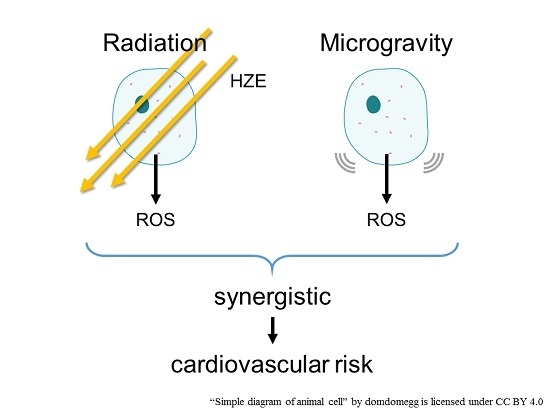
Effect of Oxidative Stress on Cardiovascular System in Response to Gravity
Abstract
:1. Introduction
2. What Are Reactive Oxygen Species (ROS)?
ROS in the Cardiovascular System
3. ROS Generation in Response to Radiation
Cardiovascular ROS Generation in Response to Radiation
4. ROS Generation in Response to Microgravity
Cardiovascular ROS Generation in Response to Microgravity
5. Combination of Radiation and Microgravity
6. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
Abbreviations
| LEO | Low Earth Orbit |
| NASA | National Aeronautics and Space Administration |
| ROS | Reactive Oxygen Species |
| NADP+ | Nicotinamide Adenine Dinucleotide Phosphate |
| NOX | Nicotinamide Adenine Dinucleotide Phosphate Oxidase |
| XO | Xanthine Oxidase |
| NOS | Nitric Oxide Synthase |
| SOD | Superoxide Dismutase |
| GPx | Glutathione Peroxidase |
| CAT | Catalase |
| HZE | High-Charge and High-Energy |
| LDR | Low-Dose Radiation |
| HLU | Hindlimb Unloading |
| eNOS | Endothelial Isoform of Nitric Oxide Synthase |
References
- Witze, A. NASA rethinks approach to Mars exploration. Nature 2016, 538, 149–150. [Google Scholar] [CrossRef] [PubMed]
- Cucinotta, F.A. Review of NASA approach to space radiation risk assessments for Mars exploration. Health Phys. 2015, 108, 131–142. [Google Scholar] [CrossRef] [PubMed]
- Messina, P.; Vennemann, D. The European space exploration programme: Current status of ESA’s plans for Moon and Mars exploration. Acta Astronaut. 2005, 57, 156–160. [Google Scholar] [CrossRef] [PubMed]
- Grimm, D.; Grosse, J.; Wehland, M.; Mann, V.; Reseland, J.E.; Sundaresan, A.; Corydon, T.J. The impact of microgravity on bone in humans. Bone 2016, 87, 44–56. [Google Scholar] [CrossRef] [PubMed]
- Stein, T.P. Weight, muscle and bone loss during space flight: Another perspective. Eur. J. Appl. Physiol. 2013, 113, 2171–2181. [Google Scholar] [CrossRef] [PubMed]
- Bullard, R.W. Physiological problems of space travel. Annu. Rev. Physiol. 1972, 34, 205–234. [Google Scholar] [CrossRef] [PubMed]
- Narici, M.V.; de Boer, M.D. Disuse of the musculo-skeletal system in space and on earth. Eur. J. Appl. Physiol. 2011, 111, 403–420. [Google Scholar] [CrossRef] [PubMed]
- Zhu, H.; Wang, H.; Liu, Z. Effects of real and simulated weightlessness on the cardiac and peripheral vascular functions of humans: A review. Int. J. Occup. Med. Environ. Health 2015, 28, 793–802. [Google Scholar] [CrossRef] [PubMed]
- Graveline, D.E. Cardiovascular deconditioning: Role of blood volume and sympathetic neurohormones. Life Sci. Space Res. 1964, 2, 287–298. [Google Scholar] [PubMed]
- Coupe, M.; Fortrat, J.O.; Larina, I.; Gauquelin-Koch, G.; Gharib, C.; Custaud, M.A. Cardiovascular deconditioning: From autonomic nervous system to microvascular dysfunctions. Respir. Physiol. Neurobiol. 2009, 169, S10–S12. [Google Scholar] [CrossRef] [PubMed]
- Delp, M.D.; Charvat, J.M.; Limoli, C.L.; Globus, R.K.; Ghosh, P. Apollo lunar astronauts show higher cardiovascular disease mortality: Possible deep space radiation effects on the vascular endothelium. Sci. Rep. 2016, 6, 29901. [Google Scholar] [CrossRef] [PubMed]
