Comparative Studies on Behavioral, Cognitive and Biomolecular Profiling of ICR, C57BL/6 and Its Sub-Strains Suitable for Scopolamine-Induced Amnesic Models
Abstract
:1. Introduction
2. Results
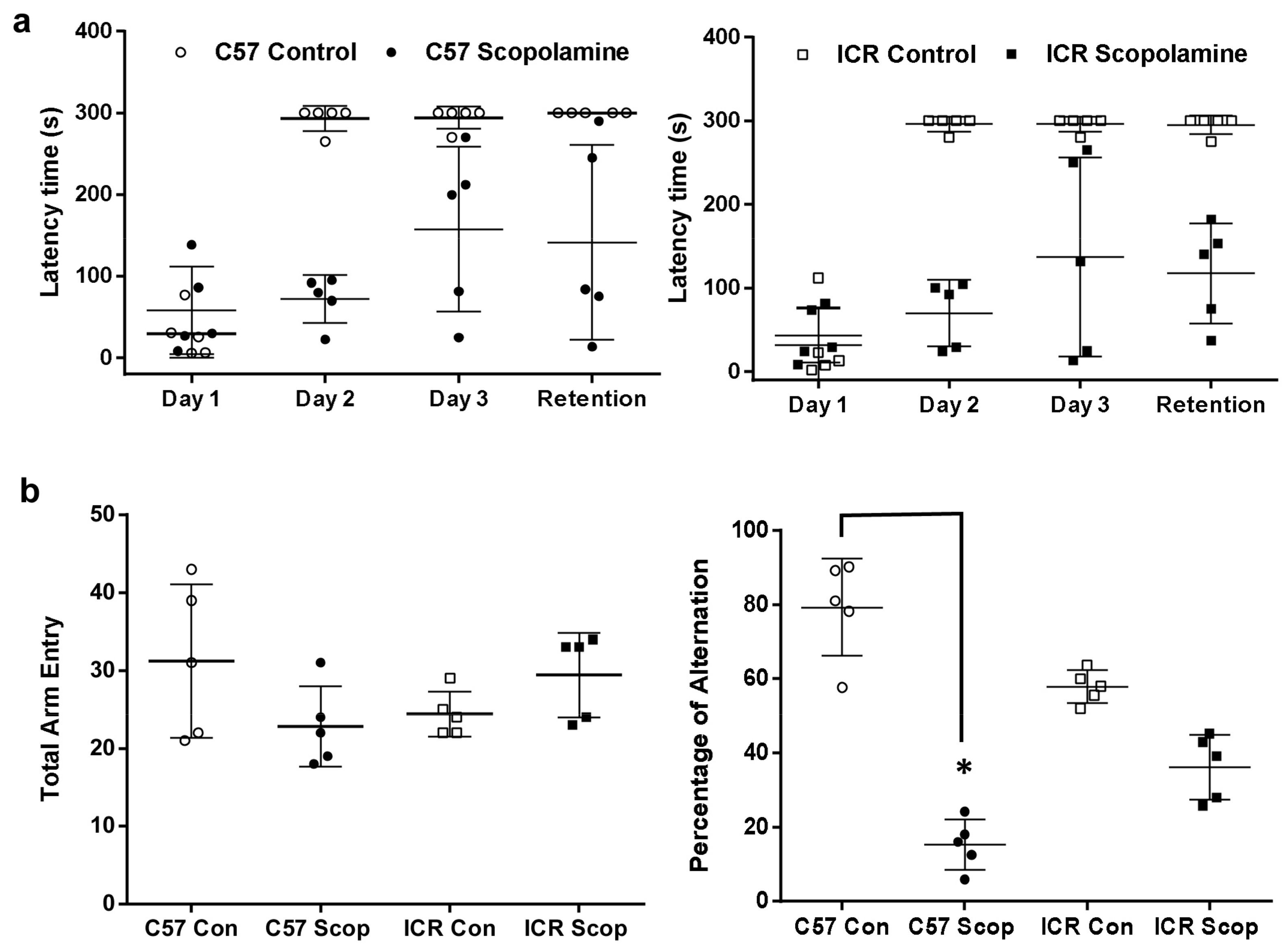
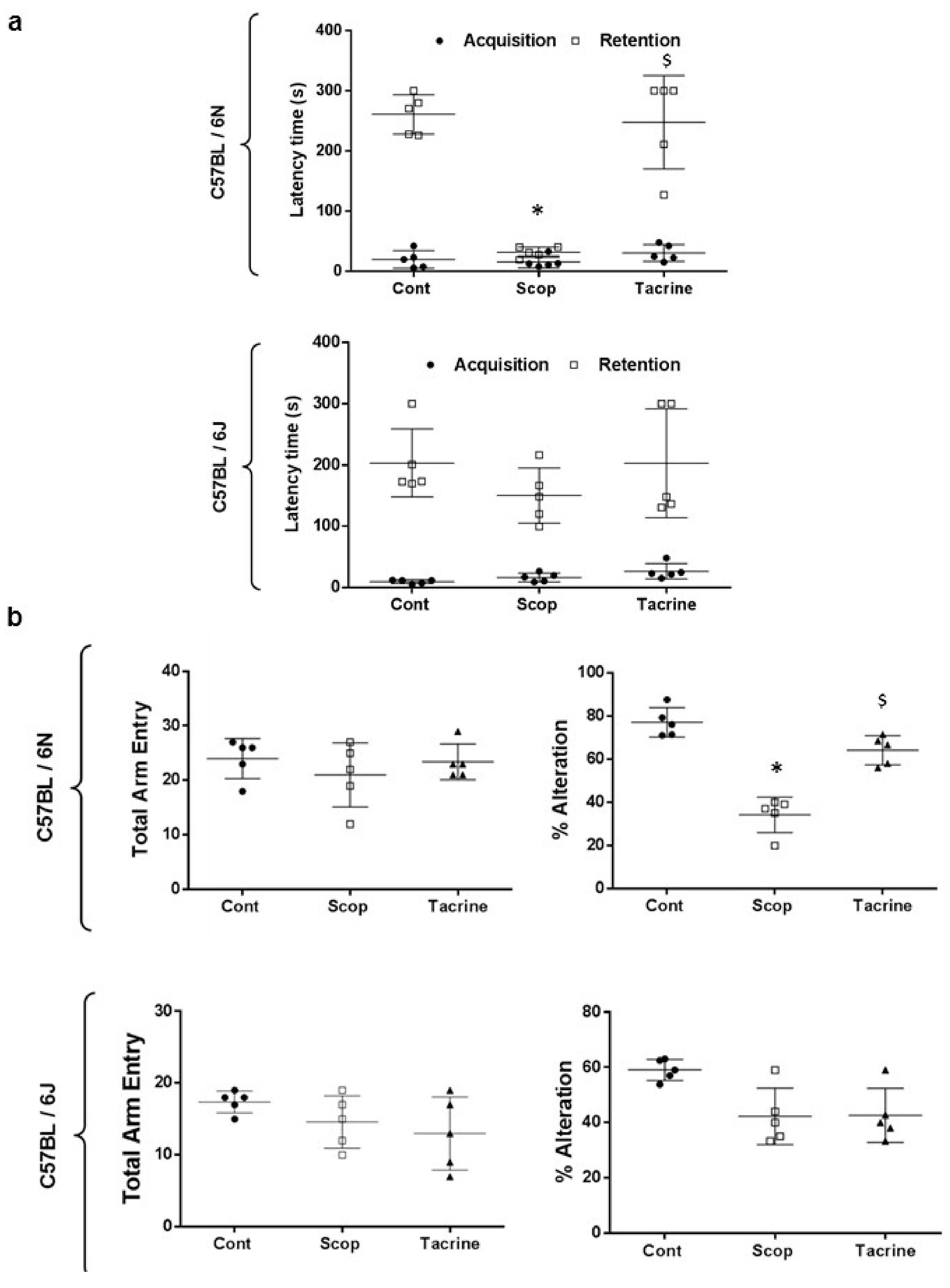
2.1. Modulations on Spatial Learning and Memory Deficits
2.1.1. ICR and C57BL/6 Comparison Study
2.1.2. C57BL/6N and C57BL/6J Comparison Study
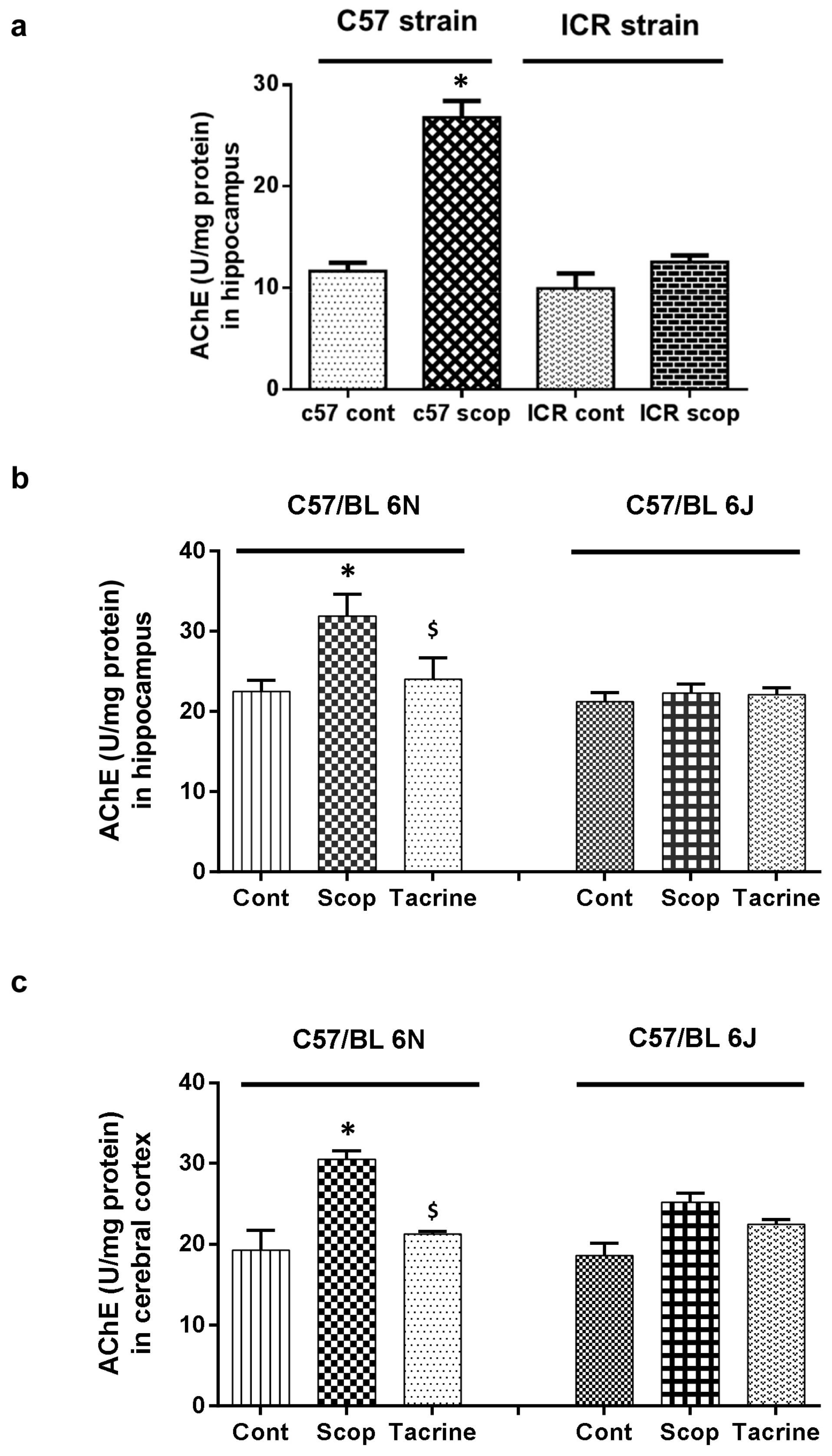
2.2. AChE Activity
2.2.1. ICR and C57BL/6 Comparison Study
2.2.2. C57BL/6N and C57BL/6J Comparison Study
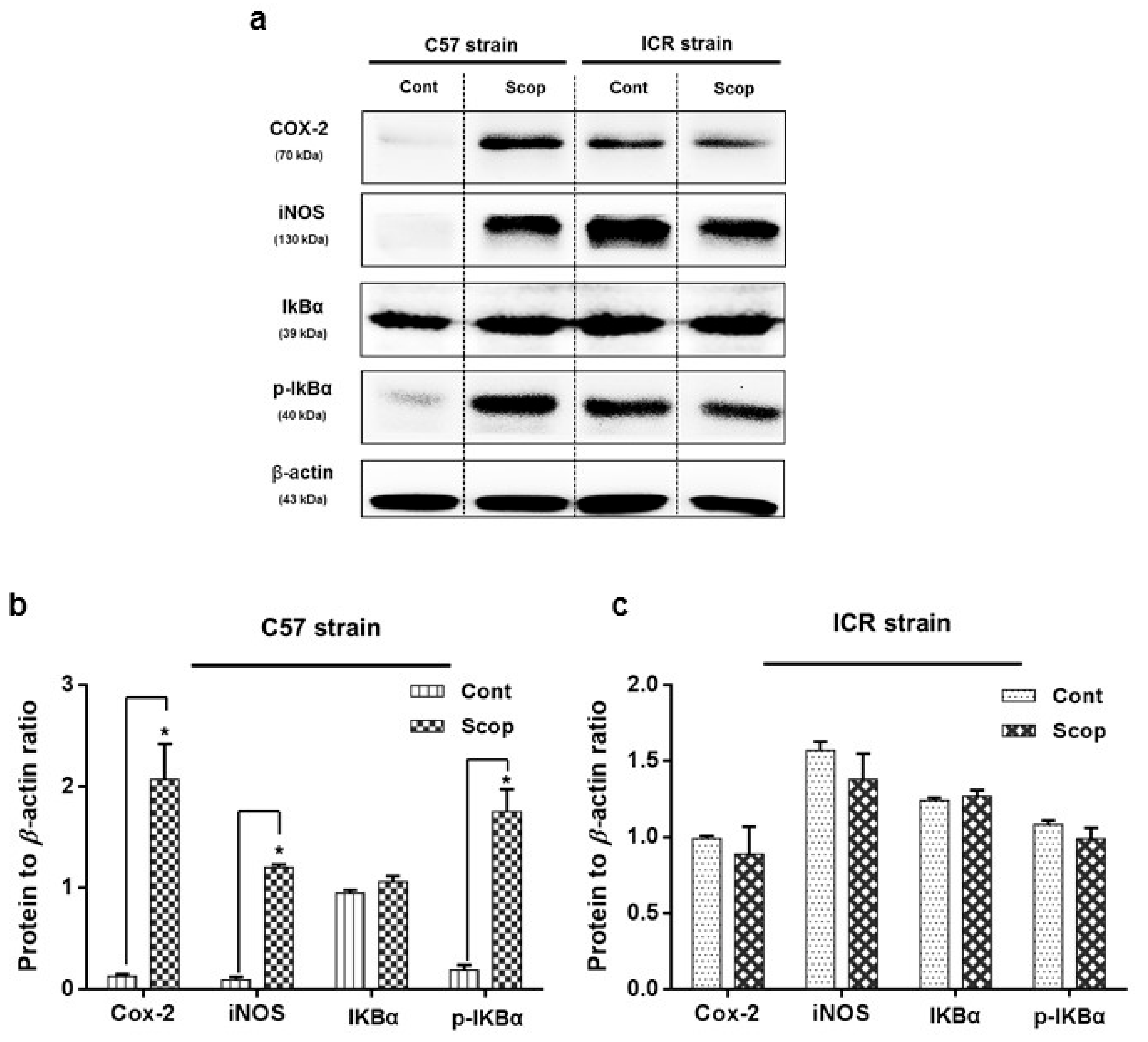
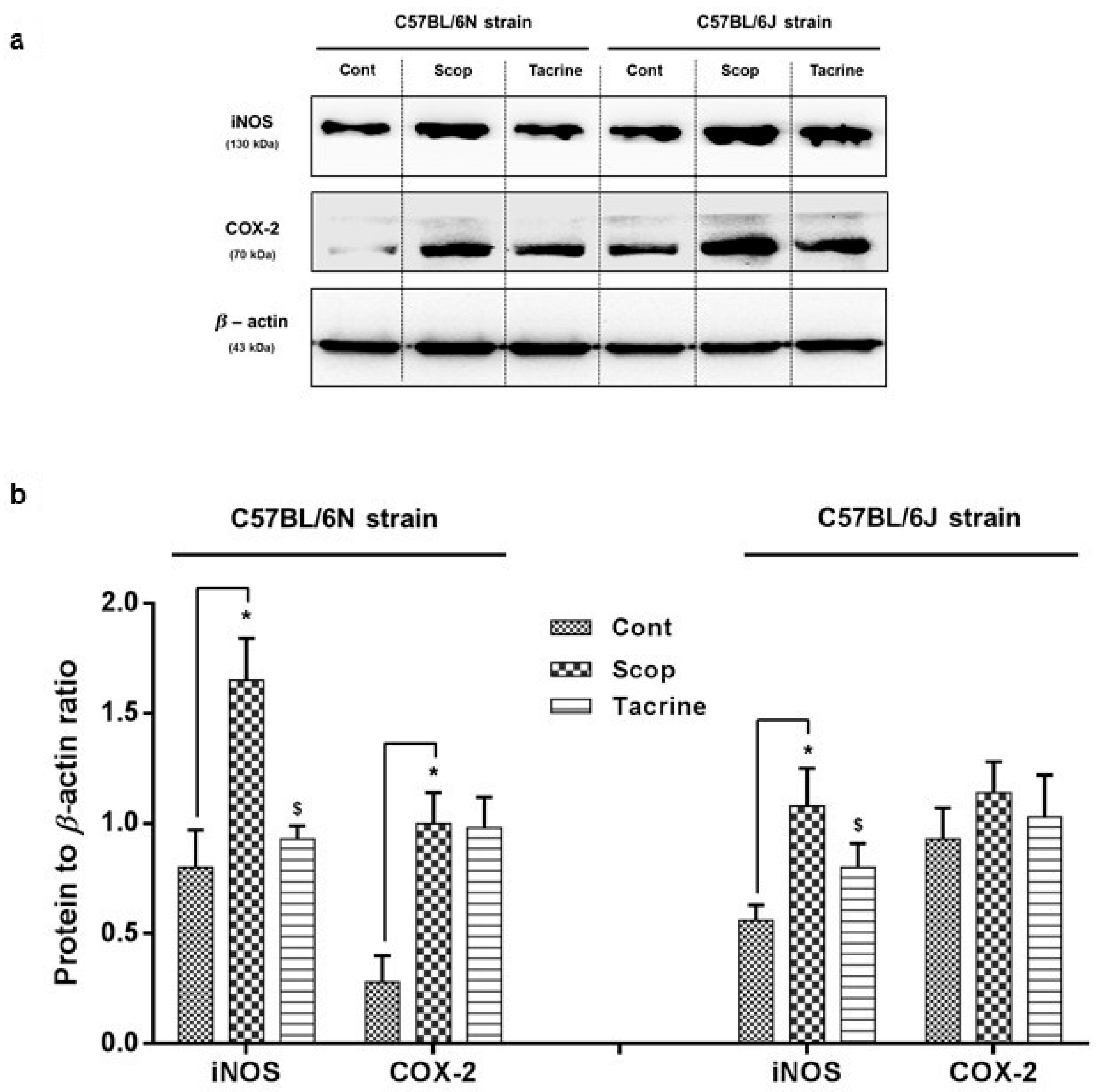
2.3. Inflammatory Protein Expressions at Hippocampus
2.3.1. ICR and C57BL/6 Comparison Study
2.3.2. C57BL/6N and C57BL/6J Comparison Study
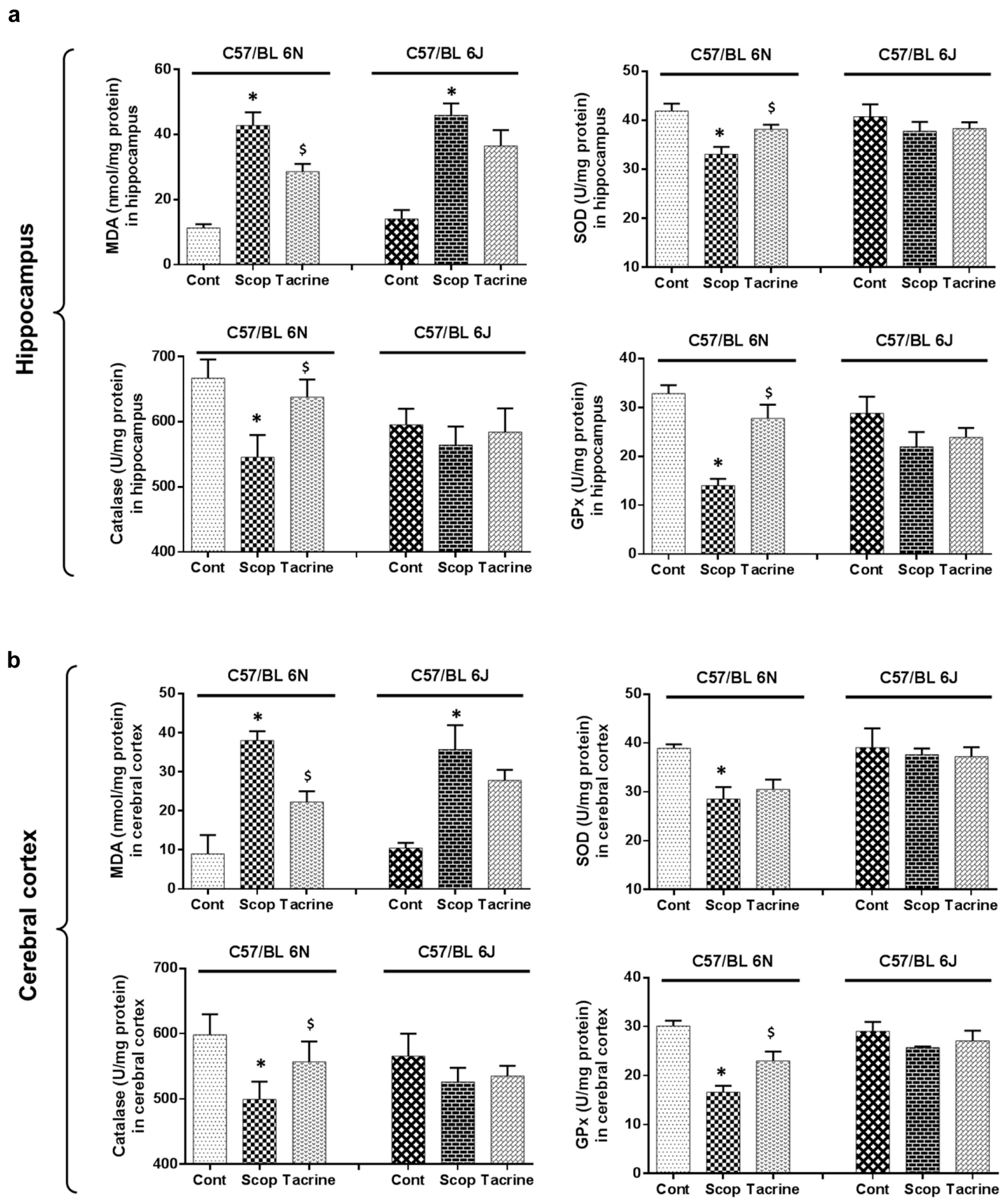
2.4. Lipid Peroxidation Activity in the Hippocampus and Cerebral Cortex
2.5. Antioxidant Biomarker Profiles in the Hippocampus and Cerebral Cortex
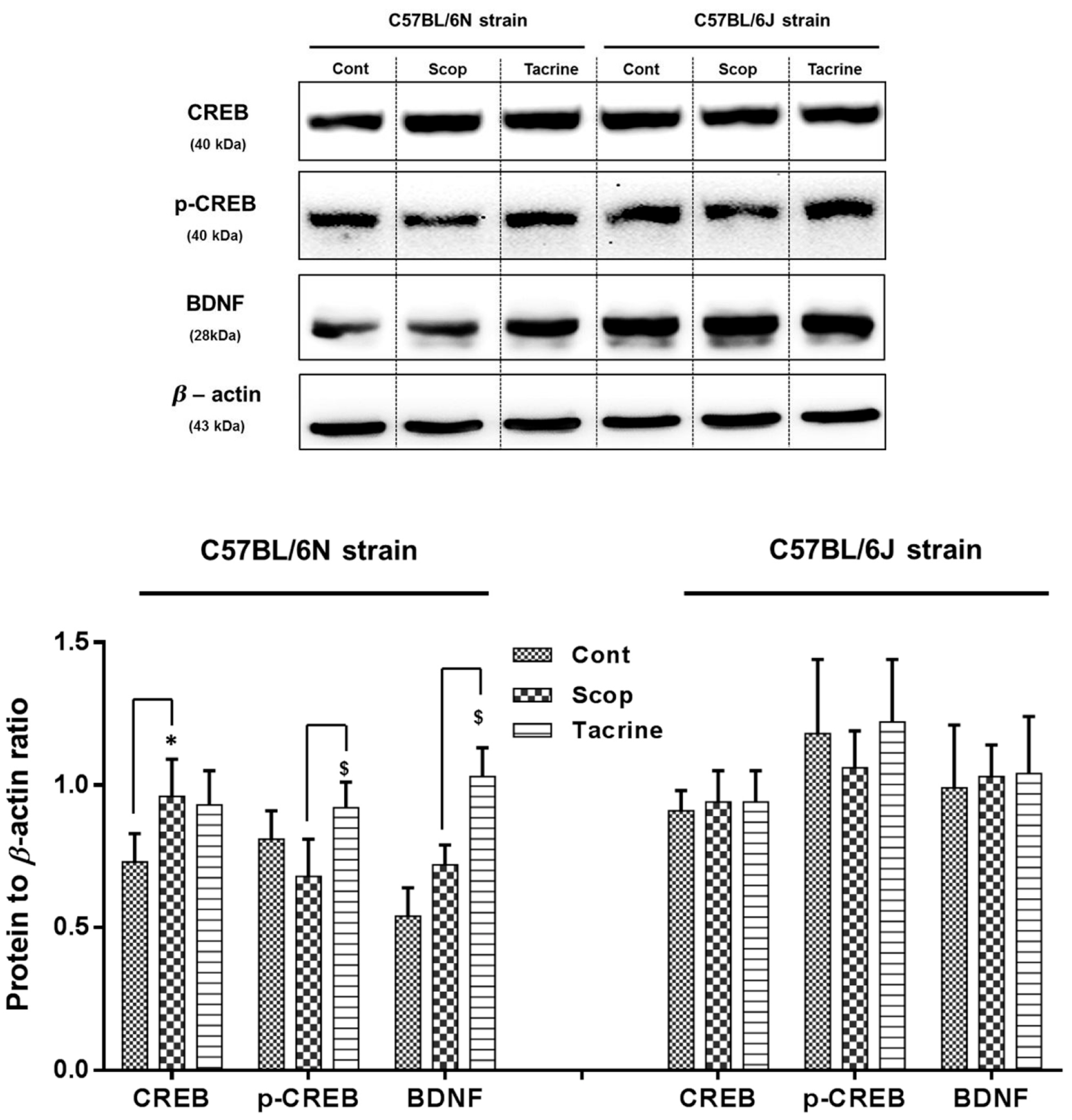
2.6. Western Blot Analysis of CREB/BDNF in the Hippocampus
3. Discussion
4. Materials and Methods
4.1. Reagents
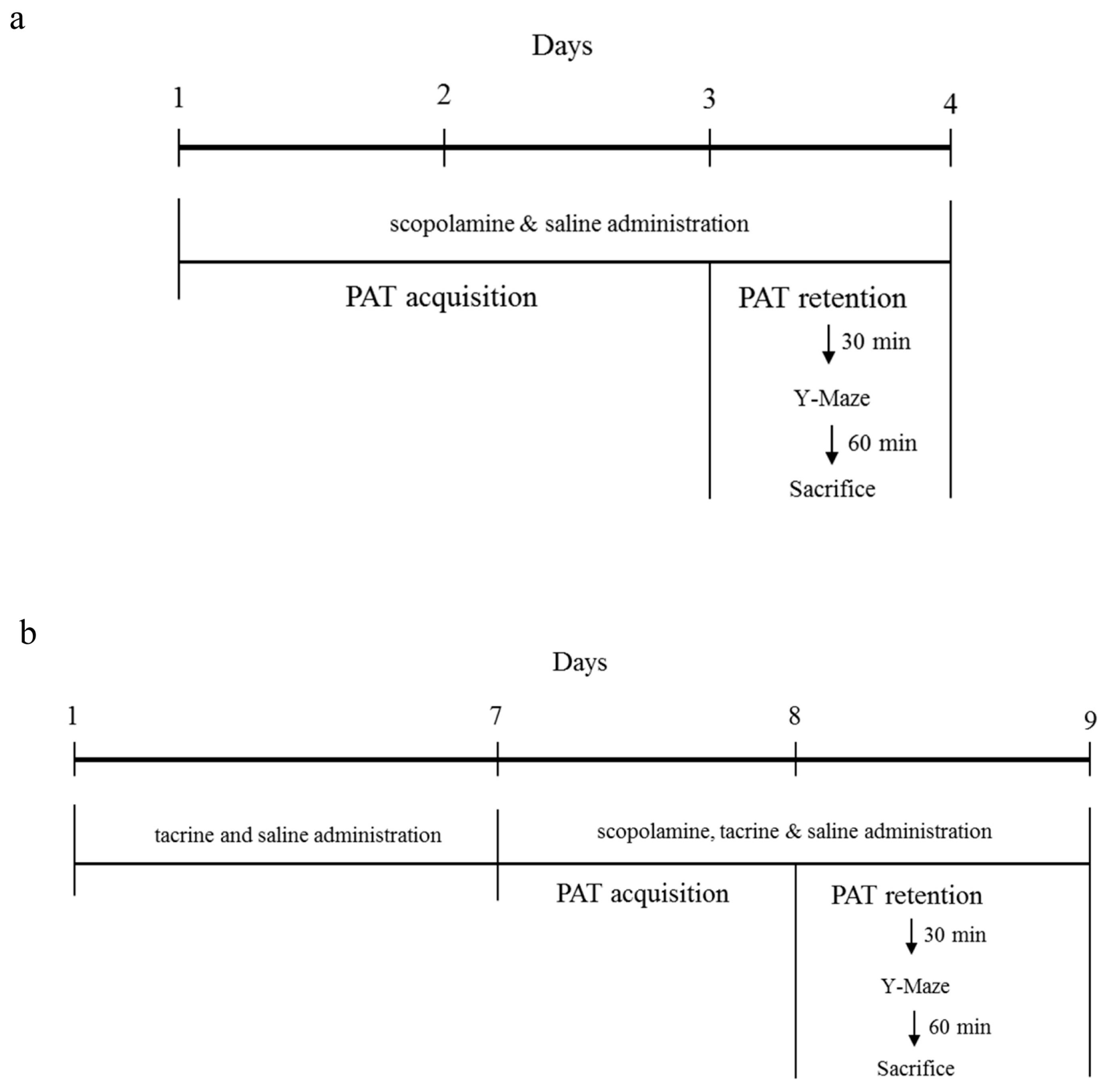
4.2. Animals and Treatment
4.2.1. ICR and C57BL/6 Comparison Study
4.2.2. C57BL/6N and C57BL/6J Comparison Study
4.3. Behavioral Studies
4.3.1. Step-Through Passive Avoidance Test
4.3.2. Spontaneous Alternation Performance (Y-Maze Test)
4.4. Tissue Procurement and Protein Quantification
4.5. AChE Activity
4.6. Lipid Peroxidation
4.7. SOD, CAT, and GPx Analysis
4.8. Western Blot Analysis
4.9. Statistical Analysis
5. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
Abbreviations
| AD | Alzheimer’s disease |
| AChE | Acetylcholine Esterase |
| BDNF | Brain-derived neurotrophic factor |
| BSA | Bovine serum albumin |
| COX-2 | Cyclooxygenase-2 |
| CAT | Catalase |
| CREB | cAMP response-element binding protein |
| GPx | Glutathione peroxidase |
| GSH | Glutathione |
| GSSG | Oxidized glutathione |
| GR | Glutathione reductase |
| IκBα | Nuclear factor of κ inhibitor |
| iNOS | Inducible nitric oxide synthase |
| LT | Time latencies |
| MDA | Malondialdehyde |
