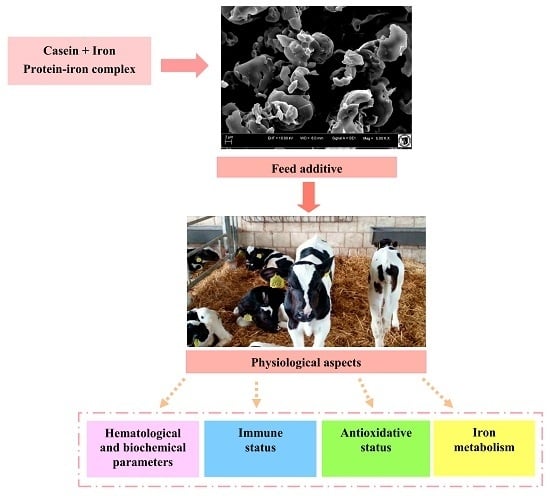
Effects of Protein-Iron Complex Concentrate Supplementation on Iron Metabolism, Oxidative and Immune Status in Preweaning Calves
Abstract
:1. Introduction
2. Results and Discussion
2.1. Hematological Parameters and Iron Metabolism
2.2. Antioxidation Status, Biochemical and Immunologial Parameters
3. Materials and Methods
3.1. Statement of Ethics
3.2. Animals and Treatments
3.3. Process of Obtaining Protein-Iron Complex
3.4. Scanning Electron Microscopy
3.5. Clinical Observations and Sampling Procedures
3.6. Laboratory Analyses
- glucose by oxidase method, reagents HORIBA ABX (Montpellier, France);
- glutathione peroxidase activity (GPx) by enzymatic method, Randox reagents Ransel RS (Crumlin, UK). The parameters determining the anti-oxidative status were also determined:
- Total antioxidant capacity (TAS) in serum by colorimetric method based on ABTS (2,2′-azine-di-[3-ethylbenzothiazoline sulfate]) method with peroxidase,
- glutathione peroxidase (GPx) in whole blood using enzymatic method,
- superoxide dismutase (SOD) in erythrocytes by the spectrophotometric, consisting of reaction with 2-(4-iodophenyl-3-(4-nitrophenol)-5-phenyltetrazoline chloride (I.N.T.)
3.7. Statistical Analysis
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
Abbreviations
| PIC | Protein-iron complex |
| MR | Milk replacers |
| IGF-1 | Insulin-like Growth Factor 1 |
| SEM | Scanning electron microscopy |
| RBC | Red blood cell |
| WBC | White blood cell |
| HCT | Hematocrit |
| HGB | Hemoglobin |
| MCV | Mean corpuscular volume |
| MCH | Mean corpuscular hemoglobin |
| MCHC | Mean corpuscular hemoglobin concentration |
| DM | Dry matter |
| PLT | Platelets |
| TIBC | Total iron binding capacity |
| UIBC | Unsaturated iron binding capacity |
| TS | Transferrin saturation |
| LFe | Experimental group receiving low iron dose |
| HFe | Experimental group receiving height iron dose |
| DMT1 | Duodenal divalent metal transporter-1 |
| TAS | Total antioxidant capacity |
| GPx | Glutathione peroxidase activity |
| SOD | Superoxide dismutase |
| MDA | Malondialdehyde |
| GSH-Px | Erythrocyte glutathione peroxidase activity |
| TNF-α | Tumor necrosis factor-α |
| IFN-γ | Interferon-γ |
| IL | Interleukin |
| IgG | Immunoglobulin G |
| IgM | Immunoglobulin M |
References
- Bami, M.H.; Mohri, M.; Seifi, H.A.; Tabatabaee, A.A. Effects of parenteral supply of iron and copper on hematology, weight gain, and health in neonatal dairy calves. Vet. Res. Commun. 2008, 32, 553–561. [Google Scholar] [CrossRef] [PubMed]
- Prodanović, R.; Kirovski, D.; Vujanac, I.; Dodovski, P.; Jovanović, L.; Šamanc, H. Relationship between serum iron and insulin-like growth factor-I concentrations in 10-day-old calves. Acta Vet. BRNO 2014, 83, 133–137. [Google Scholar] [CrossRef]
- Miltenburg, G.A.J.; Wensing, T.H.; Breukink, H.J.; Marx, J.J.M. Mucosal uptake, mucosal transfer and retention of iron in veal calves. Vet. Res. Commun. 1993, 17, 209–217. [Google Scholar] [CrossRef] [PubMed]
