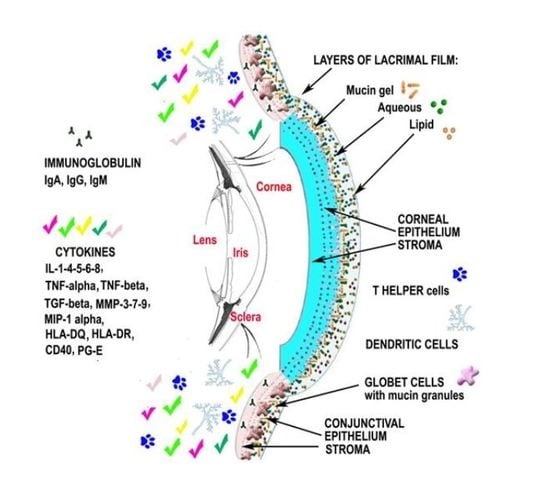
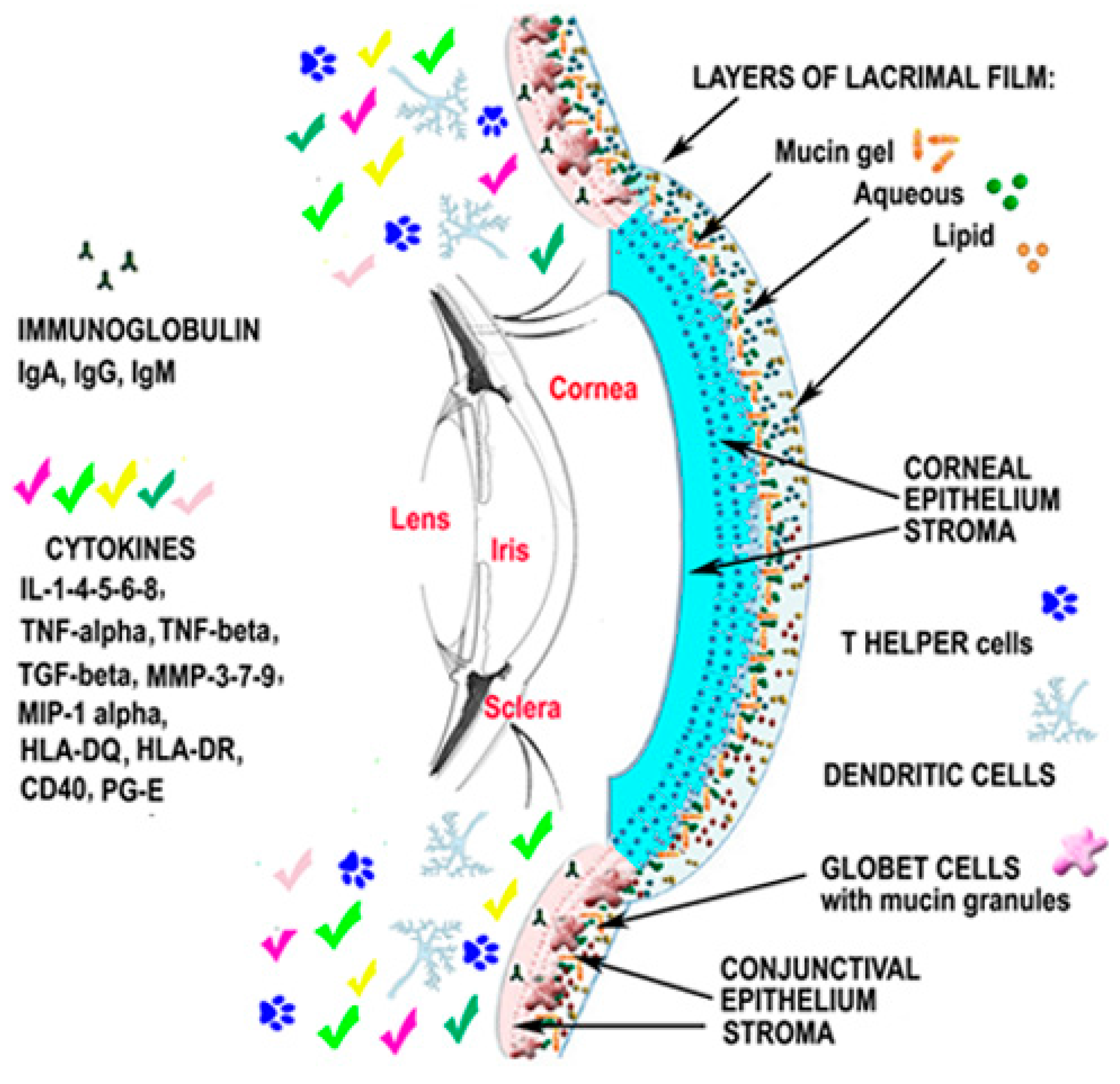
Analysis of the Pathogenic Factors and Management of Dry Eye in Ocular Surface Disorders
Abstract
:1. Introduction
2. Methods
2.1. Dysfunction of Meibomian Glands
2.1.1. Primary Dysfunction of Meibomian Glands
2.1.2. Secondary Dysfunction of Meibomian Glands to Chronic Inflammation
2.2. Dysfunction of Meibomian Glands and Aqueous Deficit: Mixed Forms
2.2.1. Aging and Lacrimal Discomfort
2.2.2. Aging and Associated Factors
- The chronic use of systemic medications, such as antidepressants, diuretics, dopamine such as those used for Parkinson's disease, and anti-metabolites commonly used to treat rheumatoid arthritis. Depending on their mechanism of action, these drugs affect lacrimal secretion. In Parkinson’s patients, for example, dopaminergic dysfunction is thought to play a role in decreasing the blink reflex that leads to DE, in addition to a physiological decrease of corneal sensitivity with age that increases the risk of exposure keratopathy. In addition, the decrease in liver and kidney function lengthens the clearance time of systemic drugs.
