Galectin-3 Performance in Histologic and Cytologic Assessment of Thyroid Nodules: A Systematic Review and Meta-Analysis
Abstract
:1. Introduction
2. Results
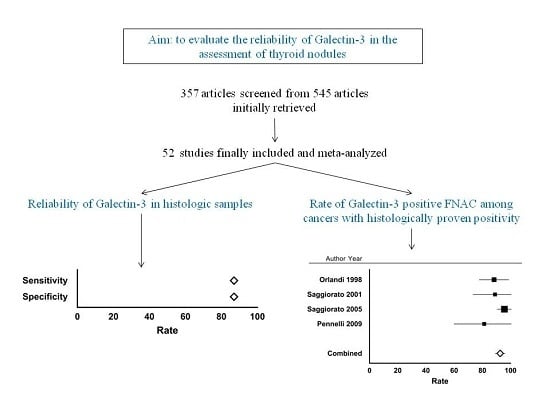
2.1. Eligible Articles and Description of the Studies
2.2. Qualitative Analysis
2.3. Quantitative Analysis (Meta-Analysis) of Gal-3 on Histology
2.4. Quantitative Analysis (Meta-Analysis) of Gal-3 on FNAC, and Concordance of Cytologic Results with Histologic Ones
3. Discussion
4. Materials and Methods
4.1. Search Strategy
4.2. Study Selection
4.3. Data Extraction
4.4. Statistical Analysis
Author Contributions
Conflicts of Interest
References
- Leffler, H.; Carlsson, S.; Hedlund, M.; Qian, Y.; Poirier, F. Introduction to galectins. Glycoconj. J. 2004, 19, 433–440. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.L.; Gray, R.M.; Haudek, K.C.; Patterson, R.J. Nucleocytoplasmic lectins. Biochim. Biophys. Acta 2004, 673, 75–93. [Google Scholar] [CrossRef] [PubMed]
- Fritsch, K.; Mernberger, M.; Nist, A.; Stiewe, T.; Brehm, A.; Jacob, R. Galectin-3 interacts with components of the nuclear ribonucleoprotein complex. BMC Cancer 2016, 16, 502. [Google Scholar] [CrossRef] [PubMed]
- Liu, F.T.; Rabinovich, G. Galectins as modulators of tumour progression. Nat. Rev. Cancer 2005, 5, 29–41. [Google Scholar] [CrossRef] [PubMed]
- Haudek, K.C.; Spronk, K.J.; Voss, P.G.; Patterson, R.J.; Wang, J.L.; Arnoys, E.J. Dynamics of Galectin-3 in the Nucleus and Cytoplasm. Biochim. Biophys. Acta 2010, 1800, 181–189. [Google Scholar] [CrossRef] [PubMed]
- Paron, I.; Scaloni, A.; Pines, A.; Bachi, A.; Liu, F.T.; Puppin, C.; Pandolfi, M.; Ledda, L.; Di Loreto, C.; Damante, G.; et al. Nuclear localization of Galectin-3 in transformed thyroid cells: A role in transcriptional regulation. Biochem. Biophys. Res. Commun. 2003, 302, 545–553. [Google Scholar] [CrossRef]
- Bartolazzi, A.; Orlandi, F.; Saggiorato, E.; Volante, M.; Arecco, F.; Rossetto, R.; Palestini, N.; Ghigo, E.; Papotti, M.; Bussolati, G.; et al. Italian Thyroid Cancer Study Group (ITCSG). Galectin-3-expression analysis in the surgical selection of follicular thyroid nodules with indeterminate fine-needle aspiration cytology: A prospective multicentre study. Lancet Oncol. 2008, 9, 543–549. [Google Scholar] [CrossRef]
- Bartolazzi, A.; Bellotti, C.; Sciacchitano, S. Methodology and technical requirements of the galectin-3 test for the preoperative characterization of thyroid nodules. Appl. Immunohistochem. Mol. Morphol. 2012, 20, 2–7. [Google Scholar] [CrossRef] [PubMed]
- Haugen, B.R.; Alexander, E.K.; Bible, K.C.; Doherty, G.M.; Mandel, S.J.; Nikiforov, Y.E.; Pacini, F.; Randolph, G.W.; Sawka, A.M.; Schlumberger, M.; et al. 2015 American Thyroid Association Management Guidelines for Adult Patients with Thyroid Nodules and Differentiated Thyroid Cancer: The American Thyroid Association Guidelines Task Force on Thyroid Nodules and Differentiated Thyroid Cancer. Thyroid 2016, 26, 1–133. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.C.; el-Naggar, A.K.; Lotan, R. Differential expression of galectin-1 and galectin-3 in thyroid tumors. Potential diagnostic implications. Am. J. Pathol. 1995, 147, 815–822. [Google Scholar] [PubMed]
