Dysfunctional Natural Killer Cells in the Aftermath of Cancer Surgery
Abstract
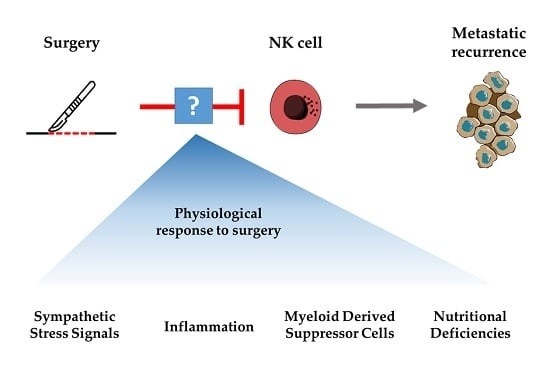
:1. Introduction
2. Natural Killer Cell Anti-Tumour Immunity
3. Natural Killer Cell Dysfunction after Surgery
4. Surgical Stress, Inflammation and Natural Killer Cell Dysfunction
4.1. Consequences of Surgical Inflammation on NK Cells
4.2. Analgesics, Anaesthetics, Blood Transfusions and Their Effect on Post-Operative NK Cell Function
4.3. The Post-Operative Hypercoaguable State Shields Cancer Cells and Blocks NK Cell Cytotoxicity
4.4. Decreased Mitochondrial Membrane Potential (ΔΨm) Increases NK Cell Susceptibility to Apoptosis
4.5. Correlating Post-Operative NK Cell Suppression to the Degree of Surgical Stress
5. Post-Operative Myeloid Derived Suppressor Cells and Natural Killer Cell Dysfunction
Post-Operative Myeloid Derived Suppressor Cells Can Suppress NK Cell Function
6. Nutritional Deficiencies Following Surgery and Natural Killer Cell Dysfunction
6.1. Arginine Deficiencies Following Surgery and NK Cell Dysfunction
7. Strategies to Improve Post-Operative NK Cell Function through Investigational Peri-Operative Therapies
8. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
Abbreviations
| Cr-51 | Chromium-51 |
| CRC | Colorectal cancer |
| CRP | C-reactive protein |
| DAMPs | Danger associated molecular patterns |
| DFS | Disease free survival |
| ERAS | Enhanced recovery after surgery |
| IL | Interleukin |
| KIRs | Killer Ig-like receptors |
| LMWH | Low molecular weight heparin |
| LRFS | Local recurrence free survival |
| LRP | Laparoscopic radical prostectomy |
| MDSC | Myeloid derived suppressor cell |
| MIC | MHC-Class I polypeptide-related sequence |
| NCR | Natural cytotoxicity receptors |
| NK | Natural Killer |
| NSAID | Non-steroidal anti-inflammatory drug |
| ORP | Open radical prostectomy |
| OS | Overall survival |
| PBL | Peripheral blood lymphocytes |
| PBMC | Peripheral blood mononuclear cells |
| PDE5 | Phosphodiesterase-5 |
| PFS | Progression free survival |
| PGE2 | Prostaglandin E2 |
| POD | Post-operation day |
| PRRs | Pattern recognition receptors |
| ULBP | UL16 Binding proteins |
| VTE | Venous thromboembolic events |
| ΔΨm | Mitochondrial membrane potential |
References
- Tyzzer, E.E. Factors in the Production and Growth of Tumor Metastases. J. Med. Res. 1913, 28, 309–333. [Google Scholar] [PubMed]
- Pollock, R.E.; Lotzová, E.; Stanford, S.D. Mechanism of Surgical Stress Impairment of Human Perioperative Natural Killer Cell Cytotoxicity. Arch. Surg. 1991, 126, 338. [Google Scholar] [CrossRef] [PubMed]
- Demicheli, R.; Valagussa, P.; Bonadonna, G. Does surgery modify growth kinetics of breast cancer micrometastases? Br. J. Cancer 2001, 85, 490–492. [Google Scholar] [CrossRef] [PubMed]
- Coffey, J.C.; Wang, J.H.; Smith, M.J. F.; Bouchier-Hayes, D.; Cotter, T.G.; Redmond, H.P. Excisional surgery for cancer cure: Therapy at a cost. Lancet Oncol. 2003, 4, 760–768. [Google Scholar] [CrossRef]
- Dominici, L.; Najita, J.; Hughes, M.; Niland, J.; Marcom, P.; Wong, Y.; Carter, B.; Javid, S.; Edge, S.; Burstein, H.; et al. Surgery of the primary tumor does not improve survival in stage IV breast cancer. Breast Cancer Res. Treat. 2011, 129, 459–465. [Google Scholar] [CrossRef] [PubMed]
