Markers of T Cell Senescence in Humans
Abstract
:1. Introduction
2. Immune System, T Cells and Cellular Senescence
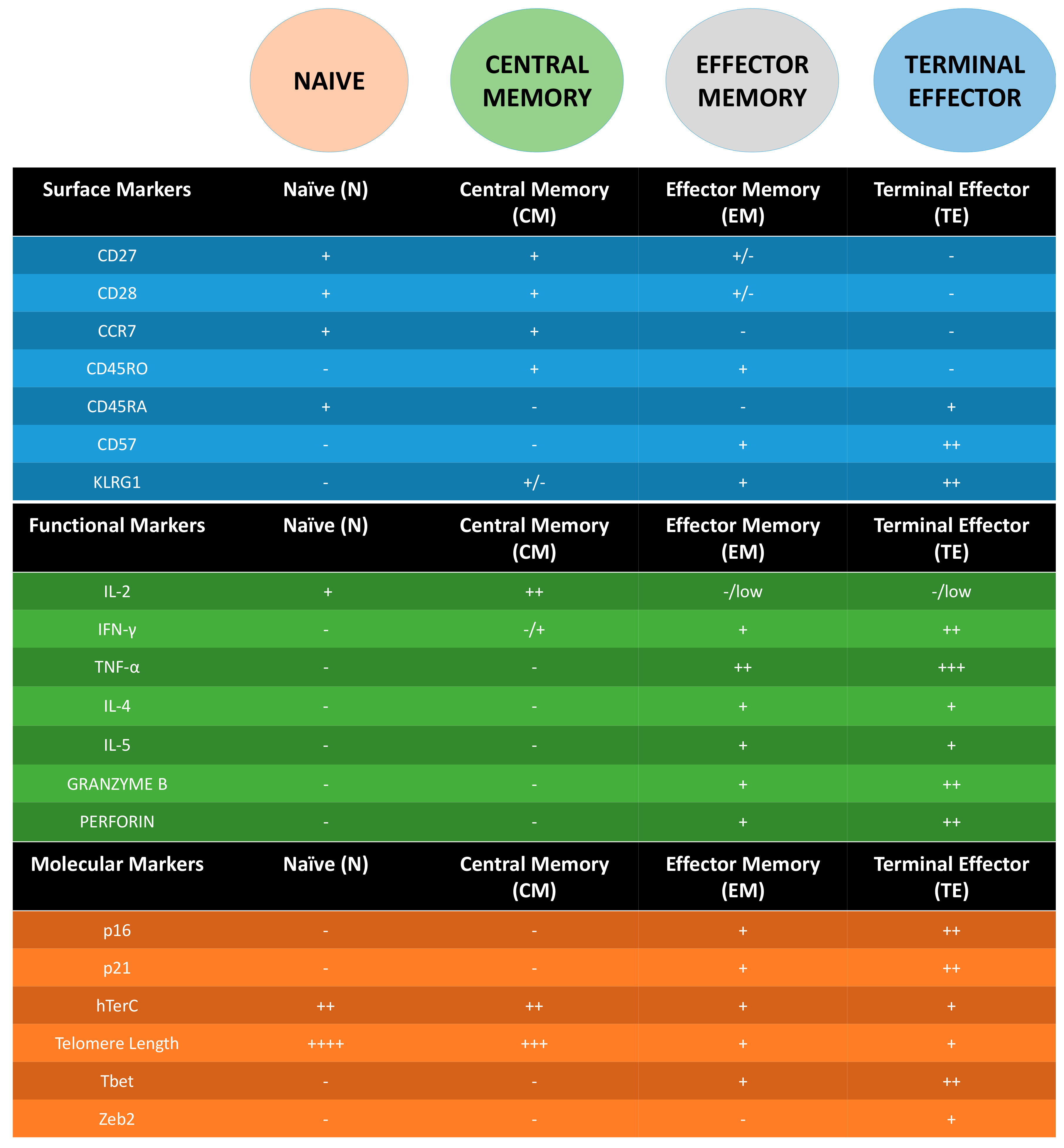
3. Following T Cell Journey through Differentiation: Surface Markers as Guides
4. Molecular Markers of Senescent T Cells
5. T Cells: Senescence Does Not Equate to Exhaustion
6. Implications of T Cells Senescence in Persistent Infections and Human Aging
7. Are the Markers Suitable for All T Cells?
8. New Players in the Field of Senescence?
9. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
Abbreviations
| APC | Antigen Presenting Cells |
| CD | Cluster of Differentiation |
| cGAS | cGMP-AMP Synthase |
| CM | Central Memory |
| CMV | Cytomegalovirus |
| CTLA-4 | Cytotoxic T Lymphocyte-Associated Protein 4 |
| DC | Dendritic Cells |
| DNA | Deoxyribonucleic Acid |
| EM | Effector Memory |
| GEM T | Germline-Encoded, Mycolyl T Cells |
| HIV | Human Immunodeficiency Virus |
| IFNγ | Interferon Gamma |
| iNKT | Invariant Natural Killer T Cells |
| IL | Interleukin |
| KLRG-1 | Killer Lectin-Like Receptor Sub Family G Protein 1 |
| LAG3 | Lymphocyte Activation Gene 3 |
| MAIT | Mucosal Associated Invariant T Cells |
| N | Naive |
| NAD | Nicotinamide |
| NK | Natural Killer |
| PD-1 | Programmed Cell Death Protein 1 |
| RNA | Ribonucleic Acid |
| RB | Retinoblastoma |
| SASP | Senescence Associated Secretory Phenotype |
| TEMRA | Terminal Effector Memory RA |
| TNFα | Tumor Necrosis Factor Alpha |
References
- OECD. Elderly Population (Indicator). 2017. Available online: https://data.oecd.org/pop/elderly-population.htm (accessed on 5 May 2017).
- World Population Ageing. Available online: http://www.un.org/en/development/desa/population/publications/pdf/ageing/WPA2015_Report.pdf (accessed on 5 May 2017).
- The World Bank. Investing in Health: World Development Indicators; Oxford University Press: New York, NY, USA, 1993. [Google Scholar]
- Wright, J.C.; Weinstein, M.C. Gains in life expectancy from medical interventions. N. Engl. J. Med. 1998, 339, 1943–1944. [Google Scholar] [CrossRef] [PubMed]
- Here’s the Visual Proof of Why Vaccines Do More Good than Harm. Available online: http://www.sciencemag.org/news/2017/04/heres-visual-proof-why-vaccines-do-more-good-harm (accessed on 15 June 2017).
- Plassman, B.L.; Langa, K.M.; Fisher, G.G.; Heeringa, S.G.; Weir, D.R.; Ofstedal, M.B.; Burke, J.R.; Hurd, M.D.; Potter, G.G.; Rodgers, W.L.; et al. Prevalence of dementia in the United States: The aging, demographics, and memory study. Neuroepidemiology 2007, 29, 125–132. [Google Scholar] [CrossRef] [PubMed]
- Kamel, H.K. Sarcopenia and Aging. Nutr. Rev. 2003, 61, 157–167. [Google Scholar] [CrossRef] [PubMed]
- Machierira-Coelho, A. Cancer and aging. Exp. Gerontol. 1986, 23, 483–495. [Google Scholar] [CrossRef]
- Lesnefsky, E.J.; Moghaddas, S.; Tandler, B.; Kerner, J.; Hoppel, C.L. Mitochondrial dysfunction in cardiac Disease: Ischemia–reperfusion, aging, and heart failure. J. Mol. Cell. Cardiol. 2001, 33, 1065–1089. [Google Scholar] [CrossRef] [PubMed]