- The chronic use of topical medications as for glaucoma. β blockers, α-adrenergics, and prostaglandins can reduce tear production. They can cause chronic irritation of the ocular surface that compromises the integrity of the lacrimal glands. It has been highlighted that a reduction of glandular density and area, and increased viscosity of the meibum, can occur. In addition, the presence of preservatives, such as benzalkonium chloride (BAK), can cause tear film instability, GCs loss, conjunctival squamous metaplasia and apoptosis, disruption of the corneal epithelium barrier, and damage to deeper ocular tissues even at low concentrations [20].
- Abnormalities in the eyelid positioning, as laxity, floppy eyelid syndrome, retraction, entropion, ectropion, and lagophthalmos. Horizontal lid laxity is the most common cause of involutional eyelid malposition. Eyelid malposition leads to corneal exposure, poor tear-film distribution, and abnormal tear outflow. As many as 50–70% of patients with this disease develop tear discomfort syndrome [21].
- Conjunctivochalasis is another notable contributor to poor tear outflow, and is characterized by a redundant bulbar conjunctiva interposed between the globe and the eyelid [22]. Pathogenesis of conjunctivochalasis is under investigation; however, elastotic degeneration from cumulative sun exposure and inflammatory degeneration from delayed tear film clearance have been proposed [23]. Once formed, the redundant folds interfere with the inferior tear meniscus, and in some cases cause the occlusion of the inferior punctum.
- A gradual reduction in corneal sensitivity has been shown to occur with increasing age, which predisposes older adults to DE. Roszkowska et al. reported that the mechanical sensitivity of the peripheral cornea decreases gradually throughout life, whereas central corneal sensitivity remains stable until 60, and then decreases sharply subsequently [24].
- Oxidative stress, a counterpart of inflammation, occurs when antioxidants are unable to counteract reactive oxygen species (ROS) that are generated in normal metabolic processes. The production of aggressive oxygen species, such as free radicals and peroxides, leads to DNA damage over time, inducing cell necrosis and the impairment of the regenerative capacity of the corneal epithelial cells. In younger, healthy subjects, low levels of ROS are counteracted by antioxidant enzymes [25].
2.3. Aqueous Deficits in Autoimmune Diseases: Sjögren’s Syndrome and Non-Sjögren’s Syndrome Dry Eye
2.4. Other Major Causes of Mixed Forms
2.4.1. Hormonal Changes
2.4.2. Use of Contact Lenses, Computers, and Interactive Environments
2.4.3. Dry Eye and Allergic Diseases
2.4.4. Dry Eye and Refractive/Cataract Surgery
2.5. Tear Film and Specific Pathologies of the Ocular Surface
2.5.1. Stevens–Johnson Syndrome and Toxic Epidermal Necrolysis
2.5.2. Keratoconus
2.5.3. Pterygium
2.6. Up-To-Date on Biochemical Mechanisms in Dry Eye Disease
2.7. Therapeutical Pragmatic Approach
2.7.1. Topical Nonsteroidal Anti-Inflammatory (NSIAD)
2.7.2. New Topical Natural Substance
2.7.3. Secretagogues
2.7.4. Topical Immunomodulators
2.7.5. Systemic Therapy
3. Conclusions
Author Contributions
Conflicts of Interest
References
- Chia, E.M.; Mitchell, P.; Rochtchina, E.; Lee, A.J.; Maroun, R.; Wang, J.J. Prevalence and associations of dry eye syndrome in an older population: The Blue Mountains Eye Study. Clin. Exp. Ophthalmol. 2003, 31, 229–232. [Google Scholar] [CrossRef] [PubMed]
- Dogru, M.; Foulks, G.N.; Kinoshita, S.; Laibson, P.; McCulley, J.; Murube, J.; Pflugfelder, S.C.; Rolando, M.; Toda, I. The definition and classification of dry eye disease: Report of the definition and classification subcommittee of the international dry eye workshop (2007). Ocul. Surf. 2007, 5, 75–92. [Google Scholar]
- Lemp, M.A.; Crews, L.A.; Bron, A.J.; Foulks, G.N.; Sullivan, B.D. Distribution of acqueous-deficient and evaporative dry eye in a clinic-based patient cohort: A retrospective study. Cornea 2012, 31, 472–478. [Google Scholar] [CrossRef] [PubMed]
- Knop, E.; Knop, N.; Millar, T.; Obata, H.; Sullivan, D.A. The International Workshop on Meibomian Gland Dysfunction: Report of the Subcommittee on Anatomy, Physiology, and Pathophysiology of the Meibomian Gland. Investig. Ophthalmol. Vis. Sci. 2011, 52, 1938–1978. [Google Scholar] [CrossRef] [PubMed]
