Association between Mastication, the Hippocampus, and the HPA Axis: A Comprehensive Review
Abstract
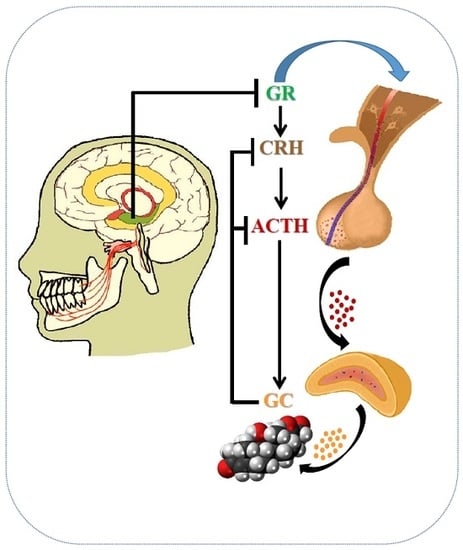
1. Introduction
2. Masticatory Dysfunction Activates the Hypothalamic-Pituitary-Adrenal (HPA) Axis
2.1. Occlusal Disharmony and the HPA Axis
2.2. Tooth Extraction and the HPA Axis
2.3. Masticatory Dysfunction and Hippocampal Function
2.4. Molecular Links between Masticatory Dysfunction and HPA Axis Hyperactivity
3. Masticatory Stimulation Preserves Hippocampal Function
4. Masticatory Stimulation Attenuates HPA Axis Activation
5. Conclusions
Acknowledgments
Conflicts of Interest
References
- McCarty, R. Learning about stress: Neural, endocrine and behavioral adaptations. Stress 2016, 19, 449–475. [Google Scholar] [CrossRef] [PubMed]
- Herman, J.P.; McKlveen, J.M.; Ghosal, S.; Kopp, B.; Wulsin, A.; Makinson, R.; Scheimann, J.; Myers, B. Regulation of the hypothalamic-pituitary-adrenocortical stress response. Compr. Physiol. 2016, 6, 603–621. [Google Scholar] [PubMed]
- Myers, B.; McKlveen, J.M.; Herman, J.P. Neural regulation of the stress response: The many faces of feedback. Cell. Mol. Neurobiol. 2012, 32, 683–694. [Google Scholar] [CrossRef] [PubMed]
- Herman, J.P. Neural control of chronic stress adaptation. Front. Behav. Neurosci. 2013, 7, 61. [Google Scholar] [CrossRef] [PubMed]
- Aguilera, G. HPA axis responsiveness to stress: Implications for healthy aging. Exp. Gerontol. 2011, 46, 90–95. [Google Scholar] [CrossRef] [PubMed]
- Chrousos, G.P. Stress and disorders of the stress system. Nat. Rev. Endocrinol. 2009, 5, 374–381. [Google Scholar] [CrossRef] [PubMed]
- Aguilera, G.; Liu, Y. The molecular physiology of CRH neurons. Front. Neuroendocrinol. 2012, 33, 67–84. [Google Scholar] [CrossRef] [PubMed]
- Gold, P.W.; Chrousos, G.P. Organization of the stress system and its dysregulation in melancholic and atypical depression: High vs. low CRH/NE states. Mol. Psychiatry 2002, 7, 254–275. [Google Scholar] [CrossRef] [PubMed]
- Herman, J.P.; Tasker, J.G. Paraventricular hypothalamic mechanisms of chronic stress adaptation. Front. Endocrinol. 2016, 7, 137. [Google Scholar] [CrossRef] [PubMed]
- Chung, S.; Son, G.H.; Kim, K. Circadian rhythm of adrenal glucocorticoid: Its regulation and clinical implications. Biochim. Biophys. Acta 2011, 1812, 581–591. [Google Scholar] [CrossRef] [PubMed]
- Oakley, R.H.; Cidlowski, J.A. The biology of the glucocorticoid receptor: New signaling mechanisms in health and disease. J. Allergy Clin. Immunol. 2013, 132, 1033–1044. [Google Scholar] [CrossRef] [PubMed]
- Finsterwald, C.; Alberini, C.M. Stress and glucocorticoid receptor-dependent mechanisms in long-term memory: From adaptive responses to psychopathologies. Neurobiol. Learn. Mem. 2014, 112, 17–29. [Google Scholar] [CrossRef] [PubMed]
- Kuo, T.; McQueen, A.; Chen, T.C.; Wang, J.C. Regulation of glucose homeostasis by glucocorticoids. Adv. Exp. Med. Biol. 2015, 872, 99–126. [Google Scholar] [PubMed]
- Fischer, A.; Gluth, M.; Weege, F.; Pape, U.F.; Wiedenmann, B.; Baumgart, D.C.; Theuring, F. Glucocorticoids regulate barrier function and claudin expression in intestinal epithelial cells via MKP-1. Am. J. Physiol. Gastrointest. Liver Physiol. 2014, 306, G218–G228. [Google Scholar] [CrossRef] [PubMed]