- Yazici, Y.; Paget, S.A. Elderly-onset rheumatoid arthritis. Rheum. Dis. Clin. N. Am. 2000, 26, 517–526. [Google Scholar] [CrossRef]
- Plotkin, S.A. Vaccines: Past, present and future. Nat. Med. 2005, 11, S5–S11. [Google Scholar] [CrossRef] [PubMed]
- Lang, P.-O.; Mendes, A.; Socquet, J.; Assir, N.; Govind, S.; Aspinall, R. Effectiveness of influenza vaccine in aging and older adults: Comprehensive analysis of the evidence. Clin. Interv. Aging 2012, 7, 55–64. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lord, J.M. The effect of aging of the immune system on vaccination responses. Hum. Vaccines Immunother. 2013, 9, 1364–1367. [Google Scholar] [CrossRef] [PubMed]
- Aspinall, R.; Giudice, G.D.; Effros, R.B.; Loebenstein, B.G.; Sambhara, S. Challenges for vaccination in the elderly. Immun. Ageing 2007, 4, 9. [Google Scholar] [CrossRef] [PubMed]
- Larbi, A.; Fulop, T. From “truly naïve” to “exhausted senescent” T cells: When markers predict functionality. Cytom. Part A 2014, 85, 25–35. [Google Scholar] [CrossRef] [PubMed]
- Wingender, G.; Kronenberg, M. OMIP-030: Characterization of human T cell subsets via surface markers. Cytom. Part A 2015, 87, 1067–1069. [Google Scholar] [CrossRef] [PubMed]
- Delves, P.J.; Roitt, I.M. The immune system: First of two parts. N. Engl. J. Med. 2000, 343, 37–49. [Google Scholar] [CrossRef] [PubMed]
- Kumar, H.; Kawai, T.; Akira, S. Pathogen recognition by the innate immune system. Int. Rev. Immunol. 2011, 30, 16–34. [Google Scholar] [CrossRef] [PubMed]
- MacLeod, M.K.L.; Kappler, J.W.; Marrack, P. Memory CD4 T cells: Generation, reactivation and re-assignment. Immunology 2010, 130, 10–15. [Google Scholar] [CrossRef] [PubMed]
- Obar, J.J.; Lefrançois, L. Memory CD8+ T cell differentiation. Ann. N. Y. Acad. Sci. 2010, 1183, 251–266. [Google Scholar] [CrossRef] [PubMed]
- Zhu, J.; Paul, W.E. CD4 T cells: Fates, functions, and faults. Immunobiology 2009, 112, 1557–1569. [Google Scholar] [CrossRef] [PubMed]
- Zhang, N.; Bevan, M.J. CD8+ T cells: Foot soldiers of the immune system. Immunity 2011, 35, 161–168. [Google Scholar] [CrossRef] [PubMed]
- Zhu, J.; Yamane, H.; Paul, W. Differentiation of effector CD4 T cell populations. Annu. Rev. Immunol. 2010, 28, 445–489. [Google Scholar] [CrossRef] [PubMed]
- Bousso, P. T-cell activation by dendritic cells in the lymph node: Lessons from the movies. Nat. Rev. Immunol. 2008, 8, 675–684. [Google Scholar] [CrossRef] [PubMed]
- Janeway, C.A., Jr.; Travers, P.; Walport, M.; Shlomchik, M.J. B-cell activation by armed helper T cells. In Immunobiology: The Immune System in Health and Disease, 5th ed.; Garland Science: New York, NY, USA, 2001. Available online: https://www.ncbi.nlm.nih.gov/books/NBK27142/ (accessed on 10 June 2017).
- Janeway, C.A., Jr.; Travers, P.; Walport, M.; Shlomchik, M.J. Macrophage activation by armed CD4 TH1 cells. In Immunobiology: The Immune System in Health and Disease, 5th ed.; Garland Science: New York, NY, USA, 2001. Available online: https://www.ncbi.nlm.nih.gov/books/NBK27153/ (accessed on 11 June 2017).