- Fernández, P.L.; Merino, M.J.; Gómez, M.; Campo, E.; Medina, T.; Castronovo, V.; Sanjuán, X.; Cardesa, A.; Liu, F.T.; Sobel, M.E. Galectin-3 and laminin expression in neoplastic and non-neoplastic thyroid tissue. J. Pathol. 1997, 181, 80–86. [Google Scholar] [CrossRef]
- Cvejic, D.; Savin, S.; Paunovic, I.; Tatic, S.; Havelka, M.; Sinadinovic, J. Immunohistochemical localization of galectin-3 in malignant and benign human thyroid tissue. Anticancer Res. 1998, 18, 2637–2641. [Google Scholar] [PubMed]
- Orlandi, F.; Saggiorato, E.; Pivano, G.; Puligheddu, B.; Termine, A.; Cappia, S.; De Giuli, P.; Angeli, A. Galectin-3 is a presurgical marker of human thyroid carcinoma. Cancer Res. 1998, 58, 3015–3020. [Google Scholar] [PubMed]
- Inohara, H.; Honjo, Y.; Yoshii, T.; Akahani, S.; Yoshida, J.; Hattori, K.; Okamoto, S.; Sawada, T.; Raz, A.; Kubo, T. Expression of galectin-3 in fine-needle aspirates as a diagnostic marker differentiating benign from malignant thyroid neoplasms. Cancer 1999, 85, 2475–2484. [Google Scholar] [CrossRef]
- Saggiorato, E.; Cappia, S.; De Giuli, P.; Mussa, A.; Pancani, G.; Caraci, P.; Angeli, A.; Orlandi, F. Galectin-3 as a presurgical immunocytodiagnostic marker of minimally invasive follicular thyroid carcinoma. J. Clin. Endocrinol. Metab. 2001, 86, 5152–5158. [Google Scholar] [CrossRef] [PubMed]
- Bartolazzi, A.; Gasbarri, A.; Papotti, M.; Bussolati, G.; Lucante, T.; Khan, A.; Inohara, H.; Marandino, F.; Orlandi, F.; Nardi, F.; et al. Thyroid Cancer Study Group. Application of an immunodiagnostic method for improving preoperative diagnosis of nodular thyroid lesions. Lancet 2001, 357, 1644–1650. [Google Scholar] [CrossRef]
- Beesley, M.F.; McLaren, K.M. Cytokeratin 19 and galectin-3 immunohistochemistry in the differential diagnosis of solitary thyroid nodules. Histopathology 2002, 41, 236–243. [Google Scholar] [CrossRef] [PubMed]
- Herrmann, M.E.; LiVolsi, V.A.; Pasha, T.L.; Roberts, S.A.; Wojcik, E.M.; Baloch, Z.W. Immunohistochemical expression of galectin-3 in benign and malignant thyroid lesions. Arch. Pathol. Lab. Med. 2002, 126, 710–713. [Google Scholar] [PubMed]
- Cvejić, D.; Savin, S.; Petrović, I.; Paunović, I.; Tatić, S.; Havelka, M. Differential expression of galectin-3 in papillary projections of malignant and non-malignant hyperplastic thyroid lesions. Acta. Chir. Iugosl. 2003, 50, 67–70. [Google Scholar] [CrossRef] [PubMed]
- Giannini, R.; Faviana, P.; Cavinato, T.; Elisei, R.; Pacini, F.; Berti, P.; Fontanini, G.; Ugolini, C.; Camacci, T.; De Ieso, K.; et al. Galectin-3 and oncofetal-fibronectin expression in thyroid neoplasia as assessed by reverse transcription-polymerase chain reaction and immunochemistry in cytologic and pathologic specimens. Thyroid 2003, 13, 765–770. [Google Scholar] [CrossRef] [PubMed]
- Jakubiak-Wielganowicz, M.; Kubiak, R.; Sygut, J.; Pomorski, L.; Kordek, R. Usefulness of galectin-3 immunohistochemistry in differential diagnosis between thyroid follicular carcinoma and follicular adenoma. Pol. J. Pathol. 2003, 54, 111–115. [Google Scholar] [PubMed]