| NADP+ | Nicotinamide adenine dinucleotide phosphate |
| Nnt | Nicotinamide nucleotide transhydrogenase |
| PAT | Passive avoidance test |
| p-IκBα | Phosphorylated-nuclear factor of κ inhibitor |
| PBS | Phosphate-buffered saline |
| PVDF | Polyvinylidene fluoride |
| ROS | Reactive oxygen species |
| SOD | Superoxide dismutase |
| TBA | Thiobarbituric acid |
| THA | Tacrine hydrochloride hydrate |
References
- Wyss-Coray, T. Ageing, neurodegeneration and brain rejuvenation. Nature 2016, 539, 180–186. [Google Scholar] [CrossRef] [PubMed]
- Ribeiro, F.M.; Camargos, E.R.d.S.; Souza, L.C.d.; Teixeira, A.L. Animal models of neurodegenerative diseases. Rev. Bras. Psiquiatr. 2013, 35, S82–S91. [Google Scholar] [CrossRef] [PubMed]
- Cummings, J.; Morstorf, T.; Lee, G. Alzheimer’s drug-development pipeline: 2016. Alzheimer’s Dement. 2016, 2, 222–232. [Google Scholar] [CrossRef]
- Coyle, J.; Price, D.; DeLong, M. Alzheimer’s disease: A disorder of cortical cholinergic innervation. Science 1983, 219, 1184–1190. [Google Scholar] [CrossRef] [PubMed]
- Cabezas-Opazo, F.A.; Vergara-Pulgar, K.; Jose Perez, M.; Jara, C.; Osorio-Fuentealba, C.; Quintanilla, R.A. Mitochondrial Dysfunction Contributes to the Pathogenesis of Alzheimer’s Disease. Oxid. Med. Cell. Longev. 2015, 2015, 12. [Google Scholar] [CrossRef] [PubMed]
- Weintraub, S.; Wicklund, A.H.; Salmon, D.P. The Neuropsychological Profile of Alzheimer Disease. Cold Spring Harb. Perspect. Med. 2012, 2, a006171. [Google Scholar] [CrossRef] [PubMed]
- Williams, S.C.P. Small nanobody drugs win big backing from pharma. Nat. Med. 2013, 19, 1355–1356. [Google Scholar] [CrossRef] [PubMed]
- Arrowsmith, J. Trial watch: Phase II failures: 2008–2010. Nat. Rev. Drug Discov. 2011, 10, 328–329. [Google Scholar] [CrossRef] [PubMed]
- Denayer, T.; Stöhr, T.; Van Roy, M. Animal models in translational medicine: Validation and prediction. New Horiz. Transl. Med. 2014, 2, 5–11. [Google Scholar] [CrossRef]
- VanGuilder, H.D.; Freeman, W.M. The Hippocampal Neuroproteome with Aging and Cognitive Decline: Past Progress and Future Directions. Front. Aging Neurosci. 2011, 3, 8. [Google Scholar] [CrossRef] [PubMed]