- Mc Culley, J.P.; Shine, W.E. Meibomian gland function and the tear lipid layer. Ocul. Surf. 2003, 1, 97–106. [Google Scholar] [CrossRef]
- Nichols, K.K.; Foulks, G.N.; Bron, A.J.; Glasgow, B.J.; Dogru, M.; Tsubota, K.; Lemp, M.A.; Sullivan, D.A. The international Workshop on Meibomian Gland Dysfunction: Executive Summar. Investig. Ophthalmol. Vis. Sci. 2011, 52, 1922–1929. [Google Scholar] [CrossRef] [PubMed]
- International Dry Eye Workshop. Report of the International Dry Eye Workshop (DEWS). Ocul. Surf. 2007, 5, 61–204. [Google Scholar]
- Butovich, I.A.; Lu, H.; McMahon, A.; Ketelson, H.; Senchyna, M.; Meadows, D.; Campbell, E.; Molai, M.; Linsenbardt, E. Biophysical and morphol ogical evaluation of human normal and dry eye meibum using hot stage polarized light microscopy. Investig. Ophthalmol. Vis. Sci. 2014, 55, 87–101. [Google Scholar] [CrossRef] [PubMed]
- Borchman, D.; Foulks, G.N.; Yappert, M.C.; Bell, J.; Wells, E.; Neravetla, S.; Greenstone, V. Human Meibum lipid conformation and thermodynamic changes with Meibomian gland dysfunction. Investig. Ophthalmol. Vis. Sci. 2011, 52, 3805–3817. [Google Scholar] [CrossRef] [PubMed]
- Rynerson, J.M.; Perry, H.D. DEBS—A unification theory for dry eye and blepharitis. Clin. Ophthalmol. 2016, 10, 2455–2467. [Google Scholar] [CrossRef] [PubMed]
- Steinhoff, M.; Schauber, J.; Leyden, J.J. New insights into rosacea pathophysiology: A review of recent findings. J. Am. Acad. Dermatol. 2013, 69, S15–S26. [Google Scholar] [CrossRef] [PubMed]
- Nebbioso, M.; Fameli, V.; Gharbiya, M.; Sacchetti, M.; Zicari, A.M.; Lambiase, A. Investigational drugs for the treatment of dry eye disease. Expert Opin. Investig. Drugs 2016, 25, 1437–1446. [Google Scholar] [CrossRef] [PubMed]
- Steinhoff, M.; Schmelz, M.; Schauber, J. Facial Erythema of Rosacea—Aetiology, different pathophysiologies and treatment options. Acta Derm. Venereol. 2016, 96, 579–586. [Google Scholar] [CrossRef] [PubMed]
- Solomon, A.; Dursun, D.; Liu, Z.; Xie, Y.; Macri, A.; Pflugfelder, S.C. Pro-and anti-inflammatory forms of interleukin-1 in the tear fluid and conjunctiva of patients with dry-eye disease. Investig. Ophthalmol. Vis. Sci. 2001, 42, 2283–2292. [Google Scholar]
- Maatta, M.; Kari, O.; Tervahartiala, T.; Peltonen, S.; Kari, M.; Saari, M.; Sorsa, T. Tear fluid levels of MMP-8 are elevated in ocular rosacea-treatment effect of oral doxycycline. Graefes Arch. Clin. Exp. Ophthalmol. 2006, 244, 957–962. [Google Scholar] [CrossRef] [PubMed]
- Nien, C.J.; Massei, S.; Lin, G.; Nabavi, C.; Tao, J.; Brown, D.J.; Paugh, J.R.; Jester, J.V. Effects of age and dysfunction on human Meibomian glands. Arch. Ophthalmol. 2011, 129, 462–469. [Google Scholar] [CrossRef] [PubMed]
- Nien, C.J.; Paugh, J.R.; Massei, S.; Wahlert, A.J.; Kao, W.W.; Jester, J.V. Age-related changes in the Meibomian gland. Exp. Eye Res. 2009, 89, 1021–1027. [Google Scholar] [CrossRef] [PubMed]
- Parfitt, G.J.; Xie, Y.; Geyfman, M.; Brown, D.J.; Jester, J.V. Absence of ductal hyper-keratinization in mouse age-related Meibomian gland dysfunction (ARMGD). Aging 2013, 5, 825–834. [Google Scholar] [CrossRef] [PubMed]
- Wei, A.; Hong, J.; Sun, X.; Xu, J. Evaluation of age-related changes in human palpebral conjunctiva and meibomian glands by in vivo confocal microscopy. Cornea 2011, 30, 1007–1012. [Google Scholar] [CrossRef] [PubMed]
- Baudouin, C.; Labbe, A.; Liang, H.; Pauly, A.; Brignole-Baudouin, F. Preservatives in eyedrops: The good, the bad and the ugly. Prog. Retin. Eye Res. 2010, 29, 312–334. [Google Scholar] [CrossRef] [PubMed]
- Damasceno, R.W.; Osaki, M.H.; Dantas, P.E.; Belfort, R., Jr. Involutional entropion and ectropion of the lower eyelid: Prevalence and associated risk factors in the elderly population. Ophthal. Plast. Reconstr. Surg. 2011, 27, 317–320. [Google Scholar] [CrossRef] [PubMed]
- Meller, D.; Tseng, S.C. Conjunctivochalasis: Literature review and possible pathophysiology. Surv. Ophthalmol. 1998, 43, 225–232. [Google Scholar] [CrossRef]
- Di Pascuale, M.A.; Espana, E.M.; Kawakita, T.; Tseng, S.C. Clinical characteristics of conjunctivochalasis with or without aqueous tear deficiency. Br. J. Ophthalmol. 2004, 88, 388–392. [Google Scholar] [CrossRef] [PubMed]
- Roszkowska, A.M.; Colosi, P.; Ferreri, F.M.B.; Galasso, S. Age-related modifications of corneal sensitivity. Ophthalmologica 2004, 218, 350–355. [Google Scholar] [CrossRef] [PubMed]