- Kubo, K.Y.; Iinuma, M.; Azuma, K. Mastication as a stress-coping behavior. BioMed Res. Int. 2015, 2015, 876409. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Iinuma, M.; Onozuka, M.; Kubo, K.Y. Chewing maintains hippocampus-dependent cognitive function. Int. J. Med. Sci. 2015, 12, 502–509. [Google Scholar] [CrossRef] [PubMed]
- Ono, Y.; Yamamoto, T.; Kubo, K.Y.; Onozuka, M. Occlusion and brain function: Mastication as a prevention of cognitive dysfunction. J. Oral Rehabil. 2010, 37, 624–640. [Google Scholar] [CrossRef] [PubMed]
- Proietti, R.; Mapelli, D.; Volpe, B.; Bartoletti, S.; Sagone, A. Mental stress and ischemic heart diseases: Evolving awareness of a complex association. Future Cardiol. 2011, 7, 425–437. [Google Scholar] [CrossRef] [PubMed]
- Reber, S.O. Stress and animal models of inflammatory bowel disease-an update on the role of the hypothalamic-pituitary-adrenal axis. Psychoneuroendocrinology 2012, 37, 1–19. [Google Scholar] [CrossRef] [PubMed]
- Furuzawa, M.; Chen, H.; Fujiwara, S.; Yamada, K.; Kubo, K.Y. Chewing ameliorates chronic mild stress-induced bone loss in senescence-accelerated mouse (SAMP8), a murine model of senile osteoporosis. Exp. Gerontol. 2014, 55, 12–18. [Google Scholar] [CrossRef] [PubMed]
- Azuma, K.; Ogura, M.; Kondo, H.; Suzuki, A.; Hayashi, S.; Iinuma, M.; Kubo, K.Y. Maternal active mastication during prenatal stress ameliorates prenatal stress-induced lower bone mass in adult mouse offspring. Int. J. Med. Sci. 2017, 14, 348–355. [Google Scholar] [CrossRef] [PubMed]
- Onishi, M.; Iinuma, M.; Tamura, Y.; Kubo, K.Y. Learning deficits and suppression of the cell proliferation in the hippocampal dentate gyrus of offspring are attenuated by maternal chewing during prenatal stress. Neurosci. Lett. 2014, 560, 77–80. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, A.; Iinuma, M.; Hayashi, S.; Sato, Y.; Azuma, K.; Kubo, K.Y. Maternal chewing during prenatal stress ameliorates stress-induced hypomyelination, synaptic alterations, and learning impairment in mouse offspring. Brain Res. 2016, 1651, 36–43. [Google Scholar] [CrossRef] [PubMed]
- Shimazaki, Y.; Soh, I.; Saito, T.; Yamashita, Y.; Koga, T.; Miyazaki, H.; Takehara, T. Influence of dentition status on physical disability, mental impairment, and mortality in institutionalized elderly people. J. Dent. Res. 2001, 80, 340–345. [Google Scholar] [CrossRef] [PubMed]
- Avlund, K.; Holm-Pedersen, P.; Schroll, M. Functional ability and oral health among older people: A longitudinal study from age 75 to 80. J. Am. Geriatr. Soc. 2001, 49, 954–962. [Google Scholar] [CrossRef] [PubMed]
- Miura, H.; Araki, Y.; Umenai, T. Chewing activity and activities of daily living in the elderly. J. Oral Rehabil. 1997, 24, 457–460. [Google Scholar] [CrossRef] [PubMed]
- Teixeira, F.B.; Pereira Fernandes, L.M.; Noronha, P.A.; dos Santos, M.A.; Gomes-Leal, W.; Ferraz Maia, C.S.; Lima, R.R. Masticatory deficiency as a risk factor for cognitive dysfunction. Int. J. Med. Sci. 2014, 11, 209–214. [Google Scholar] [CrossRef] [PubMed]
- Wu, B.; Fillenbaum, G.G.; Plassman, B.L.; Guo, L. Association between oral health and cognitive status: A systematic review. J. Am. Geriatr. Soc. 2016, 64, 739–751. [Google Scholar] [CrossRef] [PubMed]