- Erickson, K.I.; Prakash, R.S.; Voss, M.W.; Chaddock, L.; Heo, S.; McLaren, M.; Pence, B.D.; Martin, S.A.; Vieira, V.J.; Woods, J.A.; et al. Brain-derived neurotrophic factor is associated with age-related decline in hippocampal volume. J. Neurosci. 2010, 30, 5368–5375. [Google Scholar] [CrossRef] [PubMed]
- More, S.V.; Kumar, H.; Cho, D.-Y.; Yun, Y.-S.; Choi, D.-K. Toxin-Induced Experimental Models of Learning and Memory Impairment. Int. J. Mol. Sci. 2016, 17, 1447. [Google Scholar] [CrossRef] [PubMed]
- Bathina, S.; Das, U.N. Brain-derived neurotrophic factor and its clinical implications. Arch. Med. Sci. 2015, 11, 1164–1178. [Google Scholar] [CrossRef] [PubMed]
- Teich, A.F.; Nicholls, R.E.; Puzzo, D.; Fiorito, J.; Purgatorio, R.; Fa’, M.; Arancio, O. Synaptic Therapy in Alzheimer’s disease: A CREB-centric Approach. Neurotherapeutics 2015, 12, 29–41. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.D.; Wu, C.L.; Hwang, W.C.; Yang, D.I. More Insight into BDNF against Neurodegeneration: Anti-Apoptosis, Anti-Oxidation, and Suppression of Autophagy. Int. J. Mol. Sci. 2017, 18, 545. [Google Scholar] [CrossRef] [PubMed]
- Basaran, R.; Ozdamar, E.D.; Can-Eke, B. CYP2E1 and Parkinson’s disease in a MPTP-induced C57BL/6 mouse model. Mol. Neurodegener. 2013, 8 (Suppl. 1), P9. [Google Scholar] [CrossRef]
- Park, H.R.; Lee, H.; Park, H.; Cho, W.K.; Ma, J.Y. Fermented Sipjeondaebo-tang Alleviates Memory Deficits and Loss of Hippocampal Neurogenesis in Scopolamine-induced Amnesia in Mice. Sci. Rep. 2016, 6, 22405. [Google Scholar] [CrossRef] [PubMed]
- De Macêdo Medeiros, A.; Izídio, G.S.; Sousa, D.S.; Macedo, P.T.; Silva, A.F.; Shiramizu, V.K.M.; Cabral, A.; Ribeiro, A.M.; Silva, R.H. Estrogen levels modify scopolamine-induced amnesia in gonadally intact rats. Prog. Neuro Psychopharmacol. Biol. Psychiatr. 2014, 53, 99–108. [Google Scholar] [CrossRef] [PubMed]
- Falsafi, S.K.; Deli, A.; Höger, H.; Pollak, A.; Lubec, G. Scopolamine Administration Modulates Muscarinic, Nicotinic and NMDA Receptor Systems. PLoS ONE 2012, 7, e32082. [Google Scholar] [CrossRef] [PubMed]
