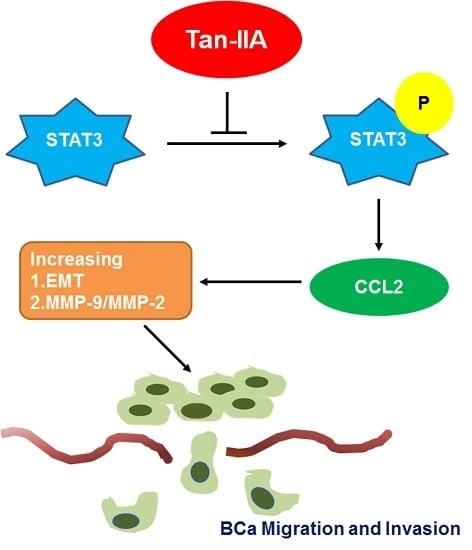
Tanshinone IIA Inhibits Epithelial-Mesenchymal Transition in Bladder Cancer Cells via Modulation of STAT3-CCL2 Signaling
Abstract
:1. Introduction
2. Results
2.1. Tan-IIA Inhibits the Migration and Invasion of Human BCa Cells
2.2. Tan-IIA Inhibits EMT in Human BCa Cells
2.3. Tan-IIA Inhibits EMT via Down-Regulated CCL2 Expression in Human BCa Cells
2.4. Tan-IIA Inhibits STAT3-CCL2 Signaling in Human BCa Cells
3. Discussion
4. Materials and Methods
4.1. Chemicals and Antibodies
4.2. Cell Culture
4.3. Western Blot Analysis
4.4. Cell Migration and Invasion Assay
4.5. RNA Extraction and Real-Time RT-PCR
4.6. Enzyme-Linked Immunosorbent Assay (ELISA)
4.7. Gelatin Zymography
4.8. Small Interfering RNA (siRNA) Transfection
4.9. Statistical Analysis
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
Abbreviations
| CCL2 | Chemokine (C-C motif) ligand 2 |
| EMT | Epithelial-mesenchymal transition |
| MMP | Matrix metalloproteinases |
| STAT3 | Signal transducer and activator of transcription 3 |
| Tan-IIA | Tanshinone IIA |
References
- Siegel, R.L.; Miller, K.D.; Jemal, A. Cancer statistics, 2016. CA Cancer J. Clin. 2016, 66, 7–30. [Google Scholar] [CrossRef] [PubMed]
- Antoni, S.; Ferlay, J.; Soerjomataram, I.; Znaor, A.; Jemal, A.; Bray, F. Bladder cancer incidence and mortality: A global overview and recent trends. Eur. Urol. 2017, 71, 96–108. [Google Scholar] [CrossRef] [PubMed]
- Jemal, A.; Siegel, R.; Ward, E.; Hao, Y.; Xu, J.; Murray, T.; Thun, M.J. Cancer statistics, 2008. CA Cancer J. Clin. 2008, 58, 71–96. [Google Scholar] [CrossRef] [PubMed]
- Lutzeyer, W.; Rubben, H.; Dahm, H. Prognostic parameters in superficial bladder cancer: An analysis of 315 cases. J. Urol. 1982, 127, 250–252. [Google Scholar] [CrossRef]
- Wu, X.R. Urothelial tumorigenesis: a tale of divergent pathways. Nat. Rev. Cancer 2005, 5, 713–725. [Google Scholar] [CrossRef] [PubMed]
- Che, A.J.; Zhang, J.Y.; Li, C.H.; Chen, X.F.; Hu, Z.D.; Chen, X.G. Separation and determination of active components in Radix Salviae miltiorrhizae and its medicinal preparations by nonaqueous capillary electrophoresis. J. Sep. Sci. 2004, 27, 569–575. [Google Scholar] [PubMed]
- Zhou, L.; Zuo, Z.; Chow, M.S. Danshen: An overview of its chemistry, pharmacology, pharmacokinetics, and clinical use. J. Clin. Pharmacol. 2005, 45, 1345–1359. [Google Scholar] [CrossRef] [PubMed]
- Wei, B.; You, M.G.; Ling, J.J.; Wei, L.L.; Wang, K.; Li, W.W.; Chen, T.; Du, Q.M.; Ji, H. Regulation of antioxidant system, lipids and fatty acid β-oxidation contributes to the cardioprotective effect of sodium tanshinone IIA sulphonate in isoproterenol-induced myocardial infarction in rats. Atherosclerosis 2013, 230, 148–156. [Google Scholar] [CrossRef] [PubMed]