- Roohafza, H.; Afshar, H.; Keshteli, A.H.; Shirani, M.J.; Afghari, P.; Vali, A.; Adibi, P. Masticatory ability with depression, anxiety, and stress: Does there exist any association? Dent. Res. J. 2016, 13, 211–216. [Google Scholar]
- Listl, S. Oral health conditions and cognitive functioning in middle and later adulthood. BMC Oral Health 2014, 14, 70. [Google Scholar] [CrossRef] [PubMed]
- Hansson, P.; Sunnegårdh-Grönberg, K.; Bergdahl, J.; Bergdahl, M.; Nyberg, L.; Nilsson, L.G. Relationship between natural teeth and memory in a healthy elderly population. Eur. J. Oral Sci. 2013, 121, 333–340. [Google Scholar] [CrossRef] [PubMed]
- Lexomboon, D.; Trulsson, M.; Wårdh, I.; Parker, M.G. Chewing ability and tooth loss: Association with cognitive impairment in an elderly population study. J. Am. Geriatr. Soc. 2012, 60, 1951–1956. [Google Scholar] [CrossRef] [PubMed]
- Weijenberg, R.A.; Lobbezoo, F.; Knol, D.L.; Tomassen, J.; Scherder, E.J. Increased masticatory activity and quality of life in elderly persons with dementia—A longitudinal matched cluster randomized single-blind multicenter intervention study. BMC Neurol. 2013, 16, 26. [Google Scholar] [CrossRef] [PubMed]
- Onozuka, M.; Watanabe, K.; Mirbod, S.M.; Ozono, S.; Nishiyama, K.; Karasawa, N.; Nagatsu, I. Reduced mastication stimulates impairment of spatial memory and degeneration of hippocampal neurons in aged SAMP8 mice. Brain Res. 1999, 826, 148–153. [Google Scholar] [CrossRef]
- Onozuka, M.; Watanabe, K.; Nagasaki, S.; Jiang, Y.; Ozono, S.; Nishiyama, K.; Kawase, T.; Karasawa, N.; Nagatsu, I. Impairment of spatial memory and changes in astroglial responsiveness following loss of molar teeth in aged SAMP8 mice. Behav. Brain Res. 2000, 108, 145–155. [Google Scholar] [CrossRef]
- Watanabe, K.; Tonosaki, K.; Kawase, T.; Karasawa, N.; Nagatsu, I.; Fujita, M.; Onozuka, M. Evidence for involvement of dysfunctional teeth in the senile process in the hippocampus of SAMP8 mice. Exp. Gerontol. 2001, 36, 283–295. [Google Scholar] [CrossRef]
- Kubo, K.Y.; Yamada, Y.; Iinuma, M.; Iwaku, F.; Tamura, Y.; Watanabe, K.; Nakamura, H.; Onozuka, M. Occlusal disharmony induces spatial memory impairment and hippocampal neuron degeneration via stress in SAMP8 mice. Neurosci. Lett. 2007, 414, 188–191. [Google Scholar] [CrossRef] [PubMed]
- Ichihashi, Y.; Arakawa, Y.; Iinuma, M.; Tamura, Y.; Kubo, K.Y.; Iwaku, F.; Sato, Y.; Onozuka, M. Occlusal disharmony attenuates glucocorticoid negative feedback in aged SAMP8 mice. Neurosci. Lett. 2007, 427, 71–76. [Google Scholar] [CrossRef] [PubMed]
- Chrousos, G.P.; Gold, P.W. The concepts of stress and stress system disorders: Overview of physical and behavioral homeostasis. JAMA 1992, 267, 1244–1252. [Google Scholar] [CrossRef] [PubMed]
- Miura, H.H.; Kariyasu, M.; Yamasaki, K.; Arai, Y.; Sumi, Y. Relationship between general health status and the change in chewing ability: A longitudinal study of the frail elderly in Japan over a 3-year period. Gerodontology 2005, 22, 200–205. [Google Scholar] [CrossRef] [PubMed]