- Bajo, R.; Pusil, S.; López, M.E.; Canuet, L.; Pereda, E.; Osipova, D.; Maestú, F.; Pekkonen, E. Scopolamine effects on functional brain connectivity: A pharmacological model of Alzheimer’s disease. Sci. Rep. 2015, 5, 9748. [Google Scholar] [CrossRef] [PubMed]
- Malaguarnera, M.; Pistone, G.; Vinci, M.; Motta, M.; di Fazio, I.; Rampello, L. Tacrine treatment of Alzheimer’s disease: Many expectations, few certainties. Neuropsychobiology 1998, 38, 226–231. [Google Scholar] [CrossRef] [PubMed]
- Qizilbash, N.; Birks, J.; Lopez-Arrieta, J.; Lewington, S.; Szeto, S. Tacrine for Alzheimer’s disease. Cochrane Database Syst. Rev. 2000, Cd000202. [Google Scholar]
- Mekada, K.; Abe, K.; Murakami, A.; Nakamura, S.; Nakata, H.; Moriwaki, K.; Obata, Y.; Yoshiki, A. Genetic differences among C57BL/6 substrains. Exp. Anim. 2009, 58, 141–149. [Google Scholar] [CrossRef] [PubMed]
- Popova, N.K.; Naumenko, V.S.; Tibeikina, M.A.; Kulikov, A.V. Serotonin transporter, 5-HT1A receptor, and behavior in DBA/2J mice in comparison with four inbred mouse strains. J. Neurosci. Res. 2009, 87, 3649–3657. [Google Scholar] [CrossRef] [PubMed]
- Norcross, M.; Poonam, M.; Enoch, A.J.; Karlsson, R.-M.; Brigman, J.L.; Cameron, H.A.; Harvey-White, J.; Holmes, A. Effects of adolescent fluoxetine treatment on fear-, anxiety- or stress-related behaviors in C57BL/6J or BALB/cJ mice. Psychopharmacology 2008, 200, 413–424. [Google Scholar] [CrossRef] [PubMed]
- Choonara, Y.E.; Pillay, V.; du Toit, L.C.; Modi, G.; Naidoo, D.; Ndesendo, V.M.K.; Sibambo, S.R. Trends in the Molecular Pathogenesis and Clinical Therapeutics of Common Neurodegenerative Disorders. Int. J. Mol. Sci. 2009, 10, 2510–2557. [Google Scholar] [CrossRef] [PubMed]
- Alzheimer’s Association. 2015 Alzheimer’s disease facts and figures. Alzheimer’s Dement. 2015, 11, 332–384. [Google Scholar]
- Mufson, E.J.; Counts, S.E.; Perez, S.E.; Ginsberg, S.D. Cholinergic system during the progression of Alzheimer’s disease: Therapeutic implications. Expert Rev. Neurother. 2008, 8, 1703–1718. [Google Scholar] [CrossRef] [PubMed]
- Bohnen, N.I.; Albin, R.L. The Cholinergic System and Parkinson Disease. Behav. Brain Res. 2011, 221, 564–573. [Google Scholar] [CrossRef] [PubMed]