- Fan, G.; Jiang, X.; Wu, X.; Fordjour, P.A.; Miao, L.; Zhang, H.; Zhu, Y.; Gao, X. Anti-Inflammatory Activity of Tanshinone IIA in LPS-Stimulated RAW264.7 Macrophages via miRNAs and TLR4-NF-κB Pathway. Inflammation 2016, 39, 375–384. [Google Scholar] [CrossRef] [PubMed]
- Huang, S.T.; Huang, C.C.; Huang, W.L.; Lin, T.K.; Liao, P.L.; Wang, P.W.; Liou, C.W.; Chuang, J.H. Tanshinone IIA induces intrinsic apoptosis in osteosarcoma cells both in vivo and in vitro associated with mitochondrial dysfunction. Sci. Rep. 2017, 7, 40382. [Google Scholar] [CrossRef] [PubMed]
- Su, C.C. Tanshinone IIA decreases the migratory ability of AGS cells by decreasing the protein expression of matrix metalloproteinases, nuclear factor κB-p65 and cyclooxygenase-2. Mol. Med. Rep. 2016, 13, 1263–1268. [Google Scholar] [PubMed]
- Xie, J.; Liu, J.; Liu, H.; Liang, S.; Lin, M.; Gu, Y.; Liu, T.; Wang, D.; Ge, H.; Mo, S.L. The antitumor effect of tanshinone IIA on anti-proliferation and decreasing VEGF/VEGFR2 expression on the human non-small cell lung cancer A549 cell line. Acta Pharm. Sin. B 2015, 5, 554–563. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.F.; Feng, J.G.; Han, J.; Zhang, B.B.; Mao, W.M. The molecular mechanisms of Tanshinone IIA on the apoptosis and arrest of human esophageal carcinoma cells. BioMed Res. Int. 2014, 2014. [Google Scholar] [CrossRef] [PubMed]
- Chiu, S.C.; Huang, S.Y.; Chen, S.P.; Su, C.C.; Chiu, T.L.; Pang, C.Y. Tanshinone IIA inhibits human prostate cancer cells growth by induction of endoplasmic reticulum stress in vitro and in vivo. Prostate Cancer Prostatic Dis. 2013, 16, 315–322. [Google Scholar] [CrossRef] [PubMed]
- Tsai, M.Y.; Yang, R.C.; Wu, H.T.; Pang, J.H.; Huang, S.T. Anti-angiogenic effect of Tanshinone IIA involves inhibition of matrix invasion and modification of MMP-2/TIMP-2 secretion in vascular endothelial cells. Cancer Lett. 2011, 310, 198–206. [Google Scholar] [CrossRef] [PubMed]
- Wu, W.Y.; Yan, H.; Wang, X.B.; Gui, Y.Z.; Gao, F.; Tang, X.L.; Qin, Y.L.; Su, M.; Chen, T.; Wang, Y.P. Sodium tanshinone IIA silate inhibits high glucose-induced vascular smooth muscle cell proliferation and migration through activation of AMP-activated protein kinase. PLoS ONE 2014, 9, e94957. [Google Scholar] [CrossRef] [PubMed]
- Yun, S.M.; Jung, J.H.; Jeong, S.J.; Sohn, E.J.; Kim, B.; Kim, S.H. Tanshinone IIA induces autophagic cell death via activation of AMPK and ERK and inhibition of mTOR and p70 S6K in KBM-5 leukemia cells. Phytother. Res. 2014, 28, 458–464. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Wei, R.X.; Zhu, X.B.; Cai, L.; Jin, W.; Hu, H. Tanshinone IIA induces apoptosis and inhibits the proliferation, migration, and invasion of the osteosarcoma MG-63 cell line in vitro. Anticancer Drugs 2012, 23, 212–219. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.Q.; Wang, B.Y.; Wu, F.; An, Y.K.; Zhou, X.Q. Influence of tanshinone IIA on the apoptosis of human esophageal Ec-109 cells. Nat. Prod. Commun. 2016, 11, 17–19. [Google Scholar] [PubMed]