- Kovács, R.B.; Földes, J.; Winkler, G.; Bodó, M.; Sápi, Z. The investigation of galectin-3 in diseases of the thyroid gland. Eur. J. Endocrinol. 2003, 149, 449–453. [Google Scholar] [CrossRef] [PubMed]
- Mehrotra, P.; Okpokam, A.; Bouhaidar, R.; Johnson, S.J.; Wilson, J.A.; Davies, B.R.; Lennard, T.W. Galectin-3 does not reliably distinguish benign from malignant thyroid neoplasms. Histopathology 2004, 45, 493–500. [Google Scholar] [CrossRef] [PubMed]
- Oestreicher-Kedem, Y.; Halpern, M.; Roizman, P.; Hardy, B.; Sulkes, J.; Feinmesser, R.; Stern, Y. Diagnostic value of galectin-3 as a marker for malignancy in follicular patterned thyroid lesions. Head Neck 2004, 26, 960–966. [Google Scholar] [CrossRef] [PubMed]
- Weber, K.B.; Shroyer, K.R.; Heinz, D.E.; Nawaz, S.; Said, M.S.; Haugen, B.R. The use of a combination of galectin-3 and thyroid peroxidase for the diagnosis and prognosis of thyroid cancer. Am. J. Clin. Pathol. 2004, 122, 524–531. [Google Scholar] [CrossRef] [PubMed]
- Saggiorato, E.; De Pompa, R.; Volante, M.; Cappia, S.; Arecco, F.; Dei Tos, A.P.; Orlandi, F.; Papotti, M. Characterization of thyroid ‘follicular neoplasms’ in fine-needle aspiration cytological specimens using a panel of immunohistochemical markers: A proposal for clinical application. Endocr. Relat. Cancer 2005, 12, 305–317. [Google Scholar] [CrossRef] [PubMed]
- De Matos, P.S.; Ferreira, A.P.; de Oliveira Facuri, F.; Assumpção, L.V.; Metze, K.; Ward, L.S. Usefulness of HBME-1, cytokeratin 19 and galectin-3 immunostaining in the diagnosis of thyroid malignancy. Histopathology 2005, 47, 391–401. [Google Scholar] [CrossRef] [PubMed]
- Galusca, B.; Dumollard, J.M.; Lassandre, S.; Niveleau, A.; Prades, J.M.; Estour, B.; Peoc’h, M. Global DNA methylation evaluation: Potential complementary marker in differential diagnosis of thyroid neoplasia. Virchows Arch. 2005, 447, 18–23. [Google Scholar] [CrossRef] [PubMed]
- Nucera, C.; Mazzon, E.; Caillou, B.; Violi, M.A.; Moleti, M.; Priolo, C.; Sturniolo, G.; Puzzolo, D.; Cavallari, V.; Trimarchi, F.; et al. Human galectin-3 immunoexpression in thyroid follicular adenomas with cell atypia. J. Endocrinol. Investig. 2005, 28, 106–112. [Google Scholar] [CrossRef]
- Prasad, M.L.; Pellegata, N.S.; Huang, Y.; Nagaraja, H.N.; de la Chapelle, A.; Kloos, R.T. Galectin-3, fibronectin-1, CITED-1, HBME1 and cytokeratin-19 immunohistochemistry is useful for the differential diagnosis of thyroid tumors. Mod. Pathol. 2005, 18, 48–57. [Google Scholar] [CrossRef] [PubMed]
- Jo, Y.S.; Li, S.; Song, J.H.; Kwon, K.H.; Lee, J.C.; Rha, S.Y.; Lee, H.J.; Sul, J.Y.; Kweon, G.R.; Ro, H.K.; et al. Influence of the BRAF V600E mutation on expression of vascular endothelial growth factor in papillary thyroid cancer. J. Clin. Endocrinol. Metab. 2006, 91, 3667–3670. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, N.; Erickson, L.A.; Jin, L.; Kajita, S.; Zhang, H.; Qian, X.; Rumilla, K.; Lloyd, R.V. Immunohistochemical separation of follicular variant of papillary thyroid carcinoma from follicular adenoma. Endocr. Pathol. 2006, 17, 213–223. [Google Scholar] [CrossRef] [PubMed]