- Lorenzini, C.A.; Baldi, E.; Bucherelli, C.; Sacchetti, B.; Tassoni, G. Role of dorsal hippocampus in acquisition, consolidation and retrieval of rat’s passive avoidance response: A tetrodotoxin functional inactivation study. Brain Res. 1996, 730, 32–39. [Google Scholar] [CrossRef]
- Kwon, S.-H.; Lee, H.-K.; Kim, J.-A.; Hong, S.-I.; Kim, H.-C.; Jo, T.-H.; Park, Y.-I.; Lee, C.-K.; Kim, Y.-B.; Lee, S.-Y.; et al. Neuroprotective effects of chlorogenic acid on scopolamine-induced amnesia via anti-acetylcholinesterase and anti-oxidative activities in mice. Eur. J. Pharmacol. 2010, 649, 210–217. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.H.; Jeon, S.J.; Son, K.H.; Jung, J.W.; Lee, S.; Yoon, B.H.; Lee, J.-J.; Cho, Y.-W.; Cheong, J.H.; Ko, K.H.; et al. The ameliorating effect of oroxylin A on scopolamine-induced memory impairment in mice. Neurobiol. Learn. Mem. 2007, 87, 536–546. [Google Scholar] [CrossRef] [PubMed]
- Sarter, M.; Bodewitz, G.; Stephens, D.N. Attenuation of scopolamine-induced impairment of spontaneous alternation behaviour by antagonist but not inverse agonist and agonist β-carbolines. Psychopharmacology 1988, 94, 491–495. [Google Scholar] [CrossRef] [PubMed]
- Drever, B.D.; Riedel, G.; Platt, B. The cholinergic system and hippocampal plasticity. Behav. Brain Res. 2011, 221, 505–514. [Google Scholar] [CrossRef] [PubMed]
- Aarsland, D.; Mosimann, U.P.; McKeith, I.G. Role of cholinesterase inhibitors in Parkinson’s disease and dementia with Lewy bodies. J. Geriatr. Psychiatr. Neurol. 2004, 17, 164–171. [Google Scholar] [CrossRef] [PubMed]
- Chinthu, R.; Anju, T.R.; Paulose, C.S. Cholinergic receptor alterations in the cerebral cortex of spinal cord injured rat. Biochem. Biophys. Rep. 2017, 10, 46–51. [Google Scholar] [CrossRef]
- Havekes, R.; Abel, T.; Van der Zee, E.A. The cholinergic system and neostriatal memory functions. Behav. Brain Res. 2011, 221, 412–423. [Google Scholar] [CrossRef] [PubMed]
- Mohler, E.G.; Ding, Z.; Rueter, L.E.; Chapin, D.; Young, D.; Kozak, R. Cross-site strain comparison of pharmacological deficits in the touchscreen visual discrimination test. Psychopharmacology 2015, 232, 4033–4041. [Google Scholar] [CrossRef] [PubMed]