- Bloom, H.J. The natural history of untreated breast cancer. Ann. N. Y. Acad. Sci. 1964, 114, 747–754. [Google Scholar] [CrossRef] [PubMed]
- Tai, L.H.; de Souza, C.T.; Belanger, S.; Ly, L.; Alkayyal, A.A.; Zhang, J.; Rintoul, J.L.; Ananth, A.A.; Lam, T.; Breitbach, C.J.; et al. Preventing Postoperative Metastatic Disease by Inhibiting Surgery-Induced Dysfunction in Natural Killer Cells. Cancer Res. 2013, 73, 97–107. [Google Scholar] [CrossRef] [PubMed]
- Seth, R.; Tai, L.H.; Falls, T.; de Souza, C.T.; Bell, J.C.; Carrier, M.; Atkins, H.; Boushey, R.; Auer, R.A. Surgical Stress Promotes the Development of Cancer Metastases by a Coagulation-Dependent Mechanism Involving Natural Killer Cells in a Murine Model. Ann. Surg. 2013, 258, 158–168. [Google Scholar] [CrossRef] [PubMed]
- Tai, L.H.; Tanese de Souza, C.; Sahi, S.; Zhang, J.; Alkayyal, A.A.; Ananth, A.A.; Auer, R.A.C. A mouse tumor model of surgical stress to explore the mechanisms of postoperative immunosuppression and evaluate novel perioperative immunotherapies. J. Vis. Exp. 2014, 85, 51253. [Google Scholar] [CrossRef] [PubMed]
- Gao, J.; Duan, Z.; Zhang, L.; Huang, X.; Long, L.; Tu, J.; Liang, H.; Zhang, Y.; Shen, T.; Lu, F. Failure recovery of circulating NKG2D + CD56 dim NK cells in HBV-associated hepatocellular carcinoma after hepatectomy predicts early recurrence. Oncoimmunology 2016, 5, e1048061. [Google Scholar] [CrossRef] [PubMed]
- Zhao, T.; Xia, W.H.; Zheng, M.Q.; Lu, C.Q.; Han, X.; Sun, Y.J. Surgical excision promotes tumor growth and metastasis by promoting expression of MMP-9 and VEGF in a breast cancer model. Exp. Oncol. 2008, 30, 60–64. [Google Scholar] [PubMed]
- Tsuchiya, Y.; Sawada, S.; Yoshioka, I.; Ohashi, Y.; Matsuo, M.; Harimaya, Y.; Tsukada, K.; Saiki, I. Increased surgical stress promotes tumor metastasis. Surgery 2003, 133, 547–555. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.-W.; Shahzad, M.M. K.; Lin, Y.G.; Armaiz-Pena, G.; Mangala, L.S.; Han, H.D.; Kim, H.S.; Nam, E.J.; Jennings, N.B.; Halder, J.; et al. Surgical stress promotes tumor growth in ovarian carcinoma. Clin. Cancer Res. 2009, 15, 2695–2702. [Google Scholar] [CrossRef] [PubMed]
- Demicheli, R.; Retsky, M.W.; Hrushesky, W.J. M.; Baum, M.; Gukas, I.D. The effects of surgery on tumor growth: A century of investigations. Ann. Oncol. 2008, 19, 1821–1828. [Google Scholar] [CrossRef] [PubMed]
- Van der Bij, G.J.; Oosterling, S.J.; Beelen, R.H. J.; Meijer, S.; Coffey, J.C.; van Egmond, M. The perioperative period is an underutilized window of therapeutic opportunity in patients with colorectal cancer. Ann. Surg. 2009, 249, 727–734. [Google Scholar] [CrossRef] [PubMed]
- Ramirez, M.F.; Ai, D.; Bauer, M.; Vauthey, J.N.; Gottumukkala, V.; Kee, S.; Shon, D.; Truty, M.; Kuerer, H.M.; Kurz, A.; et al. Innate immune function after breast, lung, and colorectal cancer surgery. J. Surg. Res. 2015, 194, 185–193. [Google Scholar] [CrossRef] [PubMed]