- Cucinotta, F.A.; Hamada, N.; Little, M.P. No evidence for an increase in circulatory disease mortality in astronauts following space radiation exposures. Life Sci. Space Res. 2016, 10, 53–56. [Google Scholar] [CrossRef] [PubMed]
- Rizzo, A.M.; Corsetto, P.A.; Montorfano, G.; Milani, S.; Zava, S.; Tavella, S.; Cancedda, R.; Berra, B. Effects of long-term space flight on erythrocytes and oxidative stress of rodents. PLoS ONE 2012, 7, e32361. [Google Scholar] [CrossRef] [PubMed]
- Guan, J.; Wan, X.S.; Zhou, Z.; Ware, J.; Donahue, J.J.; Biaglow, J.E.; Kennedy, A.R. Effects of dietary supplements on space radiation-induced oxidative stress in Sprague-Dawley rats. Radiat. Res. 2004, 162, 572–579. [Google Scholar] [CrossRef] [PubMed]
- Versari, S.; Longinotti, G.; Barenghi, L.; Maier, J.A.; Bradamante, S. The challenging environment on board the International Space Station affects endothelial cell function by triggering oxidative stress through thioredoxin interacting protein overexpression: The ESA-SPHINX experiment. FASEB J. 2013, 27, 4466–4475. [Google Scholar] [CrossRef] [PubMed]
- Mao, X.W.; Pecaut, M.J.; Stodieck, L.S.; Ferguson, V.L.; Bateman, T.A.; Bouxsein, M.; Jones, T.A.; Moldovan, M.; Cunningham, C.E.; Chieu, J.; et al. Spaceflight environment induces mitochondrial oxidative damage in ocular tissue. Radiat. Res. 2013, 180, 340–350. [Google Scholar] [CrossRef] [PubMed]
- Mao, X.W.; Pecaut, M.J.; Stodieck, L.S.; Ferguson, V.L.; Bateman, T.A.; Bouxsein, M.L.; Gridley, D.S. Biological and metabolic response in STS-135 space-flown mouse skin. Free Radic. Res. 2014, 48, 890–897. [Google Scholar] [CrossRef] [PubMed]
- Mao, X.W.; Nishiyama, N.C.; Pecaut, M.J.; Campbell-Beachler, M.; Gifford, P.; Haynes, K.E.; Becronis, C.; Gridley, D.S. Simulated microgravity and low-dose/low-dose-rate radiation induces oxidative damage in the mouse brain. Radiat. Res. 2016, 185, 647–657. [Google Scholar] [CrossRef] [PubMed]
- Wise, K.C.; Manna, S.K.; Yamauchi, K.; Ramesh, V.; Wilson, B.L.; Thomas, R.L.; Sarkar, S.; Kulkarni, A.D.; Pellis, N.R.; Ramesh, G.T. Activation of nuclear transcription factor-κB in mouse brain induced by a simulated microgravity environment. In Vitro Cell Dev. Biol. Anim. 2005, 41, 118–123. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Zhang, J.; Bai, S.; Wang, G.; Mu, L.; Sun, B.; Wang, D.; Kong, Q.; Liu, Y.; Yao, X.; et al. Simulated microgravity promotes cellular senescence via oxidant stress in rat PC12 cells. Neurochem. Int. 2009, 55, 710–716. [Google Scholar] [CrossRef] [PubMed]
- Hollander, J.; Gore, M.; Fiebig, R.; Mazzeo, R.; Ohishi, S.; Ohno, H.; Ji, L.L. Spaceflight downregulates antioxidant defense systems in rat liver. Free Radic. Biol. Med. 1998, 24, 385–390. [Google Scholar] [CrossRef]
- Baqai, F.P.; Gridley, D.S.; Slater, J.M.; Luo-Owen, X.; Stodieck, L.S.; Ferguson, V.; Chapes, S.K.; Pecaut, M.J. Effects of spaceflight on innate immune function and antioxidant gene expression. J. Appl. Physiol. 2009, 106, 1935–1942. [Google Scholar] [CrossRef] [PubMed]