- Rossi, E.D.; Raffaelli, M.; Mule’, A.; Miraglia, A.; Lombardi, C.P.; Vecchio, F.M.; Fadda, G. Simultaneous immunohistochemical expression of HBME-1 and galectin-3 differentiates papillary carcinomas from hyperfunctioning lesions of the thyroid. Histopathology 2006, 48, 795–800. [Google Scholar] [CrossRef] [PubMed]
- Scognamiglio, T.; Hyjek, E.; Kao, J.; Chen, Y.T. Diagnostic usefulness of HBME1, galectin-3, CK19, and CITED1 and evaluation of their expression in encapsulated lesions with questionable features of papillary thyroid carcinoma. Am. J. Clin. Pathol. 2006, 126, 700–708. [Google Scholar] [CrossRef] [PubMed]
- Sapio, M.R.; Guerra, A.; Posca, D.; Limone, P.P.; Deandrea, M.; Motta, M.; Troncone, G.; Caleo, A.; Vallefuoco, P.; Rossi, G.; et al. Combined analysis of galectin-3 and BRAFV600E improves the accuracy of fine-needle aspiration biopsy with cytological findings suspicious for papillary thyroid carcinoma. Endocr. Relat. Cancer 2007, 14, 1089–1097. [Google Scholar] [CrossRef] [PubMed]
- Coli, A.; Bigotti, G.; Parente, P.; Federico, F.; Castri, F.; Massi, G. Atypical thyroid nodules express both HBME-1 and Galectin-3, two phenotypic markers of papillary thyroid carcinoma. J. Exp. Clin. Cancer Res. 2007, 26, 221–227. [Google Scholar] [PubMed]
- Park, Y.J.; Kwak, S.H.; Kim, D.C.; Kim, H.; Choe, G.; Park, D.J.; Jang, H.C.; Park, S.H.; Cho, B.Y.; Park, S.Y. Diagnostic value of galectin-3, HBME-1, cytokeratin 19, high molecular weight cytokeratin, cyclin D1 and p27(kip1) in the differential diagnosis of thyroid nodules. J. Korean Med. Sci. 2007, 22, 621–628. [Google Scholar] [CrossRef] [PubMed]
- Aiad, H.A.; Kandil, M.A.; Asaad, N.Y.; El-Kased, A.M.; El-Goday, S.F. Galectin-3 immunostaining in cytological and histopathological diagnosis of thyroid lesions. J. Egypt. Natl. Cancer Inst. 2008, 20, 36–46. [Google Scholar]
- Hooft, L.; van der Veldt, A.A.; Hoekstra, O.S.; Boers, M.; Molthoff, C.F.; van Diest, P.J. Hexokinase III, cyclin A and galectin-3 are overexpressed in malignant follicular thyroid nodules. Clin. Endocrinol. Oxf. 2008, 68, 252–257. [Google Scholar] [CrossRef] [PubMed]
- Inohara, H.; Segawa, T.; Miyauchi, A.; Yoshii, T.; Nakahara, S.; Raz, A.; Maeda, M.; Miyoshi, E.; Kinoshita, N.; Yoshida, H.; et al. Cytoplasmic and serum galectin-3 in diagnosis of thyroid malignancies. Biochem. Biophys. Res. Commun. 2008, 376, 605–610. [Google Scholar] [CrossRef] [PubMed]
- Savin, S.; Cvejic, D.; Isic, T.; Paunovic, I.; Tatic, S.; Havelka, M. Thyroid peroxidase and galectin-3 immunostaining in differentiated thyroid carcinoma with clinicopathologic correlation. Hum. Pathol. 2008, 39, 1656–1663. [Google Scholar] [CrossRef] [PubMed]
- Than, T.H.; Swethadri, G.K.; Wong, J.; Ahmad, T.; Jamil, D.; Maganlal, R.K.; Hamdi, M.M.; Abdullah, M.S. Expression of Galectin-3 and Galectin-7 in thyroid malignancy as potential diagnostic indicators. Singap. Med. J. 2008, 49, 333–338. [Google Scholar]
- Pennelli, G.; Mian, C.; Pelizzo, M.R.; Naccamulli, D.; Piotto, A.; Girelli, M.E.; Mescoli, C.; Rugge, M. Galectin-3 cytotest in thyroid follicular neoplasia: A prospective, monoinstitutional study. Acta Cytol. 2009, 53, 533–539. [Google Scholar] [CrossRef] [PubMed]