- Brøchner, A.C.; Mikkelsen, S.; Hegelund, I.; Hokland, M.; Mogensen, O.; Toft, P. The immune response is affected for at least three weeks after extensive surgery for ovarian cancer. Dan. Med. J. 2016, 63, A5243. [Google Scholar] [PubMed]
- Ceelen, W.; Pattyn, P.; Mareel, M. Surgery, wound healing, and metastasis: Recent insights and clinical implications. Crit. Rev. Oncol. Hematol. 2014, 89, 16–26. [Google Scholar] [CrossRef] [PubMed]
- Horowitz, M.; Neeman, E.; Sharon, E.; Ben-Eliyahu, S. Exploiting the critical perioperative period to improve long-term cancer outcomes. Nat. Rev. Clin. Oncol. 2015, 12, 213–226. [Google Scholar] [CrossRef] [PubMed]
- Kiessling, R.; Klein, E.; Wigzell, H. “Natural” killer cells in the mouse. I. Cytotoxic cells with specificity for mouse Moloney leukemia cells. Specificity and distribution according to genotype. Eur. J. Immunol. 1975, 5, 112–117. [Google Scholar] [CrossRef] [PubMed]
- Baginska, J.; Viry, E.; Paggetti, J.; Medves, S.; Berchem, G.; Moussay, E.; Janji, B. The Critical Role of the Tumor Microenvironment in Shaping Natural Killer Cell-Mediated Anti-Tumor Immunity. Front. Immunol. 2013, 4, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Bessoles, S.; Grandclement, C.; Alari-Pahissa, E.; Gehrig, J.; Jeevan-Raj, B.; Held, W. Adaptations of Natural Killer Cells to Self-MHC Class I. Front. Immunol. 2014, 5, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Zamai, L.; Ponti, C.; Mirandola, P.; Gobbi, G.; Papa, S.; Galeotti, L.; Cocco, L.; Vitale, M. NK Cells and Cancer. J. Immunol. 2007, 178, 4011–4016. [Google Scholar] [CrossRef] [PubMed]
- Gill, S.; Olson, J.A.; Negrin, R.S. Natural Killer Cells in Allogeneic Transplantation: Effect on Engraftment, Graft- versus-Tumor, and Graft-versus-Host Responses. Biol. Blood Marrow Transplant. 2009, 15, 765–776. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.; Poursine-Laurent, J.; Truscott, S.M.; Lybarger, L.; Song, Y.J.; Yang, L.; French, A.R.; Sunwoo, J.B.; Lemieux, S.; Hansen, T.H.; et al. Licensing of natural killer cells by host major histocompatibility complex class I molecules. Nature 2005, 436, 709–713. [Google Scholar] [CrossRef] [PubMed]
- Tu, M.M.; Mahmoud, A.B.; Makrigiannis, A.P. Licensed and Unlicensed NK Cells: Differential Roles in Cancer and Viral Control. Front. Immunol. 2016, 7, 166. [Google Scholar] [CrossRef] [PubMed]
- Thomas, L.M.; Peterson, M.E.; Long, E.O. Cutting Edge: NK Cell Licensing Modulates Adhesion to Target Cells. J. Immunol. 2013, 191, 3981–3985. [Google Scholar] [CrossRef] [PubMed]
- Langers, I.; Renoux, V.; Thiry, M.; Delvenne, P.; Jacobs, N. Natural killer cells: Role in local tumor growth and metastasis. Biol. Targets Ther. 2012, 6, 73–82. [Google Scholar] [CrossRef]
- Hecht, M.L.; Rosental, B.; Horlacher, T.; Hershkovitz, O.; De Paz, J.L.; Noti, C.; Schauer, S.; Porgador, A.; Seeberger, P.H. Natural Cytotoxicity Receptors NKp30, NKp44 and NKp46 Bind to Different Heparan Sulfate/Heparin Sequences. J. Proteome Res. 2009, 8, 712–720. [Google Scholar] [CrossRef] [PubMed]
- Koch, J.; Steinle, A.; Watzl, C.; Mandelboim, O. Activating natural cytotoxicity receptors of natural killer cells in cancer and infection. Trends Immunol. 2013, 34, 182–191. [Google Scholar] [CrossRef] [PubMed]