- Dogru, M.; Wakamatsu, T.; Kojimaetal, T. The role of oxidative stress and inflammation in dry eye disease. Cornea 2009, 28, S70–S74. [Google Scholar] [CrossRef]
- Yoon, K.C.; Jeong, I.Y.; Park, Y.G.; Yang, S.Y. Interleukin-6 and tumor necrosis factor-α levels in tears of patients with dry eye syndrome. Cornea 2007, 26, 431–437. [Google Scholar] [CrossRef] [PubMed]
- Zhu, R.; Jiang, J.; Wang, T.; Xu, T.; Wu, M.; Liu, C.; Zhang, X. Expressions and clinical significance of OX40 and OX40L in peripheral blood of patients with primary Sjögren’s syndrome. Xi Bao Yu Fen Zi Mian Yi Xue Za Zhi 2013, 29, 862–865. [Google Scholar] [PubMed]
- Goto, E.; Matsumoto, Y.; Kamoi, M.; Endo, K.; Ishida, R.; Dogru, M. Tear evaporation rates in Sjögren syndrome and non-Sjögren dry eye patients. Am. J. Ophthalmol. 2007, 144, 81–85. [Google Scholar] [CrossRef] [PubMed]
- Nebbioso, M.; Belcaro, G.; Komaiha, C.; Celani, C.; Pescosolido, N. Keratoconjunctivitis by confocal microscopy after topical cyclosporine. Panminerva Med. 2012, 56, 9–13. [Google Scholar]
- Alhatem, B.; Cavalcanti, B.; Hamrah, P. In vivo confocal microscopy in dry eye disease and related conditions. Semin. Ophthalmol. 2012, 27, 138–148. [Google Scholar] [CrossRef] [PubMed]
- Villani, E.; Magnani, F.; Viola, F.; Santaniello, A.; Scorza, R.; Nucci, P.; Ratiglia, R. In vivo confocal evaluation of the ocular surface morpho-functional unit in dry eye. Optom. Vis. Sci. 2013, 90, 576–586. [Google Scholar] [CrossRef] [PubMed]
- Vitali, C.; Bombardieri, S.; Jonsson, R.; Moutsopoulos, H.M.; Alexander, E.L.; Carsons, S.E.; Daniels, T.E.; Fox, P.C.; Fox, R.I.; Kassan, S.S.; et al. European Study Group on Classification Criteria for Sjögren's Syndrome. Classification criteria for Sjögren syndrome: A revised version of the European criteria proposed by the American-European consensus group. Ann. Rheum. Dis. 2002, 61, 554–558. [Google Scholar] [CrossRef] [PubMed]
- Shiboski, S.C.; Shiboski, C.H.; Criswell, L.; Baer, A.; Challacombe, S.; Lanfranchi, H.; Schiødt, M.; Umehara, H.; Vivino, F.; Zhao, Y.; et al. Sjögren’s International Collaborative Clinical Alliance (SICCA) Research Groups. American college of rheumatology classification criteria for Sjogren’s syndrome: A data-driven, expert consensus approach in the SICCA cohort. Arthritis Care Res. 2012, 64, 475–487. [Google Scholar] [CrossRef]
- Djalilian, A.R.; Hamrah, P.; Pflugfelder, S.C. Dry eye. In Cornea, 2nd ed.; Krachmer, J.H., Mannis, M.J., Holland, E.J., Eds.; Elsevier Mosby: Philadelphia, PA, USA, 2005; pp. 521–540. [Google Scholar]
- Murray, P.I.; Rauz, S. The eye and inflammatory rheumatic diseases: The eye and rheumatoid arthritis, ankylosing spondylitis, psoriatic arthritis. Best Pract. Res. Clin. Rheumatol. 2016, 30, 802–825. [Google Scholar] [CrossRef] [PubMed]
- Rocha, E.M.; Wickham, L.A.; da Silveira, L.A.; Krenzer, K.L.; Yu, F.S.; Toda, I.; Sullivan, B.D.; Sullivan, D.A. Identification of androgen receptor protein and 5α-reductase mRNA in human ocular tissues. Br. J. Ophthalmol. 2000, 84, 76–84. [Google Scholar] [CrossRef] [PubMed]
- Nebbioso, M.; Evangelista, M.; Librando, A.; Plateroti, A.M.; Pescosolido, N. Iatrogenic dry eye disease: An eledoisin/carnitine and osmolyte drops study. Biomed. Pharmacother. 2013, 67, 659–663. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, T.; Richards, S.M.; Liu, S.; Jensen, R.V.; Sullivan, D.A. Influence of sex on gene expression in human corneal epithelial cells. Mol. Vis. 2009, 15, 2554–2569. [Google Scholar] [PubMed]
- Duarte, M.C.; Pinto, N.C.; Moreira, H.; Moreira, A.T.; Wasilewski, D. Total testosterone level in postmenopausal women with dry eye. Arq. Bras. Oftalmol. 2007, 70, 465–469. [Google Scholar] [CrossRef] [PubMed]
- Gonen, T.; Celik, C.; Oznur, M.; Abali, R.; Gonen, K.A.; Horozoglu, F.; Aksu, E.; Keskinbora, K.H. Tear osmolarity and ocular surface changes in patient with polycystic ovary syndrome. Curr. Eye Res. 2013, 38, 621–625. [Google Scholar] [CrossRef] [PubMed]
- Sriprasert, I.; Warren, D.W.; Mircheff, A.K.; Stanczyk, F.Z. Dry eye in postmenopausal women: A hormonal disorder. Menopause 2016, 23, 343–351. [Google Scholar] [CrossRef] [PubMed]
- Maziak, W.; Behrens, T.; Brasky, T.M.; Duhme, H.; Rzehak, P.; Weiland, S.K.; Keil, U. Are asthma and allergies in children and adolescents increasing? Results from ISAAC phase I and phase III surveys in Münster, Germany. Allergy 2003, 58, 572–579. [Google Scholar] [CrossRef] [PubMed]