- Barut, F.; Onak Kandemir, N.; Bektas, S.; Bahadir, B.; Keser, S.; Ozdamar, S.O. Universal markers of thyroid malignancies: Galectin-3, HBME-1, and cytokeratin-19. Endocr. Pathol. 2010, 21, 80–89. [Google Scholar] [CrossRef] [PubMed]
- Išić, T.; Savin, S.; Cvejić, D.; Marečko, I.; Tatić, S.; Havelka, M.; Paunović, I. Serum Cyfra 21.1 and galectin-3 protein levels in relation to immunohistochemical cytokeratin 19 and galectin-3 expression in patients with thyroid tumors. J. Cancer Res. Clin. Oncol. 2010, 136, 1805–1812. [Google Scholar] [CrossRef] [PubMed]
- Saleh, H.A.; Jin, B.; Barnwell, J.; Alzohaili, O. Utility of immunohistochemical markers in differentiating benign from malignant follicular-derived thyroid nodules. Diagn. Pathol. 2010, 5, 9. [Google Scholar] [CrossRef] [PubMed]
- Zhu, X.; Sun, T.; Lu, H.; Zhou, X.; Lu, Y.; Cai, X.; Zhu, X. Diagnostic significance of CK19, RET, galectin-3 and HBME-1 expression for papillary thyroid carcinoma. J. Clin. Pathol. 2010, 63, 786–989. [Google Scholar] [CrossRef] [PubMed]
- Song, Q.; Wang, D.; Lou, Y.; Li, C.; Fang, C.; He, X.; Li, J. Diagnostic significance of CK19, TG, Ki67 and galectin-3 expression for papillary thyroid carcinoma in the northeastern region of China. Diagn. Pathol. 2011, 6, 126. [Google Scholar] [CrossRef] [PubMed]
- Abulkheir, I.L.; Mohammad, D.B. Value of immunohistochemical expression of p27 and galectin-3 in differentiation between follicular adenoma and follicular carcinoma. Appl. Immunohistochem. Mol. Morphol. 2012, 20, 131–140. [Google Scholar] [CrossRef] [PubMed]
- Cui, W.; Sang, W.; Zheng, S.; Ma, Y.; Liu, X.; Zhang, W. Usefulness of cytokeratin-19, galectin-3, and Hector Battifora mesothelial-1 in the diagnosis of benign and malignant thyroid nodules. Clin. Lab. 2012, 58, 673–680. [Google Scholar] [PubMed]
- Gong, L.; Chen, P.; Liu, X.; Han, Y.; Zhou, Y.; Zhang, W.; Li, H.; Li, C.; Xie, J. Expressions of D2-40, CK19, galectin-3, VEGF and EGFR in papillary thyroid carcinoma. Gland Surg. 2012, 1, 25–32. [Google Scholar] [PubMed]
- Manivannan, P.; Siddaraju, N.; Jatiya, L.; Verma, S.K. Role of pro-angiogenic marker galectin-3 in follicular neoplasms of thyroid. Indian J. Biochem. Biophys. 2012, 49, 392–394. [Google Scholar] [PubMed]
- Mataraci, E.A.; Ozgüven, B.Y.; Kabukçuoglu, F. Expression of cytokeratin 19, HBME-1 and galectin-3 in neoplastic and nonneoplastic thyroid lesions. Pol. J. Pathol. 2012, 63, 58–64. [Google Scholar] [PubMed]
- Guerra, A.; Marotta, V.; Deandrea, M.; Motta, M.; Limone, P.P.; Caleo, A.; Zeppa, P.; Esposito, S.; Fulciniti, F.; Vitale, M. BRAF (V600E) associates with cytoplasmatic localization of p27kip1 and higher cytokeratin 19 expression in papillary thyroid carcinoma. Endocrine 2013, 44, 165–171. [Google Scholar] [CrossRef] [PubMed]
- Wu, G.; Wang, J.; Zhou, Z.; Li, T.; Tang, F. Combined staining for immunohistochemical markers in the diagnosis of papillary thyroid carcinoma: Improvement in the sensitivity or specificity? J. Int. Med. Res. 2013, 41, 975–983. [Google Scholar] [CrossRef] [PubMed]
- Abd-El Raouf, S.M.; Ibrahim, T.R. Immunohistochemical expression of HBME-1 and galectin-3 in the differential diagnosis of follicular-derived thyroid nodules. Pathol. Res. Pract. 2014, 210, 971–978. [Google Scholar] [CrossRef] [PubMed]