- Bodduluru, L.N.; Kasala, E.R.; Madhana, R.M. R.; Sriram, C.S. Natural killer cells: The journey from puzzles in biology to treatment of cancer. Cancer Lett. 2015, 357, 454–467. [Google Scholar] [CrossRef] [PubMed]
- Imai, K.; Matsuyama, S.; Miyake, S.; Suga, K.; Nakachi, K. Natural cytotoxic activity of peripheral-blood lymphocytes and cancer incidence: An 11-year follow-up study of a general population. Lancet 2000, 356, 1795–1799. [Google Scholar] [CrossRef]
- Hayashi, T.; Imai, K.; Morishita, Y.; Hayashi, I.; Kusunoki, Y.; Nakachi, K. Identification of the NKG2D haplotypes associated with natural cytotoxic activity of peripheral blood lymphocytes and cancer immunosurveillance. Cancer Res. 2006, 66, 563–570. [Google Scholar] [CrossRef] [PubMed]
- Furue, H.; Matsuo, K.; Kumimoto, H.; Hiraki, A.; Suzuki, T.; Yatabe, Y.; Komori, K.; Kanemitsu, Y.; Hirai, T.; Kato, T.; et al. Decreased risk of colorectal cancer with the high natural killer cell activity NKG2D genotype in Japanese. Carcinogenesis 2008, 29, 316–320. [Google Scholar] [CrossRef] [PubMed]
- Ishigami, S.; Natsugoe, S.; Tokuda, K.; Nakajo, A.; Che, X.; Iwashige, H.; Aridome, K.; Hokita, S.; Aikou, T. Prognostic value of intratumoral natural killer cells in gastric carcinoma. Cancer 2000, 88, 577–583. [Google Scholar] [CrossRef]
- Coca, S.; Perez-Piqueras, J.; Martinez, D.; Colmenarejo, A.; Saez, M.A.; Vallejo, C.; Martos, J.A.; Moreno, M. The prognostic significance of intratumoral natural killer cells in patients with colorectal carcinoma. Cancer 1997, 79, 2320–2328. [Google Scholar] [CrossRef]
- McGilvray, R.W.; Eagle, R.A.; Watson, N.F.S.; Al-Attar, A.; Ball, G.; Jafferji, I.; Trowsdale, J.; Durrant, L.G. NKG2D ligand expression in human colorectal cancer reveals associations with prognosis and evidence for immunoediting. Clin. Cancer Res. 2009, 15, 6993–7002. [Google Scholar] [CrossRef] [PubMed]
- Iannone, F.; Porzia, A.; Peruzzi, G.; Birarelli, P.; Milana, B.; Sacco, L.; Dinatale, G.; Peparini, N.; Prezioso, G.; Battella, S.; et al. Effect of surgery on pancreatic tumor-dependent lymphocyte asset: Modulation of natural killer cell frequency and cytotoxic function. Pancreas 2015, 44, 386–393. [Google Scholar] [CrossRef] [PubMed]
- Velásquez, J.F.; Ramírez, M.F.; Ai, D.I.; Lewis, V.; Cata, J.P. Impaired Immune Function in Patients Undergoing Surgery for Bone Cancer. Anticancer Res. 2015, 35, 5461–5466. [Google Scholar] [PubMed]
- Reinhardt, R.; Pohlmann, S.; Kleinertz, H.; Hepner-Schefczyk, M.; Paul, A.; Flohé, S.B. Invasive Surgery Impairs the Regulatory Function of Human CD56bright Natural Killer Cells in Response to Staphylococcus aureus. Suppression of Interferon-γ Synthesis. PLoS ONE 2015, 10, e0130155. [Google Scholar] [CrossRef] [PubMed]
- Ikeda, H.; Old, L.J.; Schreiber, R.D. The roles of IFNγ in protection against tumor development and cancer immunoediting. Cytokine Growth Factor Rev. 2002, 13, 95–109. [Google Scholar] [CrossRef]
- Angka, L.; Kilgour, M.K.; Sadiq, M.; Jeong, A.; de Souza, C.T.; Kennedy, M.A.; Auer, R.C. Natural Killer Cell IFN-gamma Suppression Following Major Surgery in Colorectal Cancer Patients. (manuscript in preparation).