- Pattij, T.; Janssen, M.C.; Loos, M.; Smit, A.B.; Schoffelmeer, A.N.; van Gaalen, M.M. Strain specificity and cholinergic modulation of visuospatial attention in three inbred mouse strains. Genes Brain Behav. 2007, 6, 579–587. [Google Scholar] [CrossRef] [PubMed]
- McGeer, E.G.; McGeer, P.L. Brain inflammation in Alzheimer disease and the therapeutic implications. Curr. Pharm. Des. 1999, 5, 821–836. [Google Scholar] [PubMed]
- Min, A.Y.; Doo, C.N.; Son, E.J.; Sung, N.Y.; Lee, K.J.; Sok, D.-E.; Kim, M.R. N-palmitoyl serotonin alleviates scopolamine-induced memory impairment via regulation of cholinergic and antioxidant systems, and expression of BDNF and p-CREB in mice. Chem. Biol. Interact. 2015, 242, 153–162. [Google Scholar] [CrossRef] [PubMed]
- Baarendse, P.J.; van Grootheest, G.; Jansen, R.F.; Pieneman, A.W.; Ogren, S.O.; Verhage, M.; Stiedl, O. Differential involvement of the dorsal hippocampus in passive avoidance in C57bl/6J and DBA/2J mice. Hippocampus 2008, 18, 11–19. [Google Scholar] [CrossRef] [PubMed]
- Barraclough, D.J.; Conroy, M.L.; Lee, D. Prefrontal cortex and decision making in a mixed-strategy game. Nat. Neurosci. 2004, 7, 404–410. [Google Scholar] [CrossRef] [PubMed]
- Reynolds, A.; Laurie, C.; Mosley, R.L.; Gendelman, H.E. Oxidative stress and the pathogenesis of neurodegenerative disorders. Int. Rev. Neurobiol. 2007, 82, 297–325. [Google Scholar] [PubMed]
- Moreira, P.I.; Santos, M.S.; Oliveira, C.R.; Shenk, J.C.; Nunomura, A.; Smith, M.A.; Zhu, X.; Perry, G. Alzheimer disease and the role of free radicals in the pathogenesis of the disease. CNS Neurol. Disord. Drug Targets 2008, 7, 3–10. [Google Scholar]
- Manoharan, S.; Guillemin, G.J.; Abiramasundari, R.S.; Essa, M.M.; Akbar, M.; Akbar, M.D. The Role of Reactive Oxygen Species in the Pathogenesis of Alzheimer’s Disease, Parkinson’s Disease, and Huntington’s Disease: A Mini Review. Oxid. Med. Cell. Longev. 2016, 2016, 8590578. [Google Scholar] [CrossRef] [PubMed]
- Kelsey, N.A.; Wilkins, H.M.; Linseman, D.A. Nutraceutical antioxidants as novel neuroprotective agents. Molecules 2010, 15, 7792–7814. [Google Scholar] [CrossRef] [PubMed]