- Janeway, C.A., Jr.; Travers, P.; Walport, M.; Shlomchik, M.J. T cell-mediated cytotoxicity. In Immunobiology: The Immune System in Health and Disease, 5th ed.; Garland Science: New York, NY, USA, 2001. Available online: https://www.ncbi.nlm.nih.gov/books/NBK27101/ (accessed on 12 June 2017).
- Langhoff, E.; Steinman, R.M. Clonal expansion of human T lymphocytes initiated by dendritic cells. J. Exp. Med. 1989, 169, 315–320. [Google Scholar] [CrossRef] [PubMed]
- Effros, R.B.; Pawelec, G. Replicative senescence of T cells: Does the Hayflick limit lead to immune exhaustion? Immunol. Today 1997, 18, 450–454. [Google Scholar] [CrossRef]
- Effros, R.B. Replicative senescence in the immune system: Impact of the Hayflick limit on T-cell function in the elderly. Am. J. Hum. Genet. 1998, 62, 1003–1007. [Google Scholar] [CrossRef] [PubMed]
- Hodes, R.J.; Hathcock, K.S.; Weng, N. Telomeres in T and B cells. Nat. Rev. Immunol. 2002, 2, 699–706. [Google Scholar] [CrossRef] [PubMed]
- Shay, J.W.; Wright, W.E. Hayflick, his limit and cellular aging. Nat. Rev. Mol. Cell Biol. 2000, 1, 72–76. [Google Scholar] [CrossRef] [PubMed]
- Coppé, J.-P.; Desprez, P.-Y.; Krtolica, A.; Campisi, J. The senescence-associated secretory phenotype: The dark side of tumor suppression. Annu. Rev. Pathol. Mech. Dis. 2010, 5, 99–118. [Google Scholar] [CrossRef] [PubMed]
- Mahnke, Y.D.; Brodie, T.M.; Sallusto, F.; Roederer, M.; Lugli, E. The who’s who of T-cell differentiation: Human memory T-cell subsets. Eur. J. Immunol. 2013, 43, 2797–2809. [Google Scholar] [CrossRef] [PubMed]
- King, C. New insights into the differentiation and function of T follicular helper cells. Nat. Rev. Immunol. 2009, 9, 757–766. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, R.; Bevan, M.J.; Reiner, S.L.; Fearon, D.T. The precursors of memory: Models and controversies. Nat. Rev. Immunol. 2009, 9, 662–668. [Google Scholar] [CrossRef] [PubMed]
- Rosenblum, M.D.; Way, S.S.; Abbas, A.K. Regulatory T cell memory. Nat. Rev. Immunol. 2016, 16, 90–101. [Google Scholar] [CrossRef] [PubMed]
- Kaech, S.M.; Wherry, E.J.; Ahmed, R. Effector and memory T-cell differentiation: Implications for vaccine development. Nat. Rev. Immunol. 2002, 2, 251–262. [Google Scholar] [CrossRef] [PubMed]
- Förster, R.; Davalos-Misslitz, A.C.; Rot, A. CCR7 and its ligands: Balancing immunity and tolerance. Nat. Rev. Immunol. 2008, 8, 362–371. [Google Scholar] [CrossRef] [PubMed]
- Dolfi, D.V.; Katsikis, P.D. CD28 and CD27 costimulation of CD8+ T cells: A story of survival. Adv. Exp. Med. Biol. 2007, 590, 149–170. [Google Scholar] [PubMed]
- Koch, S.; Larbi, A.; Derhovanessian, E.; Ozcelik, D.; Naumova, E.; Pawelec, G. Multiparameter flow cytometric analysis of CD4 and CD8 T cell subsets in young and old people. Immun. Ageing 2008, 5, 6. [Google Scholar] [CrossRef] [PubMed]
- Stubbe, M.; Vanderheyde, N.; Goldman, M.; Marchant, A. Antigen-specific central memory CD4+ T lymphocytes produce multiple cytokines and proliferate in vivo in humans. J. Immunol. 2006, 177, 8185–8190. [Google Scholar] [CrossRef] [PubMed]