- Budtz-Jørgensen, E. Occlusal dysfunction and stress. An experimental study in macaque monkeys. J. Oral Rehabil. 1981, 8, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Yoshihara, T.; Matsumoto, Y.; Ogura, T. Occlusal disharmony affects plasma corticosterone and hypothalamic noradrenaline release in rats. J. Dent. Res. 2001, 80, 2089–2092. [Google Scholar] [CrossRef] [PubMed]
- Kubo, K.Y.; Kojo, A.; Yamamoto, T.; Onozuka, M. The bite-raised condition in aged SAMP8 mice induces dendritic spine changes in the hippocampal region. Neurosci. Lett. 2008, 441, 141–144. [Google Scholar] [CrossRef] [PubMed]
- Katayama, T.; Mori, D.; Miyake, H.; Fujiwara, S.; Ono, Y.; Takahashi, T.; Onozuka, M.; Kubo, K.Y. Effect of bite-raised condition on the hippocampal cholinergic system of aged SAMP8 mice. Neurosci. Lett. 2012, 520, 77–81. [Google Scholar] [CrossRef] [PubMed]
- Mori, D.; Katayama, T.; Miyake, H.; Fujiwara, S.; Kubo, K.Y. Occlusal disharmony leads to learning deficits associated with decreased cellular proliferation in the hippocampal dentate gyrus of SAMP8 mice. Neurosci. Lett. 2013, 534, 228–232. [Google Scholar] [CrossRef] [PubMed]
- Miyake, H.; Mori, D.; Katayama, T.; Fujiwara, S.; Sato, Y.; Azuma, K.; Kubo, K.Y. Novel stress increases hypothalamic-pituitary-adrenal activity in mice with a raised bite. Arch. Oral Biol. 2016, 68, 55–60. [Google Scholar] [CrossRef] [PubMed]
- Mori, D.; Miyake, H.; Mizutani, K.; Shimpo, K.; Sonoda, S.; Yamamoto, T.; Fujiwara, S.; Kubo, K.Y. Effects of occlusal disharmony on the hippocampal dentate gyrus in aged senescence-accelerated mouse prone 8 (SAMP8). Arch. Oral Biol. 2016, 65, 95–101. [Google Scholar] [CrossRef] [PubMed]
- Muramoto, T.; Takano, Y.; Soma, K. Time-related changes in periodontal mechanoreceptors in rat molars after the loss of occlusal stimuli. Arch. Histol. Cytol. 2000, 63, 369–380. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, Y.; Tanaka, S.; Tsubone, H.; Atoji, Y.; Suzuki, Y. Age-related changes in sensory and secretomotor nerve endings in the larynx of F344/N rat. Arch. Gerontol. Geriatr. 2003, 36, 173–183. [Google Scholar] [CrossRef]
- Watanabe, K.; Ozono, S.; Nishiyama, K.; Saito, S.; Tonosaki, K.; Fujita, M.; Onozuka, M. The molarless condition in aged SAMP8 mice attenuates hippocampal Fos induction linked to water maze performance. Behav. Brain Res. 2002, 128, 19–25. [Google Scholar] [CrossRef]
- Onozuka, M.; Watanabe, K.; Fujita, M.; Tonosaki, K.; Saito, S. Evidence for involvement of glucocorticoid response in the hippocampal changes in aged molarless SAMP8 mice. Behav. Brain Res. 2002, 131, 125–129. [Google Scholar] [CrossRef]
- Onozuka, M.; Watanabe, K.; Fujita, M.; Tomida, M.; Ozono, S. Changes in the septohippocampal cholinergic system following removal of molar teeth in the aged SAMP8 mouse. Behav. Brain Res. 2002, 133, 197–204. [Google Scholar] [CrossRef]
- Kubo, K.Y.; Iwaku, F.; Watanabe, K.; Fujita, M.; Onozuka, M. Molarless-induced changes of spines in hippocampal region of SAMP8 mice. Brain Res. 2005, 1057, 191–195. [Google Scholar] [CrossRef] [PubMed]
- Aoki, H.; Kimoto, K.; Hori, N.; Hoshi, N.; Yamamoto, T.; Onozuka, M. Molarless condition suppresses proliferation but not differentiation rates into neurons in the rat dentate gyrus. Neurosci. Lett. 2010, 469, 44–48. [Google Scholar] [CrossRef] [PubMed]
