Ghrelin Therapy Decreases Incidents of Intracranial Hemorrhage in Mice after Whole-Body Ionizing Irradiation Combined with Burn Trauma
Abstract
:1. Introduction
2. Results
2.1. Brain Hemorrhagic Lesions in the Radiation Combined Injuries (CI) Model
2.2. Effect of Ghrelin Administration on Incidents of Hemorrhagic Lesions and the Ratio of Index of Hemorrhage Severity (IHS) in the CI Model
2.3. Ghrelin Administration Mitigates Platelet Depletion Caused by Radiation Combined with Burn Trauma (CI)
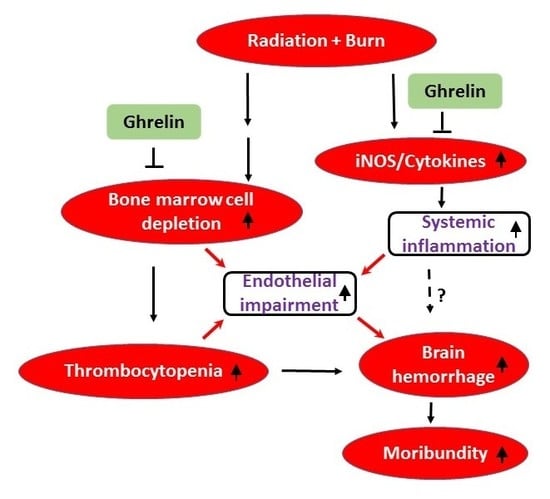
3. Discussion
4. Materials and Methods
4.1. Experimental Design
4.2. Animals
4.3. Gamma Irradiation
4.4. Skin Injury
4.5. Ghrelin Treatment
4.6. Antimicrobial Agents
4.7. Score of Intracranial Hemorrhagic Lesions using Index of Hemorrhage Severity (IHS)
4.8. Histology
4.9. Measurements of Platelet Counts
4.10. Statistical Analysis
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
Abbreviations
| ARS | Acute radiation syndrome |
| CI | Radiation combined injury |
| H-ARS | Hematopoiesis-acute radiation syndrome |
| IR | Ionizing radiation |
| IHS | Index of hemorrhage severity |
| i.p. | Intraperitoneally |
| p.o. | per os |
References
- Lawrence, J.H.; Tennant, R. The comparative effects of neutrons and X-rays on the whole body. J. Exp. Med. 1937, 66, 667–688. [Google Scholar] [CrossRef] [PubMed]
- Mettler, F.A., Jr.; Upton, A.C. Radiation effects on cell and organ systems. In Medical Effects of Ionizing Radiation, 3rd ed.; Mettler, F.A., Upton, W.B., Eds.; Elsevier: Philadelphia, PA, USA, 2008; pp. 17–21. [Google Scholar]
- Potten, C.S.; Grant, H.K. The relationship between ionizing radiation-induced apoptosis and stem cells in the small and large intestine. Br. J. Cancer 1998, 78, 993–1003. [Google Scholar] [CrossRef] [PubMed]
- Siegal, T.; Pfeffer, M.R.; Meltzer, A.; Shezen, E.; Nimrod, A.; Ezov, N.; Ovadia, H. Cellular and secretory mechanisms related to delayed radiation-induced microvessel dysfunction in the spinal cord of rats. Int. J. Radiat. Oncol. Biol. Phys. 1996, 36, 649–659. [Google Scholar] [CrossRef]
- Roth, N.M.; Sontag, M.R.; Kiani, M.F. Early effects of ionizing radiation on the microvascular networks in normal tissue. Radiat. Res. 1999, 151, 270–277. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.Q.; Chen, P.; Haimovitz-Friedman, A.; Reilly, R.M.; Wong, C.S. Endothelial apoptosis initiates acute blood-brain barrier disruption after ionizing radiation. Cancer Res. 2003, 63, 5950–5956. [Google Scholar] [PubMed]
- Barjaktarovic, Z.; Schmaltz, D.; Shyla, A.; Azimzadeh, O.; Schulz, S.; Haagen, J.; Dörr, W.; Sarioglu, H.; Schäfer, A.; Atkinson, M.J.; et al. Radiation-induced signaling results in mitochondrial impairment in mouse heart at 4 weeks after exposure to X-rays. PLoS ONE 2011, 6, e27811. [Google Scholar] [CrossRef] [PubMed]