- Ikemoto, M.; Nikawa, T.; Kano, M.; Hirasaka, K.; Kitano, T.; Watanabe, C.; Tanaka, R.; Yamamoto, T.; Kamada, M.; Kishi, K. Cysteine supplementation prevents unweighting-induced ubiquitination in association with redox regulation in rat skeletal muscle. Biol. Chem. 2002, 383, 715–721. [Google Scholar] [CrossRef] [PubMed]
- Lawler, J.M.; Song, W.; Demaree, S.R. Hindlimb unloading increases oxidative stress and disrupts antioxidant capacity in skeletal muscle. Free Radic. Biol. Med. 2003, 35, 9–16. [Google Scholar] [CrossRef]
- Finkel, T.; Holbrook, N.J. Oxidants, oxidative stress and the biology of ageing. Nature 2000, 408, 239–247. [Google Scholar] [CrossRef] [PubMed]
- Hayyan, M.; Hashim, M.A.; AlNashef, I.M. Superoxide ion: Generation and chemical implications. Chem. Rev. 2016, 116, 3029–3085. [Google Scholar] [CrossRef] [PubMed]
- Glasauer, A.; Chandel, N.S. ROS. Curr. Biol. 2013, 23, R100–R102. [Google Scholar] [CrossRef] [PubMed]
- Valko, M.; Leibfritz, D.; Moncol, J.; Cronin, M.T.; Mazur, M.; Telser, J. Free radicals and antioxidants in normal physiological functions and human disease. Int. J. Biochem. Cell. Biol. 2007, 39, 44–84. [Google Scholar] [CrossRef] [PubMed]
- Mates, J.M.; Segura, J.A.; Alonso, F.J.; Marquez, J. Intracellular redox status and oxidative stress: Implications for cell proliferation, apoptosis, and carcinogenesis. Arch. Toxicol. 2008, 82, 273–299. [Google Scholar] [CrossRef] [PubMed]
- Kamata, H.; Hirata, H. Redox regulation of cellular signalling. Cell. Signal. 1999, 11, 1–14. [Google Scholar] [CrossRef]
- Sugamura, K.; Keaney, J.F., Jr. Reactive oxygen species in cardiovascular disease. Free Radic. Biol. Med. 2011, 51, 978–992. [Google Scholar] [CrossRef] [PubMed]
- Lambeth, J.D. Nox enzymes, ROS, and chronic disease: An example of antagonistic pleiotropy. Free Radic. Biol. Med. 2007, 43, 332–347. [Google Scholar] [CrossRef] [PubMed]
- Prieto-Bermejo, R.; Hernández-Hernández, A. The Importance of NADPH oxidases and redox signaling in angiogenesis. Antioxidants 2017, 6, 32. [Google Scholar] [CrossRef] [PubMed]
- Gavazzi, G.; Banfi, B.; Deffert, C.; Fiette, L.; Schappi, M.; Herrmann, F.; Krause, K.H. Decreased blood pressure in NOX1-deficient mice. FEBS Lett. 2006, 580, 497–504. [Google Scholar] [CrossRef] [PubMed]
- Yetik-Anacak, G.; Catravas, J.D. Nitric oxide and the endothelium: History and impact on cardiovascular disease. Vasc. Pharmacol. 2006, 45, 268–276. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Youn, J.Y.; Cai, H. Mechanisms and consequences of endothelial nitric oxide synthase dysfunction in hypertension. J. Hypertens. 2015, 33, 1128–1136. [Google Scholar] [CrossRef] [PubMed]
- Rochette, L.; Lorin, J.; Zeller, M.; Guilland, J.C.; Lorgis, L.; Cottin, Y.; Vergely, C. Nitric oxide synthase inhibition and oxidative stress in cardiovascular diseases: Possible therapeutic targets? Pharmacol. Ther. 2013, 140, 239–257. [Google Scholar] [CrossRef] [PubMed]
- Sihver, L.; Ploc, O.; Puchalska, M.; Ambrozova, I.; Kubancak, J.; Kyselova, D.; Shurshakov, V. Radiation environment at aviation altitudes and in space. Radiat. Prot. Dosim. 2015, 164, 477–483. [Google Scholar] [CrossRef] [PubMed]