- Ma, H.; Xu, S.; Yan, J.; Zhang, C.; Qin, S.; Wang, X.; Li, N. The value of tumor markers in the diagnosis of papillary thyroid carcinoma alone and in combination. Pol. J. Pathol. 2014, 65, 202–209. [Google Scholar] [CrossRef] [PubMed]
- Ceyran, A.B.; Şenol, S.; Şimşek, B.Ç.; Sağıroğlu, J.; Aydın, A. Role of cd56 and e-cadherin expression in the differential diagnosis of papillary thyroid carcinoma and suspected follicular-patterned lesions of the thyroid: The prognostic importance of e-cadherin. Int. J. Clin. Exp. Pathol. 2015, 8, 3670–3680. [Google Scholar] [PubMed]
- Sumana, B.S.; Shashidhar, S.; Shivarudrappa, A.S. Galectin-3 Immunohistochemical Expression in Thyroid Neoplasms. J. Clin. Diagn. Res. 2015, 9, EC07–EC11. [Google Scholar] [CrossRef] [PubMed]
- Al-Sharaky, D.R.; Younes, S.F. Sensitivity and Specificity of Galectin-3 and Glypican-3 in Follicular-Patterned and Other Thyroid Neoplasms. J. Clin. Diagn. Res. 2016, 10, EC06–EC10. [Google Scholar] [CrossRef] [PubMed]
- Chao, T.T.; Maa, H.C.; Wang, C.Y.; Pei, D.; Liang, Y.J.; Yang, Y.F.; Chou, S.J.; Chen, Y.L. CIP2A is a poor prognostic factor and can be a diagnostic marker in papillary thyroid carcinoma. APMIS 2016, 124, 1031–1037. [Google Scholar] [CrossRef] [PubMed]
- Chiu, C.G.; Strugnell, S.S.; Griffith, O.L.; Jones, S.J.; Gown, A.M.; Walker, B.; Nabi, I.R.; Wiseman, S.M. Diagnostic utility of galectin-3 in thyroid cancer. Am. J. Pathol. 2010, 176, 2067–2081. [Google Scholar] [CrossRef] [PubMed]
- Choi, J.; Lee, H.E.; Kim, M.A.; Jang, B.G.; Lee, H.S.; Kim, W.H. Analysis of MET mRNA Expression in Gastric Cancers Using RNA In situ hybridization assay: Its clinical implication and comparison with immunohistochemistry and silver in situ hybridization. PLoS ONE 2014, 9, e111658. [Google Scholar] [CrossRef] [PubMed]
- Yu, H.; Batenchuk, C.; Badzio, A.; Boyle, T.A.; Czapiewski, P.; Chan, D.C.; Lu, X.; Gao, D.; Ellison, K.; Kowalewski, A.A.; et al. Expression by two complementary diagnostic assays and mRNA in situ hybridization in small cell lung cancer. J. Thorac. Oncol. 2017, 12, 110–120. [Google Scholar] [CrossRef] [PubMed]
- Rago, T.; Scutari, M.; Latrofa, F.; Loiacono, V.; Piaggi, P.; Marchetti, I.; Romani, R.; Basolo, F.; Miccoli, P.; Tonacchera, M.; et al. The large majority of 1520 patients with indeterminate thyroid nodule at cytology have a favorable outcome, and a clinical risk score has a high negative predictive value for a more cumbersome cancer disease. J. Clin. Endocrinol. Metab. 2014, 99, 3700–3707. [Google Scholar] [CrossRef] [PubMed]
- Trimboli, P.; Bongiovanni, M.; Rossi, F.; Guidobaldi, L.; Crescenzi, A.; Ceriani, L.; Nigri, G.; Valabrega, S.; Romanelli, F.; Giovanella, L. Differentiated thyroid cancer patients with a previous indeterminate (Thy 3) cytology have a better prognosis than those with suspicious or malignant FNAC reports. Endocrine 2015, 49, 191–195. [Google Scholar] [CrossRef] [PubMed]