- Wienk, K.J.H.; Marx, J.J.M.; Beynen, A.C. The concept of iron bioavailability and its assessment. Eur. J. Nut. 1999, 38, 51–75. [Google Scholar] [CrossRef]
- Mohri, M.; Sarrafzadeh, F.; Seifi, H.A.; Farzaneh, N. Effects of oral iron supplementation on some haematological parameters and iron biochemistry in neonatal dairy calves. Comp. Clin. Pathol. 2004, 13, 39–42. [Google Scholar] [CrossRef]
- Jones, M.L.; Allison, R.W. Evaluation of the complete blood cell count. Vet. Clin. Food Anim. 2007, 23, 377–402. [Google Scholar] [CrossRef] [PubMed]
- Volker, H.; Rotermund, L. Possibilities of oral iron supplementation for maintaining health status in calves. Dtsch. Tierarztl. Wochenschr. 2000, 107, 16–22. [Google Scholar] [PubMed]
- Mohri, M.; Poorsina, S.; Sedaghat, R. Effects of parenteral supply of iron on RBC parameters, performance, and health in neonatal dairy calves. Biol. Trace Elem. Res. 2010, 136, 33–39. [Google Scholar] [CrossRef] [PubMed]
- Atyabi, N.; Gharagozloo, F.; Nassiri, S.M. The necessity of iron supplementation for normal development of commercially reared suckling calves. Comp. Clin. Pathol. 2006, 15, 165–168. [Google Scholar] [CrossRef]
- Hansen, S.L.; Ashwell, M.S.; Moeser, A.J.; Fry, R.S.; Knutson, M.D.; Spears, J.W. High dietary iron reduces transporters involved in iron and manganese metabolism and increases intestinal permeability in calves. J. Dairy Sci. 2010, 93, 656–665. [Google Scholar] [CrossRef] [PubMed]
- Mendel, M.; Wiechetek, M. Iron poisoning in animals. Med. Weter. 2006, 62, 1357–1361. [Google Scholar]
- Smith, J.E. Iron metabolism and its diseases. In Clinical Biochemistry of Domestic Animals, 4th ed.; Kaneko, J.J., Ed.; Academic Press Inc.: San Diego, CA, USA, 1989; p. 262. [Google Scholar]
- Raja, B.K.; Jafri, E.S.; Dickson, D.; Acebron, A.; Cremonesi, P.; Fossati, G.; Simpson, R. Involvement of Iron (Ferric) Reduction in the Iron Absorption Mechanism of a Trivalent Iron-Protein Complex (Iron Protein Succinylate). Pharmacol. Toxicol. 2000, 87, 108–115. [Google Scholar] [CrossRef] [PubMed]
- Sugiarto, M.; Ye, A.; Singh, H. Characterisation of binding of iron to sodium caseinate and whey protein isolate. Food Chem. 2009, 114, 1007–1013. [Google Scholar] [CrossRef]
- Sugiarto, M.; Ye, A.; Taylor, W.M.; Singh, H. Milk protein-iron complexes: Inhibition of lipid oxidation in an emulsion. Dairy Sci. Technol. 2010, 90, 87–98. [Google Scholar] [CrossRef]
- Layrisse, M.; García-Casal, M.N.; Solano, L.; Barón, M.A.; Arguello, F.; Llovera, D.; Ramírez, J.; Leets, I.; Tropper, E. Iron bioavailability in humans from breakfasts enriched with iron bis-glycine chelate, phytates and polyphenols. J. Nutr. 2000, 130, 2195–2199. [Google Scholar] [PubMed]
- Ettle, T.; Schlegel, P.; Roth, F.X. Investigations on iron bioavailability of different sources and supply levels in piglets. J. Anim. Physiol. Anim. Nutr. 2008, 92, 35–43. [Google Scholar] [CrossRef] [PubMed]