- Cuomo, J.R.; Sharma, G.K.; Conger, P.D.; Weintraub, N.L. Novel concepts in radiation-induced cardiovascular disease. World J. Cardiol. 2016, 8, 504–519. [Google Scholar] [CrossRef] [PubMed]
- Brook, I.; Elliott, T.B.; Ledney, G.D.; Shoemaker, M.O.; Knudson, GB. Management of postirradiation infection: Lessons learned from animal models. Mil. Med. 2004, 169, 194–197. [Google Scholar] [CrossRef] [PubMed]
- Moroni, M.; Coolbaugh, T.V.; Lombardini, E.; Mitchell, J.M.; Moccia, K.D.; Shelton, L.J.; Nagy, V.; Whitnall, M.H. Hematopoietic radiation syndrome in the Gottingen minipig. Radiat. Res. 2011, 176, 89–101. [Google Scholar] [CrossRef] [PubMed]
- Gorbunov, N.V.; Sharma, P. Protracted Oxidative Alterations in the Mechanism of Hematopoietic Acute Radiation Syndrome. Antioxidants. 2015, 4, 134–152. [Google Scholar] [CrossRef] [PubMed]
- Singh, V.K.; Ducey, E.J.; Brown, D.S.; Whitnall, M.H. A Review of radiation countermeasure work ongoing at the Armed Forces Radiobiology Research Institute. Int. J. Radiat. Biol. 2012, 88, 296–310. [Google Scholar] [CrossRef] [PubMed]
- Kiang, J.G.; Smith, J.T.; Anderson, M.N.; Swift, J.M.; Gupta, P.; Balakathiresan, N.; Maheshwari, R.K. Hemorrhage exacerbates radiation effects on survival, leukocytopenia, thrombopenia, erythropenia, bone marrow cell depletion and hematopoiesis, and inflammation-associated microRNAs expression in kidney. PLoS ONE 2015, 10, e0139271. [Google Scholar] [CrossRef] [PubMed]
- Kiang, J.G.; Jiao, W.; Cary, L.; Mog, S.R.; Elliott, T.B.; Pellmar, T.C.; Ledney, G.D. Wound trauma increases radiation-induced mortality by increasing iNOS, cytokine concentrations, and bacterial infections. Radiat. Res. 2010, 173, 319–332. [Google Scholar] [CrossRef] [PubMed]
- Kiang, J.G.; Garrison, B.R.; Burns, T.M.; Zhai, M.; Dews, I.C.; Ney, P.H.; Fukumoto, R.; Cary, L.H.; Elliott, T.B.; Ledney, G.D. Wound trauma alters ionizing radiation dose assessment. Cell Biosci. 2012, 2, 20. [Google Scholar] [CrossRef] [PubMed]
- Kiang, J.G.; Ledney, G.D. Skin injuries reduce survival and modulate corticosterone, C-reactive protein, complement component 3, IgM, and prostaglandin E2 after whole-body reactor-produced mixed field (n + γ-photons) irradiation. Oxid. Med. Cell. Longev. 2013, 2013, 821541. [Google Scholar] [CrossRef] [PubMed]
- Shah, K.G.; Wu, R.; Jacob, A.; Blau, S.A.; Ji, Y.; Dong, W.; Marini, C.P.; Ravikumar, T.S.; Coppa, G.F.; Wang, P. Human ghrelin ameliorates organ injury and improves survival after radiation injury combined with severe sepsis. Mol. Med. 2009, 15, 407–414. [Google Scholar] [CrossRef] [PubMed]
- Alpen, E.L.; Sheline, G.E. The combined effects of thermal burns and whole body X irradiation on survival time and mortality. Ann. Surg. 1954, 140, 113–118. [Google Scholar] [CrossRef] [PubMed]
- Brook, I.; Ledney, G.D.; Madonna, G.S.; DeBell, R.M.; Walker, R.I. Therapies for radiation injuries: Research perspectives. Mil. Med. 1992, 157, 130–136. [Google Scholar] [PubMed]