- Cucinotta, F.A.; Durante, M. Cancer risk from exposure to galactic cosmic rays: Implications for space exploration by human beings. Lancet Oncol. 2006, 7, 431–435. [Google Scholar] [CrossRef]
- Kryston, T.B.; Georgiev, A.B.; Pissis, P.; Georgakilas, A.G. Role of oxidative stress and DNA damage in human carcinogenesis. Mutat. Res. 2011, 711, 193–201. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Boerma, M.; Zhou, D. Ionizing radiation-induced endothelial cell senescence and cardiovascular diseases. Radiat. Res. 2016, 186, 153–161. [Google Scholar] [CrossRef] [PubMed]
- LaVerne, J.A. Track effects of heavy ions in liquid water. Radiat. Res. 2000, 153, 487–496. [Google Scholar] [CrossRef]
- Gonon, G.; Groetz, J.E.; de Toledo, S.M.; Howell, R.W.; Fromm, M.; Azzam, E.I. Nontargeted stressful effects in normal human fibroblast cultures exposed to low fluences of high charge, high energy (HZE) particles: Kinetics of biologic responses and significance of secondary radiations. Radiat. Res. 2013, 179, 444–457. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Gonon, G.; Buonanno, M.; Autsavapromporn, N.; de Toledo, S.M.; Pain, D.; Azzam, E.I. Health risks of space exploration: Targeted and nontargeted oxidative injury by high-charge and high-energy particles. Antioxid. Redox Signal. 2014, 20, 1501–1523. [Google Scholar] [CrossRef] [PubMed]
- Newberg, A.B. Changes in the central nervous system and their clinical correlates during long-term spaceflight. Aviat. Space Environ. Med. 1994, 65, 562–572. [Google Scholar] [PubMed]
- DeFelipe, J.; Arellano, J.I.; Merchan-Perez, A.; Gonzalez-Albo, M.C.; Walton, K.; Llinas, R. Spaceflight induces changes in the synaptic circuitry of the postnatal developing neocortex. Cereb. Cortex 2002, 12, 883–891. [Google Scholar] [CrossRef] [PubMed]
- Van Ombergen, A.; Demertzi, A.; Tomilovskaya, E.; Jeurissen, B.; Sijbers, J.; Kozlovskaya, I.B.; Parizel, P.M.; van de Heyning, P.H.; Sunaert, S.; Laureys, S.; et al. The effect of spaceflight and microgravity on the human brain. J. Neurol. 2017, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.S.; Yang, M.; Kim, S.H.; Shin, T.; Moon, C. Neurobiological toxicity of radiation in hippocampal cells. Histol. Histopathol. 2013, 28, 301–310. [Google Scholar] [PubMed]
- Gauger, G.E.; Tobias, C.A.; Yang, T.; Whitney, M. The effect of space radiation of the nervous system. Adv. Space Res. 1986, 6, 243–249. [Google Scholar] [CrossRef]
- Darby, S.C.; Ewertz, M.; McGale, P.; Bennet, A.M.; Blom-Goldman, U.; Bronnum, D.; Correa, C.; Cutter, D.; Gagliardi, G.; Gigante, B.; et al. Risk of ischemic heart disease in women after radiotherapy for breast cancer. N. Engl. J. Med. 2013, 368, 987–998. [Google Scholar] [CrossRef] [PubMed]
- Lipshultz, S.E.; Cochran, T.R.; Franco, V.I.; Miller, T.L. Treatment-related cardiotoxicity in survivors of childhood cancer. Nat. Rev. Clin. Oncol. 2013, 10, 697–710. [Google Scholar] [CrossRef] [PubMed]
- Boerma, M.; Nelson, G.A.; Sridharan, V.; Mao, X.W.; Koturbash, I.; Hauer-Jensen, M. Space radiation and cardiovascular disease risk. World J. Cardiol. 2015, 7, 882–888. [Google Scholar] [CrossRef] [PubMed]