- Henson, S.M.; Akbar, A.N. KLRG-1—More than a marker for T cell senescence. Age (Omaha) 2009, 31, 285–291. [Google Scholar] [CrossRef] [PubMed]
- Henson, S.; Franzese, O.; Macaulay, R.; Libri, V.; Azevedo, R.I.; Kiani-Alikhan, S.; Plunkett, F.J.; Masters, J.E.; Jackson, S.; Griffiths, S.J.; et al. KLRG-1 signalling induced proliferative dysfunction and defective Aktser473 phosphorylation in highly differentiated human CD8+ T cells. Blood 2009, 113, 1–3. [Google Scholar] [CrossRef] [PubMed]
- Brenchley, J.M.; Karandikar, N.J.; Betts, M.R.; Ambrozak, D.R.; Hill, B.J.; Crotty, L.E.; Casazza, J.P.; Kuruppu, J.; Migueles, S.A.; Connors, M.; et al. Expression of CD57 defines replicative senescence and antigen-induced apoptotic death of CD8. Blood 2003, 101, 2711–2720. [Google Scholar] [CrossRef] [PubMed]
- Palmer, B.E.; Blyveis, N.; Fontenot, A.P.; Wilson, C.C. Functional and phenotypic characterization of CD57+CD4+ T cells and their association with HIV-1-induced T cell dysfunction. J. Immunol. 2005, 175, 8415–8423. [Google Scholar] [CrossRef] [PubMed]
- Chou, J.P.; Effros, R.B. T cell replicative senescence in human aging. Curr. Pharm. Des. 2013, 19, 1680–1698. [Google Scholar] [PubMed]
- Liu, Y.; Sanoff, H.K.; Cho, H.; Burd, C.E.; Torrice, C.; Ibrahim, J.G.; Thomas, N.E.; Sharpless, N.E. Expression of p16INK4a in peripheral blood T-cells is a biomarker of human aging. Aging Cell 2009, 8, 439–448. [Google Scholar] [CrossRef] [PubMed]
- Porath, I.B.; Weinberg, R.A. When cells get stressed: An integrative view of cellular senescence. J. Clin. Investig. 2004, 113, 8–13. [Google Scholar] [CrossRef]
- Shawi, M.; Autexier, C. Telomerase, senescence and ageing. Mech. Ageing Dev. 2008, 129, 3–10. [Google Scholar] [CrossRef] [PubMed]
- Qian, Y.; Chen, X. Tumor suppression by p53: Making cells senescent. Histol. Histopathol. 2010, 25, 515–526. [Google Scholar] [PubMed]
- Munoz-Espin, D.; Serrano, M. Cellular senescence: From physiology to pathology. Nat. Rev. Mol. Cell Biol. 2014, 15, 482–496. [Google Scholar] [CrossRef] [PubMed]
- Bernadotte, A.; Mikhelson, V.M.; Spivak, I.M. Markers of cellular senescence. Telomere shortening as a marker of cellular senescence. Aging (Albany NY) 2016, 8, 3–11. [Google Scholar] [CrossRef] [PubMed]
- Plunkett, F.J.; Franzese, O.; Finney, H.M.; Fletcher, J.M.; Belaramini, L.L.; Salmon, M.; Dokal, I.; Webster, D.; Lawson, A.D.G.; Akbar, A.N. The loss of telomerase activity in highly differentiated CD8+CD28−CD27− T cells is associated with decreased Akt (Ser473) phosphorylation. J. Immunol. 2007, 178, 7710–7719. [Google Scholar] [CrossRef] [PubMed]
- Lanna, A.; Henson, S.M.; Escors, D.; Akbar, A.N. The kinase p38 activated by the metabolic regulator AMPK and scaffold TAB1 drives the senescence of human T cells. Nat. Immunol. 2014, 15, 965–972. [Google Scholar] [CrossRef] [PubMed]
- Crespo, J.; Sun, H.; Welling, T.H.; Tian, Z.; Zou, W. T cell anergy, exhaustion, senescence, and stemness in the tumor microenvironment. Curr. Opin. Immunol. 2013, 25, 214–221. [Google Scholar] [CrossRef] [PubMed]