- DiCarlo, A.L.; Jackson, I.L.; Shah, J.R.; Czarniecki, C.W.; Maidment, B.W.; Williams, J.P. Development and licensure of medical countermeasures to treat lung damage resulting from a radiological or nuclear incident. Radiat. Res. 2012, 177, 717–721. [Google Scholar] [CrossRef] [PubMed]
- Murayama, T.; Tepper, O.M.; Silver, M.; Ma, H.; Losordo, D.W.; Isner, J.M.; Asahara, T.; Kalka, C. Determination of bone marrow-derived endothelial progenitor cell significance in angiogenic growth factor-induced neovascularization in vivo. Exp. Hematol. 2002, 30, 967–972. [Google Scholar] [CrossRef]
- Baker, D.G.; Krochak, R.J. The response of the microvascular system to radiation: A review. Cancer Investig. 1989, 7, 287–794. [Google Scholar] [CrossRef]
- Kiang, J.G.; Zhai, M.; Liao, P.J.; Elliott, T.B.; Gorbunov, N.V. Ghrelin therapy improves survival after whole-body ionizing irradiation or combined with burn or wound: Amelioration of leukocytopenia, thrombocytopenia, splenomegaly, and bone marrow injury. Oxid. Med. Cell Longev. 2014, 2014, 215858. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Wang, P.; Zheng, H.; Smith, R.G. Ghrelin stimulation of growth hormone release and appetite is mediated through the growth hormone secretagogue receptor. Proc. Natl. Acad. Sci. USA 2004, 101, 4679–4684. [Google Scholar] [CrossRef] [PubMed]
- Spencer, S.J.; Miller, A.A.; Andrews, Z.B. The Role of Ghrelin in Neuroprotection after Ischemic Brain Injury. Brain Sci. 2013, 3, 344–359. [Google Scholar] [CrossRef] [PubMed]
- Qi, L.; Cui, X.; Dong, W.; Barrera, R.; Nicastro, J.; Coppa, G.F.; Wang, P.; Wu, R. Ghrelin atenuates Brain Injury after Traumatic Brain Injury and Uncontrolled Hemorrhagic Shock in Rats. Mol. Med. 2012, 18, 186–193. [Google Scholar] [CrossRef] [PubMed]
- Ahluwalia, A.; Li, A.; Cheng, G.; Deng, X.; Tarnawski, A.S. Reduced ghrelin in endothelial cells plays important mechanistic role in aging-related impairment of angiogenesis. J. Physiol. Pharmacol. 2009, 60, 29–34. [Google Scholar] [PubMed]
- Frago, L.M.; Baquedano, E.; Argente, J.; Chowen, J.A. Neuroprotective Actions of Ghrelin and Growth Hormone Secretagogues. Front. Mol. Neurosci. 2011, 4, 23. [Google Scholar] [CrossRef] [PubMed]
- Johnson, K.G.; Yano, K.; Kato, H. Cerebral vascular disease in Hiroshima, Japan. J. Chron. Dis. 1967, 20, 545–559. [Google Scholar] [CrossRef]
- Fuks, Z.; Persaud, R.S.; Alfieri, A.; McLoughlin, M.; Ehleiter, D.; Schwartz, J.L.; Seddon, A.P.; Cordon-Cardo, C.; Haimovitz-Friedman, A. Basic fibroblast growth factor protects endothelial cells against radiation-induced programmed cell death in vitro and in vivo. Cancer Res. 1994, 54, 2582–2590. [Google Scholar] [PubMed]
- Li, Y.Q.; Ballinger, J.R.; Nordal, R.A.; Su, Z.F.; Wong, C.S. Hypoxia in Radiation-induced Blood-Spinal Cord Barrier Breakdown. Cancer Res. 2001, 61, 3348–3354. [Google Scholar] [PubMed]