- Nebbioso, M.; Zicari, A.M.; Celani, C.; Lollobrigida, V.; Grenga, R.; Duse, M. Pathogenesis of vernal keratoconjunctivitis and associated Factors. Semin. Ophthalmol. 2015, 30, 340–344. [Google Scholar] [CrossRef] [PubMed]
- Pflugfelder, S.C.; Jones, D.; Ji, Z.; Afonso, A.; Monroy, D. Altered cytokine balance in the tear fluid and conjunctiva of patients with Sjögren’s syndrome keratoconjunctivitis sicca. Curr. Eye Res. 1999, 19, 201–211. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Le, Q.; Zhao, F.; Hong, J.; Xu, J.; Zheng, T.; Sun, X. Application of in vivo laser scanning confocal microscopy for evaluation of ocular surface diseases: Lessons learned from pterygium, meibomian gland disease, and chemical burns. Cornea 2011, 30, S25–S28. [Google Scholar] [CrossRef] [PubMed]
- Ibrahim, O.M.; Matsumoto, Y.; Dogru, M.; Adan, E.S.; Wakamatsu, T.H.; Shimazaki, J.; Fujishima, H.; Tsubota, K. In vivo confocal microscopy evaluation of Meibomian gland dysfunction in atopic-keratoconjunctivitis patients. In Proceedings of the 64th Congress of Clinical Ophthalmology of Japan, Kobe, Japan, 12 November 2010. [Google Scholar]
- Nebbioso, M.; Zicari, A.M.; Lollobrigida, V.; Marenco, M.; Duse, M. Assessment of corneal alterations by confocal microscopy in vernal keratoconjunctivitis. Semin. Ophthalmol. 2015, 30, 40–43. [Google Scholar] [CrossRef] [PubMed]
- Battar, L.; Macri, A.; Dursun, D.; Pflugfelder, S.C. Effects of laser in situ keratomileusis on tear production, clearance, and the ocular surface. Ophthalmology 2001, 108, 1230–1235. [Google Scholar] [CrossRef]
- Li, X.M.; Hu, L.; Hu, J.; Wang, W. Investigation of dry eye disease and analysis of the pathogenic factors in patients after cataract surgery. Cornea 2007, 26, S16–S20. [Google Scholar] [CrossRef] [PubMed]
- Yu, E.Y.; Leung, A.; Rao, S.; Lam, D.S. Effect of laser in situ keratomileusis on tear stability. Ophthalmology 2000, 107, 2131–2135. [Google Scholar] [CrossRef]
- De Paiva, C.S.; Chen, Z.; Koch, D.D.; Hamill, M.B.; Manuel, F.K.; Hassan, S.S.; Wilhelmus, K.R.; Pflugfelder, S.C. The incidence and risk factors for developing dry eye after myopic Lasik. Am. J. Ophthalmol. 2006, 141, 438–445. [Google Scholar] [CrossRef] [PubMed]
- Stephenson, C.G.; Gartry, D.S.; O’Brart, D.P.; Kerr-Muir, M.G.; Marshall, J. Photorefractive keratectomy. A 6 year follow-up study. Ophthalmology 1998, 105, 273–281. [Google Scholar] [CrossRef]
- Rajan, M.S.; Jaycock, P.; O’Brart, D.; Nystrom, H.H.; Marshall, J. A long-term study of photorefractive keratectomy, 12-year follow-up. Ophthalmology 2004, 111, 1813–1824. [Google Scholar] [PubMed]
- Albietz, J.M.; Lenton, L.M.; Mc Lennan, S.G. Effect of laser in situ keratomileusis for hyperopia on tear film and ocular surface. J. Refract. Surg. 2002, 18, 113–123. [Google Scholar] [PubMed]
- Ambrosio, R.; Tervo, T., Jr.; Wilson, S.E. Lasik-associated dry eye and neurotrophic epitheliopathy: Pathophysiology and strategies for prevention and treatment. J. Refract. Surg. 2008, 24, 396–407. [Google Scholar] [PubMed]
- Richardson, J.D.; Vasko, M.R. Cellular mechanism of neurogenic infiammation. Pharmacol. Exp. Ther. 2002, 302, 839–845. [Google Scholar] [CrossRef] [PubMed]
- Wilson, S.E. Analysis of the keratocyte apoptotis, keratocyte profileration and myofibroblast transormation responses after photorefractive keratectomy and laser in situ keratomileusis. Trans. Am. Ophthalmol. Soc. 2002, 100, 411–433. [Google Scholar] [PubMed]
- Alio, J.L.; Javaloy, J. Corneal infiammation following corneal photoablative refractive surgery with excimer laser. Ophthalmology 2013, 58, 11–25. [Google Scholar]
- Patel, S.; Perez-Santonja, J.J.; Alio, J.L.; Murphy, P.J. Corneal Sensitivity and some properties of the tear film after laser in situ keratomileusis. J. Refract. Surg. 2001, 17, 17–24. [Google Scholar] [PubMed]
- Toda, I. LASIK and dry eye. Compr. Ophthalmol. Update 2007, 8, 79–89. [Google Scholar] [PubMed]
- Ueta, M.; Sotozono, C.; Inatomi, T.; Kojima, K.; Tashiro, K.; Hamuro, J.; Kinoshita, S. Toll-like receptor 3 gene polymorphisms in Japanese patients with Stevens-Johnson syndrome. Br. J. Ophthalmol. 2007, 91, 962–965. [Google Scholar] [CrossRef] [PubMed]
- Jain, R.; Sharma, N.; Basu, S.; Iyer, G.; Ueta, M.; Sotozono, C.; Kannabiran, C.; Rathi, V.M.; Gupta, N.; Kinoshita, S.; et al. Stevens-Johnson syndrome: The role of an ophthalmologist. Surv. Ophthalmol. 2016, 61, 369–399. [Google Scholar] [CrossRef] [PubMed]