- Alazawi, W.; Pirmadjid, N.; Lahiri, R.; Bhattacharya, S. Inflammatory and Immune Responses to Surgery and Their Clinical Impact. Ann. Surg. 2016, 264, 73–80. [Google Scholar] [CrossRef] [PubMed]
- Scheller, J.; Chalaris, A.; Schmidt-Arras, D.; Rose-John, S. The pro- and anti-inflammatory properties of the cytokine interleukin-6. Biochim. Biophys. Acta Mol. Cell. Res. 2011, 1813, 878–888. [Google Scholar] [CrossRef] [PubMed]
- Brøchner, A.; Toft, P. Pathophysiology of the systemic inflammatory response after major accidental trauma. Scand. J. Trauma Resusc. Emerg. Med. 2009, 17, 43–53. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Veenhof, A.A.F.A.; Vlug, M.S.; van der Pas, M.H. G.M.; Sietses, C.; van der Peet, D.L.; de Lange-de Klerk, E.S. M.; Bonjer, H.J.; Bemelman, W.A.; Cuesta, M.A. Surgical Stress Response and Postoperative Immune Function After Laparoscopy or Open Surgery With Fast Track or Standard Perioperative Care. Ann. Surg. 2012, 255, 216–221. [Google Scholar] [CrossRef] [PubMed]
- Narita, S.; Tsuchiya, N.; Kumazawa, T.; Maita, S.; Numakura, K.; Obara, T.; Tsuruta, H.; Saito, M.; Inoue, T.; Horikawa, Y.; et al. Comparison of Surgical Stress in Patients Undergoing Open Versus Laparoscopic Radical Prostatectomy by Measuring Perioperative Serum Cytokine Levels. J. Laparoendosc. Adv. Surg. Tech. 2013, 23, 33–37. [Google Scholar] [CrossRef] [PubMed]
- Shibata, J.; Ishihara, S.; Tada, N.; Kawai, K.; Tsuno, N.H.; Yamaguchi, H.; Sunami, E.; Kitayama, J.; Watanabe, T. Surgical stress response after colorectal resection: A comparison of robotic, laparoscopic, and open surgery. Tech. Coloproctol. 2015, 19, 275–280. [Google Scholar] [CrossRef] [PubMed]
- Decker, D.; Tolba, R.; Springer, W.; Lauschke, H.; Hirner, A.; von Ruecker, A. Abdominal Surgical Interventions: Local and Systemic Consequences for the Immune System–A Prospective Study on Elective Gastrointestinal Surgery1. J. Surg. Res. 2005, 126, 12–18. [Google Scholar] [CrossRef] [PubMed]
- Scheid, C.; Young, R.; McDermott, R.; Fitzsimmons, L.; Scarffe, J.H.; Stern, P.L. Immune function of patients receiving recombinant human interleukin-6 (IL-6) in a phase I clinical study: Induction of C-reactive protein and IgE and inhibition of natural killer and lymphokine-activated killer cell activity. Cancer Immunol. Immunother. 1994, 38, 119–126. [Google Scholar] [CrossRef] [PubMed]
- Kang, Y.J.; Jeung, I.C.; Park, A.; Park, Y.J.; Jung, H.; Kim, T.D.; Lee, H.G.; Choi, I.; Yoon, S.R. An increased level of IL-6 suppresses NK cell activity in peritoneal fluid of patients with endometriosis via regulation of SHP-2 expression. Hum. Reprod. 2014, 29, 2176–2189. [Google Scholar] [CrossRef] [PubMed]
- Cifaldi, L.; Prencipe, G.; Caiello, I.; Bracaglia, C.; Locatelli, F.; De Benedetti, F.; Strippoli, R. Inhibition of Natural Killer Cell Cytotoxicity by Interleukin-6: Implications for the Pathogenesis of Macrophage Activation Syndrome. Arthritis Rheumatol. 2015, 67, 3037–3046. [Google Scholar] [CrossRef] [PubMed]