- Hellwig, M.; Geissler, S.; Peto, A.; Knütter, I.; Brandsch, M.; Henle, T. Transport of free and peptide-bound pyrraline at intestinal and renal epithelial cells. J. Agric. Food Chem. 2009, 57, 6474–6480. [Google Scholar] [CrossRef] [PubMed]
- Shilpashree, B.G.; Arora, S.; Sharma, V.; Bajaj, R.K.; Tomar, S.K. Preparation of iron bound succinylated milk protein concentrate and evaluation of its stability. Food Chem. 2016, 196, 800–807. [Google Scholar] [CrossRef] [PubMed]
- Bunger, U.; Kaphangst, P.; Fiebig, U.; Schonfelder, E.; Jentsch, D.; Ponge, J.; Furcht, G. Anaemia in male calves during rearing 4. Relations between birth weight, duration of trial and body weight gain while the calves were fed on colostrums, and the blood picture during weaning. Arch. Tierernahr. 1980, 30, 611–631. [Google Scholar] [PubMed]
- Moosavian, H.R.; Mohri, M.; Seifi, H.A. Effects of parenteral over-supplementation of vitamin A and iron on hematology, iron biochemistry, weight gain, and health of neonatal dairy calves. Food Chem. Toxicol. 2010, 48, 1316–1320. [Google Scholar] [CrossRef] [PubMed]
- Veissier, I.; Butterworth, A.; Bock, B.; Roe, E. European approaches to ensure good animal welfare. Appl. Anim. Behav. Sci. 2008, 113, 279–297. [Google Scholar] [CrossRef]
- Jain, N.C. Essentials of Veterinary Hematology; Lea and Febiger: Philadelphia, PA, USA, 1993. [Google Scholar]
- Miltenburg, G.A.J.; Wensing, T.; van Vliet, J.P.M.; Schuijt, G.; van de Broek, J.; Breukink, H.J. Blood hematology, plasma iron and tissue iron in dams in late gestation, at calving, and in veal calves at delivery and later. J. Dairy Sci. 1991, 74, 3086–3094. [Google Scholar] [CrossRef]
- Knowles, T.G.; Edwards, J.E.; Bazeley, K.J.; Brown, S.N.; Butterworth, A.; Warriss, P.D. Changes in the blood biochemical and haematological profile of neonatal calves with age. Vet. Rec. 2000, 147, 593–598. [Google Scholar] [CrossRef] [PubMed]
- Hatcher, H.C.; Singh, R.N.; Torti, F.M.; Torti, S.V. Synthetic and natural iron chelators: Therapeutic potential and clinical use. Future Med. Chem. 2009, 1, 1643–1670. [Google Scholar] [CrossRef] [PubMed]
- Reece, W.O.; Hotchkiss, D.K. Blood studies and performance among calves reared by different methods. J. Dairy Sci. 1987, 70, 1601–1611. [Google Scholar] [CrossRef]
- Zhang, H.; Gilbert, E.R.; Pan, S.; Zhang, K.; Ding, X.; Wang, J.; Qiufeng, Z.; Bai, S. Dietary iron concentration influences serum concentrations of manganese in rats consuming organic or inorganic sources of manganese. Br. J. Nutr. 2016, 115, 585–593. [Google Scholar] [CrossRef] [PubMed]
- Oh, S.; Shin, P.K.; Chung, J. Effects of developmental iron deficiency and post-weaning iron repletion on the levels of iron transporter proteins in rats. Nutr. Res. Pract. 2015, 9, 613–618. [Google Scholar] [CrossRef] [PubMed]