- Wherry, E.J. T cell exhaustion. Nat. Immunol. 2011, 12, 492–499. [Google Scholar] [CrossRef] [PubMed]
- Shi, F.; Shi, M.; Zeng, Z.; Qi, R.Z.; Liu, Z.W.; Zhang, J.Y.; Yang, Y.P.; Tien, P.; Wang, F.S. PD-1 and PD-L1 upregulation promotes CD8+ T-cell apoptosis and postoperative recurrence in hepatocellular carcinoma patients. Int. J. Cancer 2011, 128, 887–896. [Google Scholar] [CrossRef] [PubMed]
- Barber, D.L.; Wherry, E.J.; Masopust, D.; Zhu, B.; Allison, J.P.; Sharpe, A.H.; Freeman, G.J.; Ahmed, R. Restoring function in exhausted CD8 T cells during chronic viral infection. Nature 2006, 439, 682–687. [Google Scholar] [CrossRef] [PubMed]
- Zarour, H.M. Reversing T-cell dysfunction and exhaustion in cancer. Clin. Cancer Res. 2016, 22, 1856–1864. [Google Scholar] [CrossRef] [PubMed]
- Weber, J. Anti CTLA-4 antibody ipilimumab: Case studies of clinical response and immune-related adverse events. Oncologist 2007, 12, 864–872. [Google Scholar] [CrossRef] [PubMed]
- Weber, J.S.; Angelo, S.P.D.; Minor, D.; Hodi, F.S.; Gutzmer, R.; Neyns, B.; Hoeller, C.; Khushalani, N.; Miller, W.H., Jr.; Lao, C.D.; et al. Nivolumab versus chemotherapy in patients with advanced melanoma who progressed after anti-CTLA-4 treatment (CheckMate 037): A randomised, controlled, open-label, phase 3 trial. Lancet Oncol. 2015, 16, 375–384. [Google Scholar] [CrossRef]
- Rizvi, N.A.; Maziaeres, J.; Planchard, D.; Stinchcombe, T.E.; Dy, G.K.; Anotonia, S.J.; Horn, L.; Lena, H.; Minenza, E.; Menncier, B.; et al. Activity and safety of nivolumab, an anti-PD-1 immune checkpoint inhibitor, for patients with advanced, refractory squamous non-small-cell lung cancer (CheckMate 063): A phase 2, single-arm trial. Lancet Oncol. 2015, 16, 257–265. [Google Scholar] [CrossRef]
- Maio, M.; Grob, J.J.; Aamdal, S.; Bondarenko, I.; Robert, C.; Thomas, L.; Garbe, C.; Chiarion-Sileni, V.; Testori, A.; Chen, T.T.; et al. Five-year survival rates for treatment-naive patients with advanced melanoma who received ipilimumab plus dacarbazine in a phase III trial. J. Clin. Oncol. 2015, 33, 1191–1196. [Google Scholar] [CrossRef] [PubMed]
- Sharma, P.; Allison, J.P. The future of immune checkpoint therapy. Science 2015, 348, 56–61. [Google Scholar] [CrossRef] [PubMed]
- Sansoni, P.; Vescovini, R.; Fagnoni, F.F.; Akbar, A.N.; Aren, R.; Chiu, Y.L.; Cicin-Sain, L.; Dechanet-Mervile, J.; Derhovanessian, E.; Ferrado-Martinez, S.; et al. New advances in CMV and immunosenescence. Exp. Gerontol. 2014, 55, 54–62. [Google Scholar] [CrossRef] [PubMed]
- Klenerman, P.; Oxenius, A. T cell responses to cytomegalovirus. Nat. Rev. Immunol. 2016, 16, 367–377. [Google Scholar] [CrossRef] [PubMed]
- Deeks, S.G. HIV infection, inflammation, immunosenescence, and aging. Annu. Rev. Med. 2011, 62, 141–155. [Google Scholar] [CrossRef] [PubMed]