- Zhang, J.; Wang, J.; Jiang, J.Y.; Liu, S.D.; Fu, K.; Liu, H.Y. Tanshinone IIA induces cytochrome c-mediated caspase cascade apoptosis in A549 human lung cancer cells via the JNK pathway. Int. J. Oncol. 2014, 45, 683–690. [Google Scholar] [PubMed]
- Tseng, P.Y.; Lu, W.C.; Hsieh, M.J.; Chien, S.Y.; Chen, M.K. Tanshinone IIA induces apoptosis in human oral cancer KB cells through a mitochondria-dependent pathway. BioMed Res. Int. 2014, 2014, 540516. [Google Scholar] [CrossRef] [PubMed]
- Gong, Y.; Li, Y.; Lu, Y.; Li, L.; Abdolmaleky, H.; Blackburn, G.L.; Zhou, J.R. Bioactive tanshinones in Salvia miltiorrhiza inhibit the growth of prostate cancer cells in vitro and in mice. Int. J. Cancer 2011, 129, 1042–1052. [Google Scholar] [CrossRef] [PubMed]
- Gong, Y.; Li, Y.; Abdolmaleky, H.M.; Li, L.; Zhou, J.R. Tanshinones inhibit the growth of breast cancer cells through epigenetic modification of aurora a expression and function. PLoS ONE 2012, 7, e33656. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, F.; Yu, G.; Wang, G.; Liu, H.; Wu, X.; Wang, Q.; Liu, M.; Liao, K.; Wu, M.; Cheng, X.; et al. An NQO1-initiated and p53-independent apoptotic pathway determines the anti-tumor effect of tanshinone IIA against non-small cell lung cancer. PLoS ONE 2012, 7, e42138. [Google Scholar] [CrossRef] [PubMed]
- Chiu, S.C.; Huang, S.Y.; Chang, S.F.; Chen, S.P.; Chen, C.C.; Lin, T.H.; Liu, H.H.; Tsai, T.H.; Lee, S.S.; Pang, C.Y.; et al. Potential therapeutic roles of tanshinone IIA in human bladder cancer cells. Int. J. Mol. Sci. 2014, 15, 15622–15637. [Google Scholar] [CrossRef] [PubMed]
- Vazquez-Lavista, L.G.; Lima, G.; Gabilondo, F.; Llorente, L. Genetic association of monocyte chemoattractant protein 1 (MCP-1)-2518 polymorphism in Mexican patients with transitional cell carcinoma of the bladder. Urology 2009, 74, 414–418. [Google Scholar] [CrossRef] [PubMed]
- Amann, B.; Perabo, F.G.; Wirger, A.; Hugenschmidt, H.; Schultze-Seemann, W. Urinary levels of monocyte chemo-attractant protein-1 correlate with tumour stage and grade in patients with bladder cancer. Br. J. Urol. 1998, 82, 118–121. [Google Scholar] [CrossRef] [PubMed]
- Brana, I.; Calles, A.; LoRusso, P.M.; Yee, L.K.; Puchalski, T.A.; Seetharam, S.; Zhong, B.; de Boer, C.J.; Tabernero, J.; Calvo, E. Carlumab, an anti-C-C chemokine ligand 2 monoclonal antibody, in combination with four chemotherapy regimens for the treatment of patients with solid tumors: An open-label, multicenter phase 1b study. Target Oncol. 2015, 10, 111–123. [Google Scholar] [CrossRef] [PubMed]