- Lyubimova, N.; Hopewell, J.W. Experimental evidence to support the hypothesis that damage to vascular endothelium plays the primary role in the development of late radiation-induced CNS injury. Br. J. Radiol. 2004, 77, 488–492. [Google Scholar] [CrossRef] [PubMed]
- Satyamitra, M.M.; DiCarlo, A.L.; Taliaferro, L. Understanding the Pathophysiology and Challenges of Development of Medical Countermeasures for Radiation-Induced Vascular/Endothelial Cell Injuries: Report of a NIAID Workshop, August 20, 2015. Radiat. Res. 2016, 186, 99–111. [Google Scholar] [CrossRef] [PubMed]
- Jacob, A.; Shah, K.G.; Wu, R.; Wang, P. Ghrelin as a novel therapy for radiation combined injury. Mol. Med. 2010, 16, 137–143. [Google Scholar] [PubMed]
- Tuttolomondo, A.; Pedone, C.; Pinto, A.; Di Raimondo, D.; Fernandez, P.; Di Sciacca, R.; Licata, G. Predictors of outcome in acute ischemic cerebrovascular syndromes: The GIFA study. Int. J. Cardiol. 2008, 125, 391–396. [Google Scholar] [CrossRef] [PubMed]
- Islam, A.; Ghimbovschi, S.; Zhai, M.; Swift, J.M. An Exploration of Molecular Correlates Relevant to Radiation Combined Skin-Burn Trauma. PLoS ONE 2015, 10, e0134827. [Google Scholar] [CrossRef] [PubMed]
- Ledney, G.D.; Elliott, T.B. Combined injury: Factors with potential to impact radiation dose assessments. Health Phys. 2010, 98, 145–152. [Google Scholar] [CrossRef] [PubMed]
- Yelverton, J.T. Pathology scoring system for blast injuries. J. Trauma 1996, 40, S111–S115. [Google Scholar] [CrossRef]
- Badea, A.; Ali-Sharief, A.A.; Johnson, G.A. Morphometric analysis of the C57BL/6J mouse brain. Neuroimage 2007, 37, 683–693. [Google Scholar] [CrossRef] [PubMed]




© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gorbunov, N.V.; Kiang, J.G. Ghrelin Therapy Decreases Incidents of Intracranial Hemorrhage in Mice after Whole-Body Ionizing Irradiation Combined with Burn Trauma. Int. J. Mol. Sci. 2017, 18, 1693. https://doi.org/10.3390/ijms18081693
Gorbunov NV, Kiang JG. Ghrelin Therapy Decreases Incidents of Intracranial Hemorrhage in Mice after Whole-Body Ionizing Irradiation Combined with Burn Trauma. International Journal of Molecular Sciences. 2017; 18(8):1693. https://doi.org/10.3390/ijms18081693
Chicago/Turabian StyleGorbunov, Nikolai V., and Juliann G. Kiang. 2017. "Ghrelin Therapy Decreases Incidents of Intracranial Hemorrhage in Mice after Whole-Body Ionizing Irradiation Combined with Burn Trauma" International Journal of Molecular Sciences 18, no. 8: 1693. https://doi.org/10.3390/ijms18081693
APA StyleGorbunov, N. V., & Kiang, J. G. (2017). Ghrelin Therapy Decreases Incidents of Intracranial Hemorrhage in Mice after Whole-Body Ionizing Irradiation Combined with Burn Trauma. International Journal of Molecular Sciences, 18(8), 1693. https://doi.org/10.3390/ijms18081693