- Iinuma, M.; Kondo, H.; Kurahashi, M.; Ohnishi, M.; Tamura, Y.; Chen, H.; Kubo, K.Y. Relationship between the early toothless condition and hippocampal functional morphology. Anat. Physiol. 2014, 4, 563–569. [Google Scholar] [CrossRef]
- Kawahata, M.; Ono, Y.; Ohno, A.; Kawamoto, S.; Kimoto, K.; Onozuka, M. Loss of molars early in life develops behavioral lateralization and impairs hippocampus-dependent recognition memory. BMC Neurosci. 2014, 15, 4. [Google Scholar] [CrossRef] [PubMed]
- Kurahashi, M.; Kondo, H.; Iinuma, M.; Tamura, Y.; Chen, H.; Kubo, K.Y. Tooth loss early in life accelerates age-related bone deterioration in mice. Tohoku J. Exp. Med. 2015, 235, 29–37. [Google Scholar] [CrossRef] [PubMed]
- Kondo, H.; Kurahashi, M.; Mori, D.; Iinuma, M.; Tamura, Y.; Mizutani, K.; Shimpo, K.; Sonoda, S.; Azuma, K.; Kubo, K.Y. Hippocampus-dependent spatial memory impairment due to molar tooth loss is ameliorated by an enriched environment. Arch. Oral Biol. 2016, 61, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Kubo, K.Y.; Murabayashi, C.; Kotachi, M.; Suzuki, A.; Mori, D.; Sato, Y.; Onozuka, M.; Azuma, K.; Iinuma, M. Tooth loss early in life suppresses neurogenesis and synaptophysin expression in the hippocampus and impairs learning in mice. Arch. Oral Biol. 2017, 74, 21–27. [Google Scholar] [CrossRef] [PubMed]
- Heard, E.; Martienssen, R.A. Transgenerational epigenetic inheritance: Myths and mechanisms. Cell 2014, 157, 95–109. [Google Scholar] [CrossRef] [PubMed]
- Konkle, A.T.; Baker, S.L.; Kentner, A.C.; Barbagallo, L.S.; Merali, Z.; Bielajew, C. Evaluation of the effects of chronic mild stressors on hedonic and physiological responses: Sex and strain compared. Brain Res. 2003, 992, 227–238. [Google Scholar] [CrossRef] [PubMed]
- Yirmiya, R.; Goshen, I.; Bajayo, A.; Kreisel, T.; Feldman, S.; Tam, J.; Trembovler, V.; Csernus, V.; Shohami, E.; Bab, I. Depression induces bone loss through stimulation of the sympathetic nervous system. Proc. Natl. Acad. Sci. USA 2006, 103, 16876–16881. [Google Scholar] [CrossRef] [PubMed]
- Kubo, K.Y.; Chen, H.; Onozuka, M. The relationship between mastication and cognition. In Senescence and Senescence-Related Disorders; Wang, Z., Inuzuka, H., Eds.; InTach: Rijeka, Croatia, 2013; pp. 115–132. [Google Scholar]
- Onozuka, M.; Hirano, Y.; Tachibana, A.; Kim, W.; Ono, Y.; Sasaguri, K.; Kubo, K.Y.; Niwa, M.; Kanematsu, K.; Watanabe, K. Interactions between chewing and brain activities in human. In Novel Trends in Brain Science; Onozula, M., Yen, C.T., Eds.; Springer: Tokyo, Japan, 2008; pp. 99–113. [Google Scholar]
- Jonas, P.; Lisman, J. Structure, function, and plasticity of hippocampal dentate gyrus microcircuits. Front. Neural Circuit 2014, 8, 107. [Google Scholar] [CrossRef] [PubMed]
- Snyder, J.S.; Soumier, A.; Brewer, M.; Pickel, J.; Cameron, H.A. Adult hippocampal neurogenesis buffers stress responses and depressive behaviour. Nature 2011, 476, 458–461. [Google Scholar] [CrossRef] [PubMed]
- Strong, R.; Hicks, P.; Hsu, L.; Bartus, R.T.; Enna, S.J. Age-related alterations in the rodent brain cholinergic system and behavior. Neurobiol. Aging 1980, 1, 59–63. [Google Scholar] [CrossRef]