- Kohanim, S.; Palioura, S.; Saeed, H.N.; Akpek, E.K.; Amescua, G.; Basu, S.; Blomquist, P.H.; Bouchard, C.S.; Dart, J.K.; Gai, X. Acute and chronic ophthalmic involvement in Stevens-Johnson syndrome/toxic epidermal necrolysis—A comprehensive review and guide to therapy. II. Ophthalmic disease. Ocul. Surf. 2016, 14, 168–188. [Google Scholar] [CrossRef] [PubMed]
- Carracedo, G.; Recchioni, A.; Alejandre-Alba, N.; Martin-Gil, A.; Crooke, A.; Jimenez-Alfaro Morote, I.; Pintor, J. Signs and symptoms of dry eye in keratoconus patients: a pilot study. Curr. Eye Res. 2015, 40, 1088–1094. [Google Scholar] [CrossRef] [PubMed]
- Dienes, L.; Kiss, H.J.; Nagy, Z.Z.; Acosta, M.C.; Gallar, J.; Kovacs, I. Corneal sensitivity and dry eye symptoms in patients with keratoconus. PLoS ONE 2015, 10, e0141621. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cho, K.J.; Mok, J.W.; Choi, M.Y.; Kim, J.Y.; Joo, C.K. Changes in corneal sensation and ocular surface in patients with asymmetrical keratoconus. Cornea 2013, 32, 205–210. [Google Scholar] [CrossRef] [PubMed]
- Al-Aquaba, M.A.; Faraj, L.; Fares, U.; Otri, A.M.; Dua, H.S. The morphologic characteristics of corneal nerves in advanced keratoconus as evaluated by acetylcholinesterase technique. Am. J. Ophthalmol. 2011, 152, 364–376. [Google Scholar] [CrossRef] [PubMed]
- Lema, I.; Duran, J.A. Infiammatory molecules in the tears of patients with keratoconus. Ophthalmology 2005, 112, 645–649. [Google Scholar] [CrossRef] [PubMed]
- Di Girolamo, N.; Chui, J.; Coroneo, M.T.; Wakeeld, D. Pathogenesis of pterygia: Role of cytokines, growth factors, and matrix metalloproteinases. Progr. Retin. Eye Res. 2004, 23, 195–228. [Google Scholar] [CrossRef] [PubMed]
- Detorakis, E.T.; Zaravinos, A.; Spandidos, D.A. Growth factor expression in ophthalmic pterygia and normal conjunctiva. Int. J. Mol. Med. 2010, 25, 513–516. [Google Scholar] [CrossRef] [PubMed]
- Li, D.; Chen, Z.; Song, J.; Luo, L.; Pugfelder, S.C. Stimulation of matrix metalloproteinases by hyperosmolarity via a JNK pathway in human corneal epithelial cells. Investig. Ophthalmol. Vis Sci. 2004, 45, 4302–4311. [Google Scholar]
- Li, M.; Zhang, M.; Lin, Y.; Xiao, Q.; Zhu, Z.; Song, S.; Lin, J.; Chen, J.; Liu, Z. Tear function and goblet cell density after pterygium excision. Eye 2007, 21, 224–228. [Google Scholar] [CrossRef] [PubMed]
- Guzman-Aranguez, A.; Gasull, X.; Diebold, Y.; Pintor, J. Purinergic receptors in ocular inflammation. Med. Inflamm. 2014, 2014, 320906. [Google Scholar] [CrossRef] [PubMed]
- Arciniega, J.C.; Nadji, E.J.; Butovich, I.A. Effects of free fatty acids on Meibomian lipid films. Exp. Eye Res. 2011, 93, 452–459. [Google Scholar] [CrossRef] [PubMed]
- Kenchegowda, S.; Bazan, H.E. Significance of lipid mediators in corneal injury and repair. J. Lipid Res. 2010, 51, 879–891. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.B.; Hu, K.M.; Seamon, K.J.; Mani, V.; Chen, Y.; Gronert, K. Estrogen negatively regulates epithelial wound healing and protective lipid mediator circuits in the cornea. FASEB J. 2012, 26, 1506–1516. [Google Scholar] [CrossRef] [PubMed]
- Leedom, A.J.; Sullivan, A.B.; Dong, B.; Lau, D.; Gronert, K. Endogenous LXA4 circuits are determinants of pathological angiogenesis in response to chronic injury. Am. J. Pathol. 2010, 176, 74–84. [Google Scholar] [CrossRef] [PubMed]
- Biteman, B.; Hassan, I.R.; Walker, E.; Leedom, A.J.; Dunn, M.; Seta, F.; Laniado-Schwartzman, M.; Gronert, K. Interdependence of lipoxin A4 and heme-oxygenase in counter-regulating inflammation during corneal wound healing. FASEB J. 2007, 21, 2257–2266. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.; Min, K.J.; Zhang, Y.B.; Su, J.; Greenwood, M.; Gronert, K. Female-specific down-regulation of tissue polymorphonuclear neutrophils drives impaired regulatory T cell and amplified effector T cell responses in autoimmune dry eye disease. J. Immunol. 2015, 195, 3086–3099. [Google Scholar] [CrossRef] [PubMed]
- Mukherjee, P.K.; Marcheselli, V.L.; Serhan, C.N.; Bazan, N.G. Neuro-protectin D1: A docosahexaenoic acid-derived docosatriene protects human retinal pigment epithelial cells from oxidative stress. Proc. Natl. Acad. Sci. USA 2004, 101, 8491–8496. [Google Scholar] [CrossRef] [PubMed]