- Sciacchitano, S.; Lavra, L.; Ulivieri, A.; Magi, F.; Porcelli, T.; Amendola, S.; De Francesco, G.P.; Bellotti, C.; Trovato, M.C.; Salehi, L.B.; et al. Combined clinical and ultrasound follow-up assists in malignancy detection in Galectin-3 negative Thy-3 thyroid nodules. Endocrine 2016, 54, 139–147. [Google Scholar] [CrossRef] [PubMed]
- Sciacchitano, S.; Lavra, L.; Ulivieri, A.; Magi, F.; Paolo De Francesco, G.; Bellotti, C.; Salehi, L.B.; Trovato, M.; Drago, C.; Bartolazzi, A. Comparative analysis of diagnostic performance, feasibility and cost of different test-methods for thyroid nodules with indeterminate cytology. Oncotarget 2017. [Google Scholar] [CrossRef] [PubMed]
- Carpi, A.; Naccarato, A.G.; Iervasi, G.; Nicolini, A.; Bevilacqua, G.; Viacava, P.; Collecchi, P.; Lavra, L.; Marchetti, C.; Sciacchitano, S.; et al. Large needle aspiration biopsy and galectin-3 determination in selected thyroid nodules with indeterminate FNA-cytology. Br. J. Cancer 2006, 95, 204–209. [Google Scholar] [CrossRef] [PubMed]
- Carpi, A.; Rossi, G.; Coscio, G.D.; Iervasi, G.; Nicolini, A.; Carpi, F.; Mechanick, J.I.; Bartolazzi, A. Galectin-3 detection on large-needle aspiration biopsy improves preoperative selection of thyroid nodules: A prospective cohort study. Ann. Med. 2010, 42, 70–78. [Google Scholar] [CrossRef] [PubMed]
- Trimboli, P.; Guidobaldi, L.; Amendola, S.; Nasrollah, N.; Romanelli, F.; Attanasio, D.; Ramacciato, G.; Saggiorato, E.; Valabrega, S.; Crescenzi, A. Galectin-3 and HBME-1 improve the accuracy of core biopsy in indeterminate thyroid nodules. Endocrine 2016, 52, 39–45. [Google Scholar] [CrossRef] [PubMed]
- Makki, F.M.; Taylor, S.M.; Shahnavaz, A.; Leslie, A.; Gallant, J.; Douglas, S.; The, E.; Trites, J.; Bullock, M.; Inglis, K.; et al. Serum biomarkers of papillary thyroid cancer. J. Otolaryngol. Head Neck Surg. 2013, 42, 16. [Google Scholar] [CrossRef] [PubMed]
- Saussez, S.; Glinoer, D.; Chantrain, G.; Pattou, F.; Carnaille, B.; André, S.; Gabius, H.J.; Laurent, G. Serum galectin-1 and galectin-3 levels in benign and malignant nodular thyroid disease. Thyroid 2008, 18, 705–712. [Google Scholar] [CrossRef] [PubMed]
- Yılmaz, E.; Karşıdağ, T.; Tatar, C.; Tüzün, S. Serum Galectin-3: Diagnostic value for papillary thyroid carcinoma. Ulus. Cerrahi Derg. 2015, 31, 192–196. [Google Scholar] [CrossRef] [PubMed]
- D’Alessandria, C.; Braesch-Andersen, S.; Bejo, K.; Reder, S.; Blechert, B.; Schwaiger, M.; Bartolazzi, A. Noninvasive In Vivo Imaging and Biologic Characterization of Thyroid Tumors by ImmunoPET Targeting of Galectin-3. Cancer Res. 2016, 76, 3583–3592. [Google Scholar] [CrossRef] [PubMed]
- Bartolazzi, A.; D’Alessandria, C.; Parisella, M.G.; Signore, A.; Del Prete, F.; Lavra, L.; Braesch-Andersen, S.; Massari, R.; Trotta, C.; Soluri, A.; et al. Thyroid cancer imaging in vivo by targeting the anti-apoptotic molecule galectin-3. PLoS ONE 2008, 3, e3768. [Google Scholar] [CrossRef] [PubMed]
- Campennì, A.; Siracusa, M.; Ruggeri, R.M.; Laudicella, R.; Pignata, S.A.; Baldari, S.; Giovanella, L. Differentiating malignant from benign thyroid nodules with indeterminate cytology by 99Tc-MIBI scan: A new quantitative method for improving diagnostic accuracy. Sci. Rep. 2017, 7, 6147. [Google Scholar] [CrossRef] [PubMed]