- Gilad, G.M. The stress-induced response of the septo-hippocampal cholinergic system. A vectorial outcome of psychoneuroendocrinological interactions. Psychoneuroendocrinology 1987, 12, 167–184. [Google Scholar] [CrossRef]
- Hu, Y.; Zhu, D.Y. Hippocampus and nitric oxide. Vitam. Horm. 2014, 96, 127–160. [Google Scholar] [PubMed]
- Zhu, L.J.; Liu, M.Y.; Li, H.; Liu, X.; Chen, C.; Han, Z.; Wu, H.Y.; Jing, X.; Zhou, H.H.; Suh, H.; et al. The different roles of glucocorticoids in the hippocampus and hypothalamus in chronic stress-induced HPA axis hyperactivity. PLoS ONE 2014, 9, e97589. [Google Scholar] [CrossRef] [PubMed]
- De Kloet, E.R.; Vreugdenhil, E.; Oitzl, M.S.; Joëls, M. Brain corticosteroid receptor balance in health and disease. Endocr. Rev. 1998, 19, 269–301. [Google Scholar] [CrossRef] [PubMed]
- Reul, J.M.; de Kloet, E.R. Two receptor systems for corticosterone in rat brain: Microdistribution and differential occupation. Endocrinology 1985, 117, 2505–2511. [Google Scholar] [CrossRef] [PubMed]
- Veenema, A.H.; Meijer, O.C.; de Kloet, E.R.; Koolhaas, J.M.; Bohus, B.G. Differences in basal and stress-induced HPA regulation of wild house mice selected for high and low aggression. Horm. Behav. 2003, 43, 197–204. [Google Scholar] [CrossRef]
- Zhou, Q.G.; Hu, Y.; Hua, Y.; Hu, M.; Luo, C.X.; Han, X.; Zhu, X.J.; Wang, B.; Xu, J.S.; Zhu, D.Y. Neuronal nitric oxide synthase contributes to chronic stress-induced depression by suppressing hippocampal neurogenesis. J. Neurochem. 2007, 103, 1843–1854. [Google Scholar] [CrossRef] [PubMed]
- Calabrese, V.; Mancuso, C.; Calvani, M.; Rizzarelli, E.; Butterfield, D.A.; Stella, A.M. Nitric oxide in the central nervous system: Neuroprotection versus neurotoxicity. Nat. Rev. Neurosci. 2007, 8, 766–775. [Google Scholar] [CrossRef] [PubMed]
- Meyer, U.; van Kampen, M.; Isovich, E.; Flügge, G.; Fuchs, E. Chronic psychosocial stress regulates the expression of both GR and MR mRNA in the hippocampal formation of tree shrews. Hippocampus 2001, 11, 329–336. [Google Scholar] [CrossRef] [PubMed]
- Juruena, M.F. Early-life stress and HPA axis trigger recurrent adulthood depression. Epilepsy Behav. 2014, 38, 148–159. [Google Scholar] [CrossRef] [PubMed]
- Porter, R.J.; Gallagher, P.; Watson, S.; Young, A.T. Corticosteroid-serotonin interactions in depression: A review of the human evidence. Psychopharmacology 2004, 173, 1–17. [Google Scholar] [CrossRef] [PubMed]
- McEuen, J.G.; Beck, S.G.; Bale, T.L. Failure to mount adaptive responses to stress results in dysregulation and cell death in the midbrain raphe. J. Neurosci. 2008, 28, 8169–8177. [Google Scholar] [CrossRef] [PubMed]
- Savitz, J.; Lucki, I.; Drevets, W.C. 5-TH1A receptor function in major depressive disorder. Prog. Neurobiol. 2009, 88, 17–31. [Google Scholar] [CrossRef] [PubMed]
- Chen, G.L.; Miller, G.M. Advances in tryptophan hydroxylase-2 gene expression regulation: New insights into serotonin-stress interaction and clinical implications. Am. J. Med. Genet. B Neuropsychiatr. Genet. 2012, 159B, 152–171. [Google Scholar] [CrossRef] [PubMed]
- Clark, J.A.; Flick, R.B.; Pai, L.Y.; Szalayova, I.; Key, S.; Conley, R.K.; Deutch, A.Y.; Hutson, P.H.; Mezey, E. Glucocorticoid modulation of tryptophan hydroxylase-2 protein in raphe nuclei and 5-hydroxytryptophan concentrations in frontal cortex of C57/BI6 mice. Mol. Psychiatry 2008, 13, 498–506. [Google Scholar] [CrossRef] [PubMed]