- Gandhi, S.Y.; Abramov, A. Mechanism of Oxidative Stress in Neurodegeneration. Oxid. Med. Cell. Longev. 2012, 2012, 11. [Google Scholar] [CrossRef] [PubMed]
- Emerit, J.; Edeas, M.; Bricaire, F. Neurodegenerative diseases and oxidative stress. Biomed. Pharmacother. 2004, 58, 39–46. [Google Scholar] [CrossRef] [PubMed]
- Hsieh, H.L.; Yang, C.M. Role of redox signaling in neuroinflammation and neurodegenerative diseases. BioMed Res. Int. 2013, 2013, 484613. [Google Scholar] [CrossRef] [PubMed]
- Wolf, S.; Hainz, N.; Beckmann, A.; Maack, C.; Menger, M.D.; Tschernig, T.; Meier, C. Brain damage resulting from postnatal hypoxic-ischemic brain injury is reduced in C57BL/6J mice as compared to C57BL/6N mice. Brain Res. 2016, 1650, 224–231. [Google Scholar] [CrossRef] [PubMed]
- Ronchi, J.A.; Figueira, T.R.; Ravagnani, F.G.; Oliveira, H.C.; Vercesi, A.E.; Castilho, R.F. A spontaneous mutation in the nicotinamide nucleotide transhydrogenase gene of C57BL/6J mice results in mitochondrial redox abnormalities. Free Radic. Biol. Med. 2013, 63, 446–456. [Google Scholar] [CrossRef] [PubMed]
- Rydstrom, J. Mitochondrial NADPH, transhydrogenase and disease. Biochim. Biophys. Acta 2006, 1757, 721–726. [Google Scholar] [CrossRef] [PubMed]
- Yamada, K.; Nabeshima, T. Brain-derived neurotrophic factor/TrkB signaling in memory processes. J. Pharmacol. Sci. 2003, 91, 267–270. [Google Scholar] [CrossRef] [PubMed]
- Mantamadiotis, T.; Lemberger, T.; Bleckmann, S.C.; Kern, H.; Kretz, O.; Martin Villalba, A.; Tronche, F.; Kellendonk, C.; Gau, D.; Kapfhammer, J.; et al. Disruption of CREB function in brain leads to neurodegeneration. Nat. Genet. 2002, 31, 47–54. [Google Scholar] [CrossRef] [PubMed]
- Zuccato, C.; Cattaneo, E. Brain-derived neurotrophic factor in neurodegenerative diseases. Nat. Rev. Neurol. 2009, 5, 311–322. [Google Scholar] [CrossRef] [PubMed]
- Joh, E.H.; Lee, I.A.; Kim, D.H. Kalopanaxsaponins A and B isolated from Kalopanax pictus ameliorate memory deficits in mice. Phytother. Res. PTR 2012, 26, 546–551. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.-S.; Kim, H.-G.; Lee, H.-W.; Han, J.-M.; Lee, S.-K.; Kim, D.-W.; Saravanakumar, A.; Son, C.-G. Hippocampal memory enhancing activity of pine needle extract against scopolamine-induced amnesia in a mouse model. Sci. Rep. 2015, 5, 9651. [Google Scholar] [CrossRef] [PubMed]