- Hansen, S.L.; Trakooljul, N.; Liu, H.C.; Moeser, A.J.; Spears, J.W. Iron transporters are differentially regulated by dietary iron, and modifications are associated with changes in manganese metabolism in young pigs. J. Nutr. 2009, 139, 1474–1479. [Google Scholar] [CrossRef] [PubMed]
- Ganz, T.; Nemeth, E. Regulation of iron acquisition and iron distribution in mammals. BBA Mol. Cell Res. 2006, 1763, 690–699. [Google Scholar] [CrossRef] [PubMed]
- Kusano, C.; Ferrari, B. Total Antioxidant Capacity: A biomarker in biomedical and nutritional studies. J. Cell. Mol. Biol. 2008, 7, 1–15. [Google Scholar]
- McDowell, L.R.; Wilkinson, N.; Madison, R.; Felix, T. Vitamins and minerals functioning as antioxidants with supplementation considerations. In Florida Ruminant Nutrition Symposium; Best Western Gateway Grand: Gainesville, FL, USA, 2007; pp. 30–31. [Google Scholar]
- Badria, F.A.; Ibrahim, A.S.; Badria, A.F.; Elmarakby, A.A. Curcumin attenuates iron accumulation and oxidative stress in the liver and spleen of chronic iron-overloaded rats. PLoS ONE 2015, 10, e0134156. [Google Scholar] [CrossRef] [PubMed]
- Bernabucci, U.; Ronchi, B.; Lacetera, N.; Nardone, A. Influence of body condition on relationships between metabolic status and oxidative stress in periparturient dairy cows. J. Dairy Sci. 2005, 88, 2017–2026. [Google Scholar] [CrossRef]
- Wang, H.; Liu, Z.; Huang, M.; Wang, S.; Cui, D.; Dong, S.; Li, S.; Qi, Z.; Liu, Y. Effects of long-term mineral block supplementation on antioxidants, immunity, and health of Tibetan sheep. Biol. Trace Elem. Res. 2016, 172, 326–335. [Google Scholar] [CrossRef] [PubMed]
- Fernández-Real, J.M.; McClain, D.; Manco, M. Mechanisms linking glucose homeostasis and iron metabolism toward the onset and progression of type 2 diabetes. Diabetes Care 2015, 38, 2169–2176. [Google Scholar] [CrossRef] [PubMed]
- Hammon, H.M.; Steinhoff-Wagner, J.; Flor, J.; Schönhusen, U.; Metges, C.C. Lactation Biology Symposium: Role of colostrum and colostrum components on glucose metabolism in neonatal calves. J. Animal. Sci. 2013, 91, 685–695. [Google Scholar] [CrossRef] [PubMed]
- Annibalini, G.; Bielli, P.; De Santi, M.; Agostini, D.; Guescini, M.; Sisti, D.; Contarelli, S.; Brandi, G.; Villarini, A.; Stocchi, V.; et al. MIR retroposon exonization promotes evolutionary variability and generates species-specific expression of IGF-1 splice variants. BBA-Gene Regul. Mech. 2016, 1859, 757–768. [Google Scholar] [CrossRef] [PubMed]
- Guilloteau, P.; Biernat, M.; Woliński, J.; Zabielski, R. Gut regulatory peptides and hormones of the small gut. In Biology of the Intestine in Growing Animals; Zabielski, R., Gregory, P.C., Weström, B., Eds.; Elsevier: Amsterdam, The Netherlands, 2002; Chapter 11; pp. 325–362. [Google Scholar]
- Steinhoff-Wagner, J.; Görs, S.; Junghans, P.; Bruckmaier, R.M.; Kanitz, E.; Metges, C.C.; Hammon, H.M. Maturation of endogenous glucose production in preterm and term calves. J. Dairy Sci. 2011, 94, 5111–5123. [Google Scholar] [CrossRef] [PubMed]