- Konno, M.; Takeda, T.; Kawakami, Y.; Suzuki, Y.; Kawano, Y.; Nakajima, K.; Ozawa, T.; Ishigami, K.; Takemura, N.; Sakatani, K. Relationships Between Gum-Chewing and Stress. Adv. Exp. Med. Biol. 2016, 876, 343–349. [Google Scholar] [PubMed]
- Hasegawa, Y.; Tachibana, Y.; Sakagami, J.; Zhang, M.; Urade, M.; Ono, T. Flavor-enhanced modulation of cerebral blood flow during gum chewing. PLoS ONE 2013, 8, e66313. [Google Scholar] [CrossRef]
- Utsugi, C.; Miyazono, S.; Osada, K.; Sasajima, H.; Noguchi, T.; Matsuda, M.; Kashiwayanagi, M. Hard-diet feeding recovers neurogenesis in the subventricular zone and olfactory functions of mice impaired by soft-diet feeding. PLoS ONE 2014, 9, e97309. [Google Scholar] [CrossRef] [PubMed]
- Ichihashi, Y.; Saito, N.; Arakawa, Y.; Kurata, C.; Iinuma, M.; Tamura, Y.; Iwaku, F.; Kubo, K.Y. The bite-raised condition in aged SAMP8 mice reduces the expression of glucocorticoid receptors in the dorsal and ventral hippocampus. Okajimas Folia Anat. Jpn. 2008, 84, 137–142. [Google Scholar] [CrossRef] [PubMed]
- Sasaguri, K.; Yoshikawa, G.; Yamada, K.; Miyake, S.; Kubo, K.Y.; Yamamoto, T. Combination of chewing and stress up-regulates hippocampal glucocorticoid receptor in contrast to the increase of mineralocorticoid receptor under stress only. Neurosci. Lett. 2012, 519, 20–25. [Google Scholar] [CrossRef] [PubMed]
- Miyake, S.; Yoshikawa, G.; Yamada, K.; Sasaguri, K.; Yamamoto, T.; Onozuka, M.; Sato, S. Chewing ameliorates stress-induced suppression of spatial memory by increasing glucocorticoid receptor expression in the hippocampus. Brain Res. 2012, 1446, 34–39. [Google Scholar] [CrossRef] [PubMed]
- Brown, R.E.; Stevens, D.R.; Haas, H.L. The physiology of brain histamine. Prog. Neurobiol. 2001, 63, 637–672. [Google Scholar] [CrossRef]
- Fujise, T.; Yoshimatsu, H.; Kurokawa, M.; Oohara, A.; Kang, M.; Nakata, M.; Sakata, T. Satiation and masticatory function modulated by brain histamine in rats. Proc. Soc. Exp. Biol. Med. 1998, 217, 228–234. [Google Scholar] [CrossRef] [PubMed]
- Sakata, T.; Yoshimatsu, H.; Masaki, T.; Tsuda, K. Anti-obesity actions of mastication driven by histamine neurons in rats. Exp. Biol. Med. 2003, 228, 1106–1110. [Google Scholar] [CrossRef]
- Mochizuki, T.; Okakura-Mochizuki, K.; Horii, A.; Yamamoto, Y.; Yamatodani, A. Histaminergic modulation of hippocampal acetylcholine release in vivo. J. Neurochem. 1994, 62, 2275–2282. [Google Scholar] [CrossRef] [PubMed]
- Ono, Y.; Kataoka, T.; Miyake, S.; Sasaguri, K.; Sato, S.; Onozuka, M. Chewing rescues stress-suppressed hippocampal long-term potentiation via activation of histamine H1 receptor. Neurosci. Res. 2009, 64, 385–390. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, T.; Yoshida, M.; Yokoo, H.; Tomita, M.; Tanaka, M. Expression of aggression attenuates both stress-induced gastric ulcer formation and increases in noradrenaline release in the rat amygdala assessed by intracerebral microdialysis. Pharmacol. Biochem. Behav. 1998, 59, 27–31. [Google Scholar] [CrossRef]