- Soucy, K.G.; Lim, H.K.; Kim, J.H.; Oh, Y.; Attarzadeh, D.O.; Sevinc, B.; Kuo, M.M.; Shoukas, A.A.; Vazquez, M.E.; Berkowitz, D.E. HZE 56Fe-ion irradiation induces endothelial dysfunction in rat aorta: Role of xanthine oxidase. Radiat. Res. 2011, 176, 474–485. [Google Scholar] [CrossRef] [PubMed]
- Datta, K.; Suman, S.; Kallakury, B.V.; Fornace, A.J., Jr. Exposure to heavy ion radiation induces persistent oxidative stress in mouse intestine. PLoS ONE 2012, 7, e42224. [Google Scholar] [CrossRef] [PubMed]
- Qu, L.; Chen, H.; Liu, X.; Bi, L.; Xiong, J.; Mao, Z.; Li, Y. Protective effects of flavonoids against oxidative stress induced by simulated microgravity in SH-SY5Y cells. Neurochem. Res. 2010, 35, 1445–1454. [Google Scholar] [CrossRef] [PubMed]
- Gambara, G.; Salanova, M.; Ciciliot, S.; Furlan, S.; Gutsmann, M.; Schiffl, G.; Ungethuem, U.; Volpe, P.; Gunga, H.C.; Blottner, D. Gene expression profiling in slow-type calf soleus muscle of 30 days space-flown mice. PLoS ONE 2017, 12, e0169314. [Google Scholar] [CrossRef] [PubMed]
- Salanova, M.; Gambara, G.; Moriggi, M.; Vasso, M.; Ungethuem, U.; Belavy, D.L.; Felsenberg, D.; Cerretelli, P.; Gelfi, C.; Blottner, D. Vibration mechanosignals superimposed to resistive exercise result in baseline skeletal muscle transcriptome profiles following chronic disuse in bed rest. Sci. Rep. 2015, 5, 17027. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Javed, I.; Liu, Y.; Lu, S.; Peng, G.; Zhang, Y.; Qing, H.; Deng, Y. Effect of prolonged simulated microgravity on metabolic proteins in rat hippocampus: Steps toward safe space travel. J. Proteome Res. 2016, 15, 29–37. [Google Scholar] [CrossRef] [PubMed]
- Zhang, R.; Bai, Y.G.; Lin, L.J.; Bao, J.X.; Zhang, Y.Y.; Tang, H.; Cheng, J.H.; Jia, G.L.; Ren, X.L.; Ma, J. Blockade of AT1 receptor partially restores vasoreactivity, NOS expression, and superoxide levels in cerebral and carotid arteries of hindlimb unweighting rats. J. Appl. Physiol. 2009, 106, 251–258. [Google Scholar] [CrossRef] [PubMed]
- Zhang, R.; Ran, H.H.; Peng, L.; Xu, F.; Sun, J.F.; Zhang, L.N.; Fan, Y.Y.; Peng, L.; Cui, G. Mitochondrial regulation of NADPH oxidase in hindlimb unweighting rat cerebral arteries. PLoS ONE 2014, 9, e95916. [Google Scholar] [CrossRef] [PubMed]
- Peng, L.; Ran, H.H.; Zhang, Y.; Zhao, Y.; Fan, Y.Y.; Peng, L.; Zhang, R.; Cao, F. NADPH Oxidase Accounts for Changes in Cerebrovascular Redox Status in Hindlimb Unweighting Rats. Biomed. Environ. Sci. 2015, 28, 799–807. [Google Scholar] [CrossRef]
- Ran, F.; An, L.; Fan, Y.; Hang, H.; Wang, S. Simulated microgravity potentiates generation of reactive oxygen species in cells. Biophys. Rep. 2016, 2, 100–105. [Google Scholar] [CrossRef] [PubMed]
- Dikalov, S. Cross talk between mitochondria and NADPH oxidases. Free Radic. Biol. Med. 2011, 51, 1289–1301. [Google Scholar] [CrossRef] [PubMed]
- Stein, T.P. Space flight and oxidative stress. Nutrition 2002, 18, 867–871. [Google Scholar] [CrossRef]
- Stein, T.P.; Leskiw, M.J. Oxidant damage during and after spaceflight. Am. J. Physiol. Endocrinol. Metab. 2000, 278, E375–E382. [Google Scholar] [PubMed]