- Mitchem, J.B.; Brennan, D.J.; Knolhoff, B.L.; Belt, B.A.; Zhu, Y.; Sanford, D.E.; Belaygorod, L.; Carpenter, D.; Collins, L.; Piwnica-Worms, D.; et al. Targeting tumor-infiltrating macrophages decreases tumor-initiating cells, relieves immunosuppression, and improves chemotherapeutic responses. Cancer Res. 2013, 73, 1128–1141. [Google Scholar] [CrossRef] [PubMed]
- Mitchem, J.B.; DeNardo, D.G. Battle over CCL2 for control of the metastatic niche: neutrophils versus monocytes. Breast Cancer Res. 2012, 14, 315. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.C.; Ho, H.C.; Su, Y.C.; Lee, M.S.; Hung, S.K.; Lin, C.H. MCP1-Induced Epithelial-Mesenchymal Transition in Head and Neck Cancer by AKT Activation. Anticancer Res. 2015, 35, 3299–3306. [Google Scholar] [PubMed]
- Rao, Q.; Chen, Y.; Yeh, C.R.; Ding, J.; Li, L.; Chang, C.; Yeh, S. Recruited mast cells in the tumor microenvironment enhance bladder cancer metastasis via modulation of ERβ/CCL2/CCR2 EMT/MMP9 signals. Oncotarget 2016, 7, 7842–7855. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.; Gao, Q.; Han, S.; Pan, F.; Fan, W. The CCL2/CCR2 axis enhances IL-6-induced epithelial-mesenchymal transition by cooperatively activating STAT3-Twist signaling. Tumor Biol. 2015, 36, 973–981. [Google Scholar] [CrossRef] [PubMed]
- Hsu, Y.L.; Hung, J.Y.; Tsai, Y.M.; Tsai, E.M.; Huang, M.S.; Hou, M.F.; Kuo, P.L. 6-shogaol, an active constituent of dietary ginger, impairs cancer development and lung metastasis by inhibiting the secretion of CC-chemokine ligand 2 (CCL2) in tumor-associated dendritic cells. J. Agric. Food Chem. 2015, 63, 1730–1738. [Google Scholar] [CrossRef] [PubMed]
- Kortylewski, M.; Yu, H. Stat3 as a potential target for cancer immunotherapy. J. Immunother. 2007, 30, 131–139. [Google Scholar] [CrossRef] [PubMed]
- Yu, H.; Pardoll, D.; Jove, R. STATs in cancer inflammation and immunity: A leading role for STAT3. Nat. Rev. Cancer 2009, 9, 798–809. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.L.; Cen, L.; Kohout, J.; Hutzen, B.; Chan, C.; Hsieh, F.C.; Loy, A.; Huang, V.; Cheng, G.; Lin, J. Signal transducer and activator of transcription 3 activation is associated with bladder cancer cell growth and survival. Mol. Cancer 2008, 7, 78. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.; Zhang, W.; Wang, L.; Kazobinka, G.; Han, X.; Li, B.; Hou, T. Musashi-2 promotes migration and invasion in bladder cancer via activation of the JAK2/STAT3 pathway. Lab. Investig. 2016, 96, 950–958. [Google Scholar] [CrossRef] [PubMed]
- Chiu, H.Y.; Sun, K.H.; Chen, S.Y.; Wang, H.H.; Lee, M.Y.; Tsou, Y.C.; Jwo, S.C.; Sun, G.H.; Tang, S.J. Autocrine CCL2 promotes cell migration and invasion via PKC activation and tyrosine phosphorylation of paxillin in bladder cancer cells. Cytokine 2012, 59, 423–432. [Google Scholar] [CrossRef] [PubMed]