- Campennì, A.; Giovanella, L.; Siracusa, M.; Alibrandi, A.; Pignata, S.A.; Giovinazzo, S.; Trimarchi, F.; Ruggeri, R.M.; Baldari, S. (99 m)Tc-Methoxy-Isobutyl-Isonitrile Scintigraphy Is a Useful Tool for Assessing the Risk of Malignancy in Thyroid Nodules with Indeterminate Fine-Needle Cytology. Thyroid 2016, 26, 1101–1109. [Google Scholar] [CrossRef] [PubMed]
- Piccardo, A.; Puntoni, M.; Treglia, G.; Foppiani, L.; Bertagna, F.; Paparo, F.; Massollo, M.; Dib, B.; Paone, G.; Arlandini, A.; et al. Thyroid nodules with indeterminate cytology: Prospective comparison between 18F-FDG-PET/CT, multiparametric neck ultrasonography, 99 mTc-MIBI scintigraphy and histology. Eur. J. Endocrinol. 2016, 174, 693–703. [Google Scholar] [CrossRef] [PubMed]
- Heinzel, A.; Müller, D.; Behrendt, F.F.; Giovanella, L.; Mottaghy, F.M.; Verburg, F.A. Thyroid nodules with indeterminate cytology: Molecular imaging with ⁹⁹mTc-methoxyisobutylisonitrile (MIBI) is more cost-effective than the Afirma gene expression classifier. Eur. J. Nucl. Med. Mol. Imaging 2014, 41, 1497–1500. [Google Scholar] [CrossRef] [PubMed]
- Giovanella, L.; Campenni, A.; Treglia, G.; Verburg, F.A.; Trimboli, P.; Ceriani, L.; Bongiovanni, M. Molecular imaging with (99 m)Tc-MIBI and molecular testing for mutations in differentiating benign from malignant follicular neoplasm: A prospective comparison. Eur. J. Nucl. Med. Mol. Imaging 2016, 43, 1018–1026. [Google Scholar] [CrossRef] [PubMed]



| Samples | Total Lesions | Carcinomas | Carcinomas with Gal-3+ (%) | Benign Lesions | Benign Lesions with Gal-3− (%) |
|---|---|---|---|---|---|
| Histologic data | 8172 | 4237 | 3654 (86%) | 3935 | 3341 (85%) |
| Cytologic data (cell-block preparation from FNAC samples) | 358 | 142 | 129 (91%) | 216 | 194 (90%) |
| Samples | Malignant | Benign | Borderline | Positive Predictive Value |
|---|---|---|---|---|
| Galectin-3 positive | 101 | 22 | 11 | 82% |
| Galectin-3 negative | 280 | 29 | 22 | 91% |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Trimboli, P.; Virili, C.; Romanelli, F.; Crescenzi, A.; Giovanella, L. Galectin-3 Performance in Histologic and Cytologic Assessment of Thyroid Nodules: A Systematic Review and Meta-Analysis. Int. J. Mol. Sci. 2017, 18, 1756. https://doi.org/10.3390/ijms18081756
Trimboli P, Virili C, Romanelli F, Crescenzi A, Giovanella L. Galectin-3 Performance in Histologic and Cytologic Assessment of Thyroid Nodules: A Systematic Review and Meta-Analysis. International Journal of Molecular Sciences. 2017; 18(8):1756. https://doi.org/10.3390/ijms18081756
Chicago/Turabian StyleTrimboli, Pierpaolo, Camilla Virili, Francesco Romanelli, Anna Crescenzi, and Luca Giovanella. 2017. "Galectin-3 Performance in Histologic and Cytologic Assessment of Thyroid Nodules: A Systematic Review and Meta-Analysis" International Journal of Molecular Sciences 18, no. 8: 1756. https://doi.org/10.3390/ijms18081756
APA StyleTrimboli, P., Virili, C., Romanelli, F., Crescenzi, A., & Giovanella, L. (2017). Galectin-3 Performance in Histologic and Cytologic Assessment of Thyroid Nodules: A Systematic Review and Meta-Analysis. International Journal of Molecular Sciences, 18(8), 1756. https://doi.org/10.3390/ijms18081756