- Lee, B.; Cao, R.; Choi, Y.S.; Cho, H.Y.; Rhee, A.D.; Hah, C.K.; Hoyt, K.R.; Obrietan, K. The CREB/CRE transcriptional pathway: Protection against oxidative stress-mediated neuronal cell death. J. Neurochem. 2009, 108, 1251–1265. [Google Scholar] [CrossRef] [PubMed]
- Kumar, H.; Kim, B.W.; Song, S.Y.; Kim, J.S.; Kim, I.S.; Kwon, Y.S.; Koppula, S.; Choi, D.K. Cognitive enhancing effects of α asarone in amnesic mice by influencing cholinergic and antioxidant defense mechanisms. Biosci. Biotechnol. Biochem. 2012, 76, 1518–1522. [Google Scholar] [CrossRef] [PubMed]
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Karthivashan, G.; Park, S.-Y.; Kim, J.-S.; Cho, D.-Y.; Ganesan, P.; Choi, D.-K. Comparative Studies on Behavioral, Cognitive and Biomolecular Profiling of ICR, C57BL/6 and Its Sub-Strains Suitable for Scopolamine-Induced Amnesic Models. Int. J. Mol. Sci. 2017, 18, 1735. https://doi.org/10.3390/ijms18081735
Karthivashan G, Park S-Y, Kim J-S, Cho D-Y, Ganesan P, Choi D-K. Comparative Studies on Behavioral, Cognitive and Biomolecular Profiling of ICR, C57BL/6 and Its Sub-Strains Suitable for Scopolamine-Induced Amnesic Models. International Journal of Molecular Sciences. 2017; 18(8):1735. https://doi.org/10.3390/ijms18081735
Chicago/Turabian StyleKarthivashan, Govindarajan, Shin-Young Park, Joon-Soo Kim, Duk-Yeon Cho, Palanivel Ganesan, and Dong-Kug Choi. 2017. "Comparative Studies on Behavioral, Cognitive and Biomolecular Profiling of ICR, C57BL/6 and Its Sub-Strains Suitable for Scopolamine-Induced Amnesic Models" International Journal of Molecular Sciences 18, no. 8: 1735. https://doi.org/10.3390/ijms18081735
APA StyleKarthivashan, G., Park, S.-Y., Kim, J.-S., Cho, D.-Y., Ganesan, P., & Choi, D.-K. (2017). Comparative Studies on Behavioral, Cognitive and Biomolecular Profiling of ICR, C57BL/6 and Its Sub-Strains Suitable for Scopolamine-Induced Amnesic Models. International Journal of Molecular Sciences, 18(8), 1735. https://doi.org/10.3390/ijms18081735