- Vredevoe, D.L.; Widawski, M.; Fonarow, G.C.; Hamilton, M.; Martínez-Maza, O.; Gage, J.R. Interleukin-6 (IL-6) expression and natural killer (NK) cell dysfunction and anergy in heart failure. Am. J. Cardiol. 2004, 93, 1007–1011. [Google Scholar] [CrossRef] [PubMed]
- Joshi, P.C.; Zhou, X.; Cuchens, M.; Jones, Q. Prostaglandin E2 Suppressed IL-15-Mediated Human NK Cell Function Through Down-Regulation of Common-Chain. J. Immunol. 2001, 166, 885–891. [Google Scholar] [CrossRef] [PubMed]
- Shariat, S.F.; Kattan, M.W.; Traxel, E.; Andrews, B.; Zhu, K.; Wheeler, T.M.; Slawin, K.M. Association of pre- and postoperative plasma levels of transforming growth factor beta(1) and interleukin 6 and its soluble receptor with prostate cancer progression. Clin. Cancer Res. 2004, 10, 1992–1999. [Google Scholar] [CrossRef] [PubMed]
- Rook, A.H.; Kehrl, J.H.; Wakefield, L.M.; Roberts, A.B.; Sporn, M.B.; Burlington, D.B.; Lane, H.C.; Fauci, A.S. Effects of transforming growth factor beta on the functions of natural killer cells: Depressed cytolytic activity and blunting of interferon responsiveness. J. Immunol. 1986, 136, 3916–3920. [Google Scholar] [PubMed]
- Bellone, G.; Aste-Amezaga, M.; Trinchieri, G.; Rodeck, U. Regulation of NK cell functions by TGF-beta 1. J. Immunol. 1995, 155, 1066–1073. [Google Scholar] [PubMed]
- Malygin, A.M.; Meri, S.; Timonen, T. Regulation of natural killer cell activity by transforming growth factor-beta and prostaglandin E2. Scand. J. Immunol. 1993, 37, 71–76. [Google Scholar] [CrossRef] [PubMed]
- Viel, S.; Marçais, A.; Guimaraes, F.S.F.; Loftus, R.; Rabilloud, J.; Grau, M.; Degouve, S.; Djebali, S.; Sanlaville, A.; Charrier, E.; et al. TGF-β inhibits the activation and functions of NK cells by repressing the mTOR pathway. Sci. Signal. 2016, 9, ra19. [Google Scholar] [CrossRef] [PubMed]
- Crane, C.A.; Han, S.J.; Barry, J.J.; Ahn, B.J.; Lanier, L.L.; Parsa, A.T. TGF-beta downregulates the activating receptor NKG2D on NK cells and CD8+ T cells in glioma patients. Neuro-Oncology 2010, 12, 7–13. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.C.; Lee, K.M.; Kim, D.W.; Heo, D.S. Elevated TGF-β1 Secretion and Down-Modulation of NKG2D Underlies Impaired NK Cytotoxicity in Cancer Patients. J. Immunol. 2004, 172, 7335–7340. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.L.; Topley, N.; Ito, T.; Phillips, A. Interleukin-6 regulation of transforming growth factor (TGF)-beta receptor compartmentalization and turnover enhances TGF-beta1 signaling. J. Biol. Chem. 2005, 280, 12239–12245. [Google Scholar] [CrossRef] [PubMed]
- Turner, M.; Chantry, D.; Feldmann, M. Transforming growth factor beta induces the production of interleukin 6 by human peripheral blood mononuclear cells. Cytokine 1990, 2, 211–216. [Google Scholar] [CrossRef]
- Ulich, T.R.; Yin, S.; Guo, K.; Yi, E.S.; Remick, D.; del Castillo, J. Intratracheal injection of endotoxin and cytokines. II. Interleukin-6 and transforming growth factor beta inhibit acute inflammation. Am. J. Pathol. 1991, 138, 1097–10101. [Google Scholar] [PubMed]
