 0.791 | 0.791 | |
| rs308379 | TT | 65.02 ± 3.55 | 58.03 ± 1.48 | 52.82 ± 2.28 | 59.17 ± 5.36 |
| TA | 65.29 ± 3.58 | 58.16 ± 1.53 | 52.52 ± 1.7 | 59.24 ± 5.76 | |
| AA | 64.82 ± 3.12 | 58.08 ± 1.49 | 52.88 ± 1.62 | 59.70 ± 5.37 | |
| p | 0.715 | 0.846 | 0.545 | 0.593 | |
| rs308442 | TT | 64.96 ± 3.45 | 58.11 ± 1.48 | 52.68 ± 1.98 | 61.04 ± 5.81 |
| TA | 65.24 ± 3.62 | 58.03 ± 1.52 | 52.67 ± 1.72 | 58.75 ± 5.45 | |
| AA | 65.68 ± 3.25 | 58.48 ± 1.65 | 52.69 ± 1.62 | 60.59 ± 5.55 | |
| p | 0.624 | 0.630 | 1.000 | 0.03 | |
| rs3747676 | TT | 64.83 ± 2.85 | 57.82 ± 1.44 | 52.51 ± 1.85 | 59.67 ± 5.05 |
| CT | 65.57 ± 3.76 | 58.16 ± 1.52 | 52.57 ± 1.98 | 59.34 ± 5.93 | |
| CC | 64.53 ± 3.53 | 58.34 ± 1.52 | 53.22 ± 1.4 | 59.22 ± 5.30 | |
| p | 0.140 | 0.137 | 0.191 | 0.757 | |
| rs3789138 | AA | 64.73 ± 2.87 | 57.83 ± 1.44 | 52.51 ± 1.85 | 59.25 ± 5.27 |
| GA | 65.54 ± 3.7 | 58.16 ± 1.52 | 52.6 ± 1.97 | 59.38 ± 5.91 | |
| GG | 64.64 ± 3.65 | 58.32 ± 1.53 | 53.15 ± 1.42 | 59.58 ± 5.09 | |
| p | 0.167 | 0.161 | 0.301 | 0.873 |
| Mutant loci | rs308379 Case/Control Group | rs308442 Case/Control Group | rs3747676 Case/Control Group | rs3789138 Case/Control Group |
|---|---|---|---|---|
| rs12644427 | 0.144/0.162 | 0.553/0.524 | 0.922/0.875 | 0.966/0.811 |
| rs308379 | 0.124/0.107 | 0.182/0.210 | 0.183/0.174 | |
| rs308442 | 0.238/0.291 | 0.230/0.214 | ||
| rs3747676 | 0.997/0.895 |
| Mutant loci | rs308379 | rs308442 | rs3747676 | rs3789138 |
|---|---|---|---|---|
| rs12644427 | 0.016 | 0.097 | 0.725 | 0.767 |
| rs308379 | 0.007 | 0.022 | 0.021 | |
| rs308442 | 0.021 | 0.020 | ||
| rs3747676 | 0.959 |
| Polymorphic Loci | Forward Primer (5’–3‘) | Reverse Primer (5’–3‘) | Extension Primer (5’–3‘) |
|---|---|---|---|
| rs12644427 | TTCACCATTTATGAAACACTGACTTG | GGGATCATCCAGTACACCTTCCCTAT | TTTTTTTTTTTTTTTTTTTGAAACACTGACTTGTCTGTTTCCA |
| rs3789138 | TCCCTTGCCAATACCTTGTCAT | TTGCAGCCATGTGATTGGTGTC | TTTTTTTTTTTTTTTTTTTTTTTTTTTTTTTTTTTTTTGTGAGTTTTGAGCTAAGTTTTGGAGTA |
| rs308379 | TCCCGTATTTGTTACCTTCTGTCCA | TCCAGCAATTAGGTAGCATGGAGTG | TTTTTTTTTTTTTTTTTTTTTTTTTTTTTTTTTTTTCCAGCCTCATTTAGTCCCCC |
| rs308442 | CCCTTCACGGAATTCCCCAATA | CATCCAGCAAGCATTTATGAAGCAC | TTTTTTTTTTTTTTTTTTTTTTTTTTTTTTTTTTTCAAGCATTTATGAAGCACTCATTG |
| rs3747676 | GGGGACATGCATATTAAGGAAAAGG | TCTCAACTGAGAAATAATCCCCTAACACA | TTTTTTTTTTTTTTTTTTCAAATACATTGATTTGTCATGATACACA |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bin, X.; Lin, C.; Huang, X.; Zhou, Q.; Wang, L.; Xian, C.J. FGF-2 Gene Polymorphism in Osteoporosis among Guangxi’s Zhuang Chinese. Int. J. Mol. Sci. 2017, 18, 1358. https://doi.org/10.3390/ijms18071358
Bin X, Lin C, Huang X, Zhou Q, Wang L, Xian CJ. FGF-2 Gene Polymorphism in Osteoporosis among Guangxi’s Zhuang Chinese. International Journal of Molecular Sciences. 2017; 18(7):1358. https://doi.org/10.3390/ijms18071358
Chicago/Turabian StyleBin, Xiaoyun, Chaowen Lin, Xiufeng Huang, Qinghui Zhou, Liping Wang, and Cory J. Xian. 2017. "FGF-2 Gene Polymorphism in Osteoporosis among Guangxi’s Zhuang Chinese" International Journal of Molecular Sciences 18, no. 7: 1358. https://doi.org/10.3390/ijms18071358
APA StyleBin, X., Lin, C., Huang, X., Zhou, Q., Wang, L., & Xian, C. J. (2017). FGF-2 Gene Polymorphism in Osteoporosis among Guangxi’s Zhuang Chinese. International Journal of Molecular Sciences, 18(7), 1358. https://doi.org/10.3390/ijms18071358