- Da Silva, M.S.; Zimmerman, P.M.; Meguid, M.M.; Nandi, J.; Ohinata, K.; Xu, Y.; Chen, C.; Tada, T.; Inui, A. Anorexia in space and possible etiologies: An overview. Nutrition 2002, 18, 805–813. [Google Scholar] [CrossRef]
- Heer, M.; Boerger, A.; Kamps, N.; Mika, C.; Korr, C.; Drummer, C. Nutrient supply during recent European missions. Pflügers Arch. Eur. J. Physiol. 2000, 441, R8–R14. [Google Scholar] [CrossRef]
- Biolo, G.; Heer, M.; Narici, M.; Strollo, F. Microgravity as a model of ageing. Curr. Opin. Clin. Nutr. Metab. Care 2003, 6, 31–40. [Google Scholar] [CrossRef] [PubMed]
- Adrian, A.; Schoppmann, K.; Sromicki, J.; Brungs, S.; von der Wiesche, M.; Hock, B.; Kolanus, W.; Hemmersbach, R.; Ullrich, O. The oxidative burst reaction in mammalian cells depends on gravity. Cell. Commun. Signal. 2013, 11, 98. [Google Scholar] [CrossRef] [PubMed]
- Veglia, F.; Cighetti, G.; de Franceschi, M.; Zingaro, L.; Boccotti, L.; Tremoli, E.; Cavalca, V. Age- and gender-related oxidative status determined in healthy subjects by means of OXY-SCORE, a potential new comprehensive index. Biomarkers 2006, 11, 562–573. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Q.; Li, L.; Zhao, B.; Guan, K.L. The hippo pathway in heart development, regeneration, and diseases. Circ. Res. 2015, 116, 1431–1447. [Google Scholar] [CrossRef] [PubMed]
- Porazinski, S.; Wang, H.; Asaoka, Y.; Behrndt, M.; Miyamoto, T.; Morita, H.; Hata, S.; Sasaki, T.; Krens, S.F.; Osada, Y.; et al. YAP is essential for tissue tension to ensure vertebrate 3D body shape. Nature 2015, 521, 217–221. [Google Scholar] [CrossRef] [PubMed]
- Asaoka, Y.; Nishina, H.; Furutani-Seiki, M. YAP is essential for 3D organogenesis withstanding gravity. Dev. Growth Differ. 2017, 59, 52–58. [Google Scholar] [CrossRef] [PubMed]
- Vernikos, J.; Schneider, V.S. Space, gravity and the physiology of aging: Parallel or convergent disciplines? A mini-review. Gerontology 2010, 56, 157–166. [Google Scholar] [CrossRef] [PubMed]
- Hohn, A.; Weber, D.; Jung, T.; Ott, C.; Hugo, M.; Kochlik, B.; Kehm, R.; Konig, J.; Grune, T.; Castro, J.P. Happily (n)ever after: Aging in the context of oxidative stress, proteostasis loss and cellular senescence. Redox Biol. 2017, 11, 482–501. [Google Scholar] [CrossRef] [PubMed]

© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Takahashi, K.; Okumura, H.; Guo, R.; Naruse, K. Effect of Oxidative Stress on Cardiovascular System in Response to Gravity. Int. J. Mol. Sci. 2017, 18, 1426. https://doi.org/10.3390/ijms18071426
Takahashi K, Okumura H, Guo R, Naruse K. Effect of Oxidative Stress on Cardiovascular System in Response to Gravity. International Journal of Molecular Sciences. 2017; 18(7):1426. https://doi.org/10.3390/ijms18071426
Chicago/Turabian StyleTakahashi, Ken, Hiroki Okumura, Rui Guo, and Keiji Naruse. 2017. "Effect of Oxidative Stress on Cardiovascular System in Response to Gravity" International Journal of Molecular Sciences 18, no. 7: 1426. https://doi.org/10.3390/ijms18071426
APA StyleTakahashi, K., Okumura, H., Guo, R., & Naruse, K. (2017). Effect of Oxidative Stress on Cardiovascular System in Response to Gravity. International Journal of Molecular Sciences, 18(7), 1426. https://doi.org/10.3390/ijms18071426