- Cortina, M.S.; He, J.; Li, N.; Bazan, N.G.; Bazan, H.E. Recovery of corneal sensitivity, calcitonin gene-related peptide-positive nerves, and increased wound healing induced by pigment epithelial-derived factor plus docosahexaenoic acid after experimental surgery. Arch. Ophthalmol. 2012, 130, 76–83. [Google Scholar] [CrossRef] [PubMed]
- Cortina, M.S.; He, J.; Li, N.; Bazan, N.G.; Bazan, H.E. Neuroprotectin D1 synthesis and corneal nerve regeneration after experimental surgery and treatment with PEDF plus DHA. Investig. Ophthalmol. Vis. Sci. 2010, 51, 804–810. [Google Scholar] [CrossRef] [PubMed]
- Serhan, C.N. Resolution phase of inflammation: Novel endogenous anti-inflammatory and proresolving lipid mediators and pathways. Annu. Rev. Immunol. 2007, 25, 101–137. [Google Scholar] [CrossRef] [PubMed]
- Li, N.; He, J.; Schwartz, C.E.; Gjorstrup, P.; Bazan, H.E. Resolvin E1 improves tear production and decreases inflammation in a dry eye mouse model. J. Ocul. Pharmacol. Ther. 2010, 26, 431–439. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Wang, S.; Kao, A.A.; Long, Q. The effect of topical pranoprofen 0.1% on the clinical evaluation and conjunctival HLA-DR expression in dry eyes. Cornea 2012, 31, 1235–1239. [Google Scholar] [CrossRef] [PubMed]
- Fujishima, H.; Fuseya, M.; Ogata, M.; Murat, D. Efficacy of bromfenac sodium ophtlamic solution for treatment of dry eye disease. Asia Pac. J. Ophtalmol. (Phila.) 2015, 4, 9–13. [Google Scholar] [CrossRef] [PubMed]
- Nassar, A.; Tabbara, K.F.; Aljurf, M. Ocular manifestations of graft versus disease. Saudi J. Opthalmol. 2013, 27, 215–222. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, T.A.; Sullivan, D.A.; Knop, E.; Richards, S.M.; Knop, N.; Liu, S.; Sahin, A.; Darabad, R.R.; Morrison, S.; Kam, W.R.; Sullivan, B.D. Transcription, translation, and function of lubricin, a boundary lubricant, at the ocular surface. JAMA Ophthalmol. 2013, 13, 766–776. [Google Scholar] [CrossRef] [PubMed]
- Samsom, M.L.; Morrison, S.; Masala, N.; Sullivan, B.D.; Sullivan, D.A.; Sheardown, H.; Schmidt, T.A. Characterization of full-length recombinant human Proteoglycan 4 as an ocular surface boundary lubricant. Exp. Eye Res. 2014, 127, 14–19. [Google Scholar] [CrossRef] [PubMed]
- Lambiase, A.; Sullivan, B.D.; Schmidt, T.A.; Sullivan, D.A.; Jay, G.D.; Truitt, E.R., 3rd; Bruscolini, A.; Sacchetti, M.; Mantelli, F. A two-week, randomized, double-masked study to evaluate safety and efficacy of lubricin (150 μg/mL) eye drops versus sodium hyaluronate (HA) 0.18% eye drops (Vismed®) in patients with moderate dry eye disease. Ocul. Surf. 2016, 15, 77–87. [Google Scholar] [CrossRef] [PubMed]
- Meerovitch, K.; Torkildsen, G.; Lonsdale, J.; Goldfarb, H.; Lama, T.; Cumberlidge, G.; Ousler, G.W., 3rd. Safety and efficacy of MIM-D3 ophthalmic solutions in a randomized, placebo-controlled phase 2 clinical trial in patients with dry eye. Clin. Ophthalmol. 2013, 7, 1275–1285. [Google Scholar] [CrossRef] [PubMed]
- Bremond-Gignac, D.; Gicquel, J.J.; Chiambaretta, F. Pharmacokinetic evaluation of diquafosol tetrasodium for the treatment of Sjögren’s syndrome. Expert Opin. Drug. Metab. Toxicol. 2014, 10, 905–913. [Google Scholar] [CrossRef] [PubMed]
- Stevenson, W.; Chauhan, S.K.; Dana, R. Dry eye disease: An immune-mediated ocular surface disorder. Arch. Ophthalmol. 2012, 130, 90–100. [Google Scholar] [CrossRef] [PubMed]
- Wan, K.H.; Chen, L.J.; Young, A.L. Efficacy and safety of topical 0.05% cyclosporine eye drops in the treatment of dry eye syndrome: A systematic review and meta-analysis. Ocul. Surf. 2015, 13, 213–225. [Google Scholar] [CrossRef] [PubMed]
- Semba, C.P.; Torkildsen, G.L.; Lonsdale, J.D.; McLaurin, E.B.; Geffin, J.A.; Mundorf, T.K.; Kennedy, K.S.; Ousler, G.W. A phase 2 randomized, double-masked, placebo-controlled study of a novel integrin antagonist (sar 1118) for the treatment of dry eye. Am. J. Ophthalmol. 2012, 153, 1050–1060. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.Q.; Wei, R.L. Topical cyclosporine a in the treatment of dry eye: A systematic review and meta-analysis. Cornea 2014, 33, 760–767. [Google Scholar] [CrossRef] [PubMed]
- Agarwal, P.; Rupenthal, I.D. Modern approaches to the ocular delivery of cyclosporine A. Drug Discov. Today 2016, 21, 977–988. [Google Scholar] [CrossRef] [PubMed]
- Perez, V.L.; Pflugfelder, S.C.; Zhang, S.; Shojaei, A.; Haque, R. Lifitegrast, a novel integrin antagonist for treatment of dry eye disease. Ocul. Surf. 2016, 14, 207–215. [Google Scholar] [CrossRef] [PubMed]