- Tran, P.V.; Fretham, S.J.; Wobken, J.; Miller, B.S.; Georgieff, M.K. Gestational-neonatal iron deficiency suppresses and iron treatment reactivates IGF signaling in developing rat hippocampus. Am. J. Physiol. Endocrinol. Metab. 2012, 302, 316–324. [Google Scholar] [CrossRef] [PubMed]
- Soliman, A.T.; De Sanctis, V.; Yassin, M.; Adel, A. Growth and growth hormone–Insulin Like Growth Factor–I (GH-IGF-I) axis in chronic anemias. Acta Biomed. 2017, 88, 101–111. [Google Scholar] [CrossRef] [PubMed]
- Tan, Y.; Liu, J.; Deng, Y.; Cao, H.; Xu, D.; Cu, F.L.; Lei, Y.Y.; Magdalou, J.; Wu, M.; Chen, L.; et al. Caffeine-induced fetal rat over-exposure to maternal glucocorticoid and histone methylation of liver IGF-1 might cause skeletal growth retardation. Toxicol. Lett. 2012, 214, 279–287. [Google Scholar] [CrossRef] [PubMed]
- Brown, E.G.; VandeHaar, M.J.; Daniels, K.M.; Liesman, J.S.; Chapin, L.T.; Keisler, D.H.; Nielsen, M.W. Effect of increasing energy and protein intake on body growth and carcass composition of heifer calves. J. Dairy Sci. 2005, 88, 585–594. [Google Scholar] [CrossRef]
- Quigley, J.D.; Wolfe, T.A.; Elsasser, T.H. Effects of additional milk replacer feeding on calf health, growth, and selected blood metabolites in calves. J. Dairy Sci. 2006, 89, 207–216. [Google Scholar] [CrossRef]
- Cui, K.; Tu, Y.; Wang, Y.C.; Zhang, N.F.; Ma, T.; Diao, Q.Y. Effects of a limited period of iron supplementation on the growth performance and meat colour of dairy bull calves for veal production. Anim. Prod. Sci. 2016, 57, 778–784. [Google Scholar] [CrossRef]
- Rodríguez, F.; González, J.F.; Arbelo, M.; Zucca, D.; Fernández, A. Cytokine expression in lungs of calves spontaneously infected with Mycoplasma bovis. Vet. Res. Commun. 2015, 39, 69–72. [Google Scholar] [CrossRef] [PubMed]
- Bednarski, M.; Kupczyński, R.; Sobiech, P. Acid-base disorders in calves with chronic diarrhea. Pol. J. Vet. Sci. 2015, 18, 207–215. [Google Scholar] [CrossRef] [PubMed]
- Hulbert, L.E.; Cobb, C.J.; Carroll, J.A.; Ballou, M.A. Effects of changing milk replacer feedings from twice to once daily on Holstein calf innate immune responses before and after weaning. J. Dairy Sci. 2011, 94, 2557–2565. [Google Scholar] [CrossRef] [PubMed]
- Sun, F.; Cao, Y.; Cai, C.; Li, S.; Yu, C.; Yao, J. Regulation of Nutritional Metabolism in Transition Dairy Cows: Energy Homeostasis and Health in Response to Post-Ruminal Choline and Methionine. PLoS ONE 2016, 11, e0160659. [Google Scholar] [CrossRef] [PubMed]
- Association of Official Analytical Chemists. AOAC Official Methods of Analysis, 15th ed.; Association of Official Analytical Chemists: Washington, DC, USA, 1990; p. 105. [Google Scholar]


| Item | Treatment | SEM | p-Value 1 | ||||
|---|---|---|---|---|---|---|---|
| Control | Low Iron Dose (LFe) | Height Iron Dose (HFe) | D | T | D × T | ||
| WBC (G/L) | 9.95 | 9.48 | 7.93 | 0.28 | <0.01 | 0.11 | 0.88 |
| RBC (T/L) | 8.04 | 8.02 | 8.14 | 0.10 | 0.87 | 0.26 | 0.92 |
| HGB (mmol/L) | 6.01 | 6.21 | 6.25 | 0.09 | 0.60 | 0.25 | 0.79 |