- Izumi, K.; Fang, L.Y.; Mizokami, A.; Namiki, M.; Li, L.; Lin, W.J.; Chang, C. Targeting the androgen receptor with siRNA promotes prostate cancer metastasis through enhanced macrophage recruitment via CCL2/CCR2-induced STAT3 activation. EMBO Mol. Med. 2013, 5, 1383–1401. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Cheng, M.K.; Griffiths, T.R.; Mellon, J.K.; Kai, B.; Kriajevska, M.; Manson, M.M. Inhibition of STAT signalling in bladder cancer by diindolylmethane: Relevance to cell adhesion, migration and proliferation. Curr. Cancer Drug Targets 2013, 13, 57–68. [Google Scholar] [CrossRef] [PubMed]
- Sung, W.J.; Kim, H.; Park, K.K. The biological role of epithelial-mesenchymal transition in lung cancer (Review). Oncol. Rep. 2016, 36, 1199–1206. [Google Scholar] [CrossRef] [PubMed]
- Horejs, C.M. Basement membrane fragments in the context of the epithelial-to-mesenchymal transition. Eur. J. Cell Biol. 2016, 95, 427–440. [Google Scholar] [CrossRef] [PubMed]
- Deshmane, S.L.; Kremlev, S.; Amini, S.; Sawaya, B.E. Monocyte chemoattractant protein-1 (MCP-1): An overview. J. Interferon Cytokine Res. 2009, 29, 313–326. [Google Scholar] [CrossRef] [PubMed]
- Raman, D.; Baugher, P.J.; Thu, Y.M.; Richmond, A. Role of chemokines in tumor growth. Cancer Lett. 2007, 256, 137–165. [Google Scholar] [CrossRef] [PubMed]
- Arabzadeh, A.; Dupaul-Chicoine, J.; Breton, V.; Haftchenary, S.; Yumeen, S.; Turbide, C.; Saleh, M.; McGregor, K.; Greenwood, C.M.; Akavia, U.D.; et al. Carcinoembryonic Antigen Cell Adhesion Molecule 1 long isoform modulates malignancy of poorly differentiated colon cancer cells. Gut 2016, 65, 821–829. [Google Scholar] [CrossRef] [PubMed]
- Ding, L.; Wang, S.; Wang, W.; Lv, P.; Zhao, D.; Chen, F.; Meng, T.; Dong, L.; Qi, L. Tanshinone IIA affects autophagy and apoptosis of glioma cells by inhibiting phosphatidylinositol 3-Kinase/Akt/Mammalian target of rapamycin signaling pathway. Pharmacology 2017, 99, 188–195. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Li, Z.; Li, X.; Liu, B.; Liu, Z. Mechanisms of tanshinone II a inhibits malignant melanoma development through blocking autophagy signal transduction in A375 cell. BMC Cancer 2017, 17, 357. [Google Scholar] [CrossRef] [PubMed]
- Sui, H.; Zhao, J.; Zhou, L.; Wen, H.; Deng, W.; Li, C.; Ji, Q.; Liu, X.; Feng, Y.; Chai, N.; et al. Tanshinone IIA inhibits β-catenin/VEGF-mediated angiogenesis by targeting TGF-β1 in normoxic and HIF-1α in hypoxic microenvironments in human colorectal cancer. Cancer Lett. 2017, 403, 86–97. [Google Scholar] [CrossRef] [PubMed]
- Zhong, C.; Zhang, Y.F.; Huang, J.H.; Wang, Z.Y.; Chen, Q.Y.; Su, L.T.; Liu, Z.T.; Xiong, C.M.; Tao, Z.; Guo, R.P. The Chinese medicine, Jianpi Huayu Decoction, inhibits the epithelial mesenchymal transition via the regulation of the Smad3/Smad7 cascade. Am. J. Transl. Res. 2017, 9, 2694–2711. [Google Scholar] [PubMed]