- Sada, P.R.; Isenberg, D.; Ciurtin, C. Biologic treatment in Sjögren’s syndrome. Rheumatology 2015, 54, 219–230. [Google Scholar] [CrossRef] [PubMed]
- St Clair, E.W.; Levesque, M.C.; Prak, E.T.; Vivino, F.B.; Alappatt, C.J.; Spychala, M.E.; Wedgwood, J.; McNamara, J.; Moser Sivils, K.L.; Fisher, L.; et al. Autoimmunity centers of excellence. Rituximab therapy for primary Sjögren’s syndrome: An open-label clinical trial and mechanistic analysis. Arthritis Rheum. 2013, 65, 1097–1106. [Google Scholar] [CrossRef] [PubMed]
- Nebbioso, M.; Rusciano, D.; Pucci, B.; Zicari, A.M.; Grenga, R.; Pescocolido, N. Treatment of glaucomatous patients by means of food supplement to reduce the ocular discomfort: A double blind randomized trial. Eur. Rev. Med. Pharmacol. Sci. 2013, 17, 1117–1122. [Google Scholar] [PubMed]
- Pescosolido, N.; Giannotti, R.; Plateroti, A.M.; Pascarella, A.; Nebbioso, M. Curcumin: Therapeutical potential in ophthalmology. Planta Med. 2014, 80, 249–254. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Chen, Y.T.; Hayashida, Y.; Blanco, G.; Kheirkah, A.; He, H.; Chen, S.Y.; Liu, C.Y.; Tseng, S.C. Down-regulation of PAX6 is associated with abnormal differentiation of corneal epithelial cells in severe ocular surface diseases. J. Pathol. 2008, 214, 114–122. [Google Scholar] [CrossRef] [PubMed]
- Ouyang, H.; Goldberg, J.L.; Chen, S.; Li, W.; Xu, G.T.; Li, W.; Zhang, K.; Nussenblatt, R.B.; Liu, Y.; Xie, T.; et al. Ocular stem cell research from basic science to clinical application: A report from zhongshan ophthalmic center ocular stem cell symposium. Int. J. Mol. Sci. 2016, 17, 415. [Google Scholar] [CrossRef] [PubMed]


| Common Dry Eye Symptoms | |
| Irritation | Burning or stinging sensation |
| Dryness or grittiness | Foreign body sensation |
| Itching and Redness | Tearing |
| Fluctuation of vision | Contact lens intolerance |
| Increased blinking frequency | Blurry vision |
| Photophobia | Mucous discharge |
| Dry Eye Diagnostic Tests | |
| Tear Break Up Time (BUT) | Tear film meniscus height |
| Ocular surface staining with fluorescein, lissamine green, and rose bengal dye | Schirmer test I and II |
| Other tests: tear film osmolarity, lactoferrin, lysozyme immunoglobulin, and albumin dosage, and impression cytology. | |
| Meibomian Glands Dysfunction | Principal Mixed Forms | Other Major Mixed Forms | Aqueous Deficits | Ocular Surface Diseases |
|---|---|---|---|---|
| Primary or DE blepharitis syndrome (DEBS). Secondary to skin diseases such as rosacea, psoriasis, lupus, ichthyosis, rheumatoid arthritis, etc. | Oxidative stress, aging and/or associated factors. Corneal hypoesthesia. Systemic/topical medications, conjunctivochalasis, abnormal eyelid position, etc. | Hormonal changes, menopause, pregnancy. Allergic diseases, bacterial or viral conjunctivitis, use of contact lenses or computer, ocular surface trauma or tumor or surgery, environmental factors, etc. | Autoimmune pathologies: Sjögren’s syndrome dry eye (SS). Non-Sjögren’s syndrome dry eye (non-SSDE) | Stevens Johnson syndrome (SJS), toxic epidermal necrolysis (TEN), graft-versus-host disease (GVHD). Neurotrophic deficiencies, Keratoconus (KC), pinguecula, pterygium, corneal dystrophies, etc. |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nebbioso, M.; Del Regno, P.; Gharbiya, M.; Sacchetti, M.; Plateroti, R.; Lambiase, A. Analysis of the Pathogenic Factors and Management of Dry Eye in Ocular Surface Disorders. Int. J. Mol. Sci. 2017, 18, 1764. https://doi.org/10.3390/ijms18081764
Nebbioso M, Del Regno P, Gharbiya M, Sacchetti M, Plateroti R, Lambiase A. Analysis of the Pathogenic Factors and Management of Dry Eye in Ocular Surface Disorders. International Journal of Molecular Sciences. 2017; 18(8):1764. https://doi.org/10.3390/ijms18081764
Chicago/Turabian StyleNebbioso, Marcella, Paola Del Regno, Magda Gharbiya, Marta Sacchetti, Rocco Plateroti, and Alessandro Lambiase. 2017. "Analysis of the Pathogenic Factors and Management of Dry Eye in Ocular Surface Disorders" International Journal of Molecular Sciences 18, no. 8: 1764. https://doi.org/10.3390/ijms18081764
APA StyleNebbioso, M., Del Regno, P., Gharbiya, M., Sacchetti, M., Plateroti, R., & Lambiase, A. (2017). Analysis of the Pathogenic Factors and Management of Dry Eye in Ocular Surface Disorders. International Journal of Molecular Sciences, 18(8), 1764. https://doi.org/10.3390/ijms18081764