| HCT (L/L) | 0.31 | 0.27 | 0.32 | 0.07 | <0.01 | 0.37 | 0.41 |
| PLT (G/L) | 757.47 | 770.93 | 822.34 | 30.22 | 0.64 | <0.01 | 0.89 |
| MCV (fl) | 38.39 | 33.64 | 39.29 | 0.28 | <0.01 | <0.01 | 0.14 |
| MCH (fmol) | 0.77 | 0.76 | 0.77 | 0.04 | 0.40 | 0.12 | 0.65 |
| MCHC (mmol/L) | 19.36 | 23.00 | 19.53 | 0.14 | <0.01 | <0.01 | 0.54 |
| Item | Treatment | SEM | p-Value 1 | ||||
|---|---|---|---|---|---|---|---|
| Control | Low Iron Dose (LFe) | Height Iron Dose (HFe) | D | T | D × T | ||
| Iron (µmol/L) | 15.06 | 16.59 | 14.70 | 0.57 | 0.35 | 0.01 | 0.88 |
| UIBC (µmol/L) | 6.68 | 5.47 | 6.15 | 0.46 | 0.62 | <0.01 | 0.98 |
| TIBC (µmol/L) | 19.99 | 18.30 | 18.72 | 0.56 | 0.76 | 0.11 | 0.52 |
| Transferrin saturation (%) | 70.484 | 73.77 | 79.10 | 0.94 | 0.03 | 0.02 | 0.99 |
| Transferrin (mg/mL) | 2.041 | 3.59 | 5.53 | 0.42 | <0.01 | <0.01 | 0.01 |
| Item | Treatment | SEM | p-Value 1 | ||||
|---|---|---|---|---|---|---|---|
| Control | Low Iron Dose (LFe) | Height Iron Dose (HFe) | D | T | D × T | ||
| TAS (mmol/L) | 1.15 | 0.91 | 0.86 | 0.22 | <0.01 | 0.79 | 0.13 |
| GPx (U/L) | 59,716.22 | 60,833.10 | 50,422.23 | 324.11 | 0.01 | 0.01 | 0.88 |
| SOD (U/mL) | 1168.6 | 1041.8 | 1249.4 | 30.45 | 0.31 | 0.36 | 0.99 |
| Insulin (ng/mL) | 0.496 | 0.589 | 0.620 | 0.03 | 0.12 | 0.18 | 0.11 |
| Glucose (mmol/L) | 5.992 | 5.783 | 5.459 | 0.11 | 0.16 | 0.01 | 0.66 |
| IGF-1 (ng/mL) | 45.251 | 50.761 | 40.849 | 1.42 | 0.30 | 0.51 | 0.98 |
| TNF-α (pg/mL) | 103.76 | 108.79 | 95.891 | 1.14 | 0.49 | 0.15 | 0.71 |
| IgG (mg/mL) | 12.355 | 13.502 | 11.256 | 0.54 | 0.23 | 0.09 | 0.86 |
| IgM (mg/mL) | 0.70125 | 0.64937 | 0.78187 | 0.05 | 0.52 | 0.67 | 0.17 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kupczyński, R.; Bednarski, M.; Śpitalniak, K.; Pogoda-Sewerniak, K. Effects of Protein-Iron Complex Concentrate Supplementation on Iron Metabolism, Oxidative and Immune Status in Preweaning Calves. Int. J. Mol. Sci. 2017, 18, 1501. https://doi.org/10.3390/ijms18071501
Kupczyński R, Bednarski M, Śpitalniak K, Pogoda-Sewerniak K. Effects of Protein-Iron Complex Concentrate Supplementation on Iron Metabolism, Oxidative and Immune Status in Preweaning Calves. International Journal of Molecular Sciences. 2017; 18(7):1501. https://doi.org/10.3390/ijms18071501
Chicago/Turabian StyleKupczyński, Robert, Michał Bednarski, Kinga Śpitalniak, and Krystyna Pogoda-Sewerniak. 2017. "Effects of Protein-Iron Complex Concentrate Supplementation on Iron Metabolism, Oxidative and Immune Status in Preweaning Calves" International Journal of Molecular Sciences 18, no. 7: 1501. https://doi.org/10.3390/ijms18071501
APA StyleKupczyński, R., Bednarski, M., Śpitalniak, K., & Pogoda-Sewerniak, K. (2017). Effects of Protein-Iron Complex Concentrate Supplementation on Iron Metabolism, Oxidative and Immune Status in Preweaning Calves. International Journal of Molecular Sciences, 18(7), 1501. https://doi.org/10.3390/ijms18071501