- Calon, A.; Espinet, E.; Palomo-Ponce, S.; Tauriello, D.V.; Iglesias, M.; Cespedes, M.V.; Sevillano, M.; Nadal, C.; Jung, P.; Zhang, X.H.; et al. Dependency of colorectal cancer on a TGF-β-driven program in stromal cells for metastasis initiation. Cancer Cell 2012, 22, 571–584. [Google Scholar] [CrossRef] [PubMed]
- Liu, R.Y.; Zeng, Y.; Lei, Z.; Wang, L.; Yang, H.; Liu, Z.; Zhao, J.; Zhang, H.T. JAK/STAT3 signaling is required for TGF-β-induced epithelial-mesenchymal transition in lung cancer cells. Int. J. Oncol. 2014, 44, 1643–1651. [Google Scholar] [CrossRef] [PubMed]
- Luwor, R.B.; Baradaran, B.; Taylor, L.E.; Iaria, J.; Nheu, T.V.; Amiry, N.; Hovens, C.M.; Wang, B.; Kaye, A.H.; Zhu, H.J. Targeting Stat3 and Smad7 to restore TGF-β cytostatic regulation of tumor cells in vitro and in vivo. Oncogene 2013, 32, 2433–2441. [Google Scholar] [CrossRef] [PubMed]





| Gene | Primers |
|---|---|
| CCL2 | sense: 5′-GATCTCAGTGCAGAGGCTCG-3′ antisense: 5′-TGCTTGTCCAGGTGGTCCAT-3′ |
| E-cadherin | sense: 5′-ACGTCGTAATCACCACACTGA-3′ antisense: 5′-TTCGTCACTGCTACGTGTAGAA-3′ |
| N-cadherin | sense: 5′-ACAGTGGCCACCTACAAAGG-3′ antisense: 5′-CCGAGATGGGGTTGATAATG-3′ |
| Fibronectin | sense: 5′-CCCACCGTCTCAACATGCTTAG-3′ antisense: 5′-CTCGGCTTCCTCCATAACAAGTAC-3′ |
| Vimentin | sense: 5′-CTTCGCCAACTACATCGACA-3′ antisense: 5′-GCTTCAACGGCAAAGTTCTC-3′ |
| Snail | sense: 5′-TCGTCCTTCTCCTCTACTTC-3′ antisense: 5′-TTCCTTGTTGCAGTATTTGC-3′ |
| Slug | sense: 5′-TGTTGCAGTGAGGGCAAGAA-3′ antisense: 5′-GACCCTGGTTGCTTCAAGGA-3′ |
| GAPDH | sense: 5′-CCATGGAGAAGGCTGGGG-3′ antisense: 5′-CAAAGTTGTCATGGATGACC-3′ |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huang, S.-Y.; Chang, S.-F.; Liao, K.-F.; Chiu, S.-C. Tanshinone IIA Inhibits Epithelial-Mesenchymal Transition in Bladder Cancer Cells via Modulation of STAT3-CCL2 Signaling. Int. J. Mol. Sci. 2017, 18, 1616. https://doi.org/10.3390/ijms18081616
Huang S-Y, Chang S-F, Liao K-F, Chiu S-C. Tanshinone IIA Inhibits Epithelial-Mesenchymal Transition in Bladder Cancer Cells via Modulation of STAT3-CCL2 Signaling. International Journal of Molecular Sciences. 2017; 18(8):1616. https://doi.org/10.3390/ijms18081616
Chicago/Turabian StyleHuang, Sung-Ying, Shu-Fang Chang, Kuan-Fu Liao, and Sheng-Chun Chiu. 2017. "Tanshinone IIA Inhibits Epithelial-Mesenchymal Transition in Bladder Cancer Cells via Modulation of STAT3-CCL2 Signaling" International Journal of Molecular Sciences 18, no. 8: 1616. https://doi.org/10.3390/ijms18081616
APA StyleHuang, S. -Y., Chang, S. -F., Liao, K. -F., & Chiu, S. -C. (2017). Tanshinone IIA Inhibits Epithelial-Mesenchymal Transition in Bladder Cancer Cells via Modulation of STAT3-CCL2 Signaling. International Journal of Molecular Sciences, 18(8), 1616. https://doi.org/10.3390/ijms18081616