- Fülöp, T.; Larbi, A.; Pawelec, G. Human T cell aging and the impact of persistent viral infections. Front. Immunol. 2013, 4, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Palmer, D.B. The effect of age on thymic function. Front. Immunol. 2013, 4, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Aspinall, R.; Pitts, D.; Lapenna, A.; Mitchell, W. Immunity in the elderly: The role of the thymus. J. Comp. Pathol. 2010, 142 (Suppl. 1), S111–S115. [Google Scholar] [CrossRef] [PubMed]
- Pang, W.W.; Price, E.A.; Sahoo, D.; Beerman, I.; Maloney, W.J.; Rossi, D.J.; Schrier, S.L.; Weissman, I.L. Human bone marrow hematopoietic stem cells are increased in frequency and myeloid-biased with age. Proc. Natl. Acad. Sci. USA 2011, 108, 20012–20017. [Google Scholar] [CrossRef] [PubMed]
- Linton, P.J.; Dorshkind, K. Age-related changes in lymphocyte development and function. Nat. Immunol. 2004, 5, 133–139. [Google Scholar] [CrossRef] [PubMed]
- Taub, D.D.; Longo, D.L. Insights into thymic aging and regeneration. Immunol. Rev. 2005, 205, 72–93. [Google Scholar] [CrossRef] [PubMed]
- Bredenkamp, N.; Nowell, C.S.; Blackburn, C.C. Regeneration of the aged thymus by a single transcription factor. Development 2014, 141, 1627–1637. [Google Scholar] [CrossRef] [PubMed]
- Franceschi, C.; Campisi, J. Chronic inflammation (inflammaging) and its potential contribution to age-associated diseases. J. Gerontol. Ser. A Biol. Sci. Med. Sci. 2014, 69, S4–S9. [Google Scholar] [CrossRef] [PubMed]
- Khansari, N.; Shakiba, Y.; Mahmoudi, M. Chronic inflammation and oxidative stress as a major cause of age-related diseases and cancer. Recent Pat. Inflamm. Allergy Drug Discov. 2009, 3, 73–80. [Google Scholar] [CrossRef] [PubMed]
- Nikolich-Zugich, J. Ageing and life-long maintenance of T-cell subsets in the face of latent persistent infections. Nat. Rev. Immunol. 2008, 8, 512–522. [Google Scholar] [CrossRef] [PubMed]
- Goronzy, J.J.; Fang, F.; Cavanagh, M.M.; Qi, Q.; Weyand, C.M. Naive T cell maintenance and function in human aging. J. Immunol. 2015, 194, 4073–4080. [Google Scholar] [CrossRef] [PubMed]
- Demaria, M.; Ohtani, N.; Youssef, S.A.; Rodier, F.; Toussaint, W.; Mitchell, J.R.; Laberge, R.M.; Vijg, J.; Steeg, H.V.; Dolle, M.E.T.; et al. An essential role for senescent cells in optimal wound healing through secretion of PDGF-AA. Dev. Cell 2014, 31, 722–733. [Google Scholar] [CrossRef] [PubMed]
- Jun, J.-I.; Lau, L.F. Cellular senescence controls fibrosis in wound healing. Aging (Albany NY) 2010, 2, 627–631. [Google Scholar] [CrossRef] [PubMed]
- Lanier, L.L. Shades of grey—The blurring view of innate and adaptive immunity. Nat. Rev. Immunol. 2013, 13, 73–74. [Google Scholar] [CrossRef] [PubMed]
- Wencker, M.; Turchinovich, G.; Barros, R.D.M.; Deban, L.; Jandke, A.; Cope, A.; Hayday, A.C. Innate-like T cells straddle innate and adaptive immunity by altering antigen-receptor responsiveness. Nat. Immunol. 2014, 15, 80–87. [Google Scholar] [CrossRef] [PubMed]
- Dadi, S.; Chhangawala, S.; Whitlock, B.M.; Huse, M.; Leslie, C.S.; Li, M.O. Cancer immunosurveillance by tissue-resident innate lymphoid cells and innate-like T cells. Cell 2016, 164, 365–377. [Google Scholar] [CrossRef] [PubMed]