- Tsuda, A.; Tanaka, M.; Ida, Y.; Shirao, I.; Gond, Y.; Oguchi, M.; Yoshida, M. Expression of aggression attenuates stress-induced increases in rat brain noradrenaline turnover. Brain Res. 1988, 474, 174–180. [Google Scholar] [CrossRef]
- Stalnaker, T.A.; España, R.A.; Berridge, C.W. Coping behavior causes asymmetric changes in neuronal activation in the prefrontal cortex and amygdala. Synapse 2009, 63, 82–85. [Google Scholar] [CrossRef] [PubMed]
- Berridge, C.W.; Mitton, E.; Clark, W.; Roth, R.H. Engagement in a non-escape (displacement) behavior elicits a selective and lateralized suppression of frontal cortical dopaminergic utilization in stress. Synapse 1999, 32, 187–197. [Google Scholar] [CrossRef]
- Gómez, F.M.; Giralt, M.T.; Sainz, B.; Arrúe, A.; Prieto, M.; García-Vallejo, P. A possible attenuation of stress-induced increases in striatal dopamine metabolism by the expression of non-functional masticatory activity in the rat. Eur. J. Oral Sci. 1999, 107, 461–467. [Google Scholar] [CrossRef] [PubMed]
- Ono, Y.; Kataoka, T.; Miyake, S.; Cheng, S.J.; Tachibana, A.; Sasaguri, K.I.; Onozuka, M. Chewing ameliorates stress-induced suppression of hippocampal long-term potentiation. Neuroscience 2008, 154, 1352–1359. [Google Scholar] [CrossRef] [PubMed]
- Lee, T.; Saruta, J.; Sasaguri, K.; Sato, S.; Tsukinoki, K. Allowing animals to bite reverses the effects of immobilization stress on hippocampal neurotrophin expression. Brain Res. 2008, 1195, 43–49. [Google Scholar] [CrossRef] [PubMed]
- Conner, R.L.; Vernikos-Danellis, J.; Levine, S. Stress, fighting and neuroendocrine function. Nature 1971, 234, 564–566. [Google Scholar] [CrossRef] [PubMed]
- Sapolsky, R.M.; Krey, L.C.; McEwen, B.S. Stress down-regulates corticosterone receptors in a site-specific manner in the brain. Endocrinology 1984, 114, 287–292. [Google Scholar] [CrossRef] [PubMed]
- Hori, N.; Yuyama, N.; Tamura, K. Biting suppresses stress-induced expression of corticotropin-releasing factor (CRF) in the rat hypothalamus. J. Dent. Res. 2004, 83, 124–128. [Google Scholar] [CrossRef] [PubMed]
- Goncharova, N.D. Stress responsiveness of the hypothalamic-pituitary-adrenal axis: Age-related features of the vasopressinergic regulation. Front. Endocrinol. 2013, 4, 26. [Google Scholar] [CrossRef] [PubMed]

© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Azuma, K.; Zhou, Q.; Niwa, M.; Kubo, K.-y. Association between Mastication, the Hippocampus, and the HPA Axis: A Comprehensive Review. Int. J. Mol. Sci. 2017, 18, 1687. https://doi.org/10.3390/ijms18081687
Azuma K, Zhou Q, Niwa M, Kubo K-y. Association between Mastication, the Hippocampus, and the HPA Axis: A Comprehensive Review. International Journal of Molecular Sciences. 2017; 18(8):1687. https://doi.org/10.3390/ijms18081687
Chicago/Turabian StyleAzuma, Kagaku, Qian Zhou, Masami Niwa, and Kin-ya Kubo. 2017. "Association between Mastication, the Hippocampus, and the HPA Axis: A Comprehensive Review" International Journal of Molecular Sciences 18, no. 8: 1687. https://doi.org/10.3390/ijms18081687
APA StyleAzuma, K., Zhou, Q., Niwa, M., & Kubo, K.-y. (2017). Association between Mastication, the Hippocampus, and the HPA Axis: A Comprehensive Review. International Journal of Molecular Sciences, 18(8), 1687. https://doi.org/10.3390/ijms18081687