- Novak, J.; Dobrovolny, J.; Novakova, L.; Kozak, T. The decrease in number and change in phenotype of mucosal-associated invariant T cells in the elderly and differences in men and women of reproductive age. Scand. J. Immunol. 2014, 80, 271–275. [Google Scholar] [CrossRef] [PubMed]
- Vasudev, A.; Tan, C.T.Y.; Ayyadhury, S.; Puan, K.J.; Andiappan, A.K.; Nyunt, M.S.Z.; Shadan, N.B.; Mustafa, S.; Low, I.; Rotzschke, O.; et al. γ/δ T cell subsets in human aging using the classical α/β T cell model. J. Leukoc. Biol. 2014, 96, 647–655. [Google Scholar] [CrossRef] [PubMed]
- Tan, C.; Wistuba-Hamprecht, K.; Xu, W.; Nyunt, M.S.Z.; Vasudev, A.; Lee, B.T.K.; Pawalec, G.; Puan, K.J.; Rotzschke, O.; Ng, T.P.; et al. Vδ2+ and α/ß T cells show divergent trajectories during human aging. Oncotarget 2016, 7, 44906–44918. [Google Scholar] [CrossRef] [PubMed]
- Ryan, P.L.; Sumaria, N.; Holland, C.J.; Bradford, C.M.; Izotova, N.; Grandjean, C.L.; Jawad, A.S.; Bergmeier, L.A.; Pennington, D.J. Heterogeneous yet stable Vδ2(+) T-cell profiles define distinct cytotoxic effector potentials in healthy human individuals. Proc. Natl. Acad. Sci. USA 2016, 113, 14378–14383. [Google Scholar] [CrossRef] [PubMed]
- Eberl, M.; Engel, R.; Aberle, S.; Fisch, P.; Jomaa, H. Human Vγ9/Vδ2 effector memory T cells express the killer cell lectin-like receptor G1 (KLRG1). J. Leukoc. Biol. 2005, 77, 16–19. [Google Scholar]
- Wistuba-Hamprecht, K.; Frasca, D.; Blomberg, B.; Pawelec, G.; Derhovanessian, E. Age-associated alterations in gammadelta T-cells are present predominantly in individuals infected with cytomegalovirus. Immun. Ageing 2013, 10, 26. [Google Scholar] [CrossRef] [PubMed]
- Davey, M.S.; Willcox, C.R.; Joyce, S.P.; Ladell, K.; Kasatskaya, S.A.; McLaren, J.E.; Hunter, S.; Salin, M.; Mohammed, F.; Price, D.A.; et al. Clonal selection in the human Vδ1 T cell repertoire indicates γδ TCR-dependent adaptive immune surveillance. Nat. Commun. 2017, 8, 14760. [Google Scholar] [CrossRef] [PubMed]
- Dominguez, C.X.; Amezquita, R.A.; Guan, T.; Marshall, H.D.; Joshi, N.S.; Kleinstein, S.H.; Kaech, S.M. The transcription factors Zeb2 and T-bet cooperate to program cytotoxic T cell terminal differentiation in response to LCMV viral infection. J. Exp. Med. 2015, 212, 2041–2056. [Google Scholar] [CrossRef] [PubMed]
- Yu, D.M.; Jung, S.H.; An, H.T.; Lee, S.; Hong, J.; Park, J.S.; Lee, H.; Bahn, M.S.; Lee, H.C.; Han, N.K.; et al. Caveolin-1 deficiency induces premature senescence with mitochondrial dysfunction. Aging Cell 2017, 16, 773–784. [Google Scholar] [CrossRef] [PubMed]
- Yu, D.; Du, Z.; Pian, L.; Li, T.; Wen, X.; Li, W.; Kim, S.J.; Xiao, J.; Cohen, P.; Cui, J.; et al. Mitochondrial DNA hypomethylation is a biomarker associated with induced senescence in human fetal heart mesenchymal stem Cells. Stem Cells Int. 2017, 2017, 1764549. [Google Scholar] [CrossRef] [PubMed]
- Ademowo, O.S.; Dias, H.K.I.; Burton, D.G.A.; Griffiths, H.R. Lipid (per) oxidation in mitochondria: An emerging target in the ageing process? Biogerontology 2017. [Google Scholar] [CrossRef] [PubMed]
- Mendelsohn, A.R.; Larrick, J.A. NAD+/PARP1/SIRT1 axis in aging. Rejuvenation Res. 2017, 20, 244–247. [Google Scholar] [CrossRef] [PubMed]
- Chatuvedi, P.; Tyagi, S.C. NAD+: A big player in cardiac and skeletal muscle remodeling and aging. J. Cell Physiol. 2017. [Google Scholar] [CrossRef]
- Verdin, E. NAD⁺ in aging, metabolism, and neurodegeneration. Science 2015, 350, 1208–1213. [Google Scholar] [CrossRef] [PubMed]
- Panda, A.C.; Abdelmohsen, K.; Gorospe, M. SASP regulation by noncoding RNA. Mech. Ageing Dev. 2017. [Google Scholar] [CrossRef] [PubMed]
- Xing, W.; Gao, W.; Mao, G.; Zhang, J.; Lv, X.; Wang, G.; Yan, J. Long non-coding RNAs in aging organs and tissues. Clin. Exp. Pharmacol. Physiol. 2017. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Xiong, K.; Shi, J.; Cui, Q.; Xue, L. A potential role of microRNAs in protein accumulation in cellular senescence analyzed by bioinformatics. PLoS ONE 2017, 12, e0179034. [Google Scholar] [CrossRef] [PubMed]
- Bu, H.; Wedel, S.; Cavinato, M.; Jansen-Dürr, P. MicroRNA regulation of oxidative stress-induced cellular senescence. Oxid. Med. Cell. Longev. 2017, 2017, 2398696. [Google Scholar] [CrossRef] [PubMed]
- Moskowitz, D.M.; Zhang, D.W.; Hu, B.; Le Saux, S.; Yanes, R.E.; Ye, Z.; Buenrostro, J.D.; Weyand, C.M.; Greenleaf, W.J.; Goronzy, J.J. Epigenomics of human CD8 T cell differentiation and aging. Sci. Immunol. 2017, 2, eaag0192. [Google Scholar] [CrossRef] [PubMed]
- Durek, P.; Nordstrom, K.; Gasparoni, G.; Salhab, A.; Kressler, K.C.; de Almeida, M.; Bassler, K.; Ulas, T.; Schmidt, F.; Xiong, J.; et al. Epigenomic profiling of human CD4+ T cells supports a linear differentiation model and highlights molecular regulators of memory development. Immunity 2016, 45, 1148–1161. [Google Scholar] [CrossRef] [PubMed]
- Schulyer, R.P.; Merkel, A.; Raineri, E.; Altucci, L.; Vellenga, E.; Martens, J.H.; Pourfarzad, F.; Kuijpers, T.W.; Burden, F.; Farrow, S.; et al. Distinct trends of DNA methylation patterning in the innate and adaptive immune systems. Cell Rep. 2016, 17, 2101–2111. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.; Wang, H.; Ren, J.; Chen, Q.; Chen, Z.J. cGAS is essential for cellular senescence. Proc. Natl. Acad. Sci. USA 2017, 114, E4612–E4620. [Google Scholar] [CrossRef] [PubMed]
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xu, W.; Larbi, A. Markers of T Cell Senescence in Humans. Int. J. Mol. Sci. 2017, 18, 1742. https://doi.org/10.3390/ijms18081742
Xu W, Larbi A. Markers of T Cell Senescence in Humans. International Journal of Molecular Sciences. 2017; 18(8):1742. https://doi.org/10.3390/ijms18081742
Chicago/Turabian StyleXu, Weili, and Anis Larbi. 2017. "Markers of T Cell Senescence in Humans" International Journal of Molecular Sciences 18, no. 8: 1742. https://doi.org/10.3390/ijms18081742
APA StyleXu, W., & Larbi, A. (2017). Markers of T Cell Senescence in Humans. International Journal of Molecular Sciences, 18(8), 1742. https://doi.org/10.3390/ijms18081742