- Kopp, H.G.; Placke, T.; Salih, H.R. Platelet-derived transforming growth factor-beta down-regulates NKG2D thereby inhibiting natural killer cell antitumor reactivity. Cancer Res. 2009, 69, 7775–7783. [Google Scholar] [CrossRef] [PubMed]
- Cucina, A.; Sterpetti, A. V; Borrelli, V.; Pagliei, S.; Cavallaro, A.; D’Angelo, L.S. Shear stress induces transforming growth factor-beta 1 release by arterial endothelial cells. Surgery 1998, 123, 212–217. [Google Scholar] [CrossRef]
- Zhao, Y.; Hu, J.; Li, R.; Song, J.; Kang, Y.; Liu, S.; Zhang, D. Enhanced NK cell adoptive antitumor effects against breast cancer in vitro via blockade of the transforming growth factor-β signaling pathway. OncoTargets Ther. 2015, 8, 1553–1559. [Google Scholar] [CrossRef] [PubMed]
- Narahara, H.; Kadoi, Y.; Hinohara, H.; Kunimoto, F.; Saito, S. Comparative effects ofComparative effects of flurbiprofen and fentanyl on natural killer cell cytotoxicity, lymphocyte subsets and cytokine concentrations in post-surgical intensive care unit patients: Prospective, randomized study. flurbiprofen and fenta. J. Anesth. 2013, 27, 676–683. [Google Scholar] [CrossRef] [PubMed]
- Beilin, B.; Martin, F.C.; Shavit, Y.; Gale, R.P.; Liebeskind, J.C. Suppression of natural killer cell activity by high-dose narcotic anesthesia in rats. Brain Behav. Immun. 1989, 3, 129–137. [Google Scholar] [CrossRef]
- Forget, P.; Collet, V.; Lavand’homme, P.; De Kock, M. Does analgesia and condition influence immunity after surgery? Effects of fentanyl, ketamine and clonidine on natural killer activity at different ages. Eur. J. Anaesthesiol. 2010, 27, 233–240. [Google Scholar] [CrossRef] [PubMed]
- Martucci, C.; Panerai, A.E.; Sacerdote, P. Chronic fentanyl or buprenorphine infusion in the mouse: Similar analgesic profile but different effects on immune responses. Pain 2004, 110, 385–392. [Google Scholar] [CrossRef] [PubMed]
- Hutchinson, M.R.; Zhang, Y.; Shridhar, M.; Evans, J.H.; Buchanan, M.M.; Zhao, T.X.; Slivka, P.F.; Coats, B.D.; Rezvani, N.; Wieseler, J.; et al. Evidence that opioids may have toll-like receptor 4 and MD-2 effects. Brain Behav. Immun. 2010, 24, 83–95. [Google Scholar] [CrossRef] [PubMed]
- Borner, C.; Warnick, B.; Smida, M.; Hartig, R.; Lindquist, J.A.; Schraven, B.; Hollt, V.; Kraus, J. Mechanisms of Opioid-Mediated Inhibition of Human T Cell Receptor Signaling. J. Immunol. 2009, 183, 882–889. [Google Scholar] [CrossRef] [PubMed]
- Williams, J.P.; Thompson, J.P.; McDonald, J.; Barnes, T.A.; Cote, T.; Rowbotham, D.J.; Lambert, D.G. Human Peripheral Blood Mononuclear Cells Express Nociceptin/Orphanin FQ, but Not μ, δ, or κ Opioid Receptors. Anesth. Analg. 2007, 105, 998–1005. [Google Scholar] [CrossRef] [PubMed]
- Boland, J.W.; McWilliams, K.; Ahmedzai, S.H.; Pockley, A.G. Effects of opioids on immunologic parameters that are relevant to anti-tumour immune potential in patients with cancer: A systematic literature review. Br. J. Cancer 2014, 111, 866–873. [Google Scholar] [CrossRef] [PubMed]
- Yeager, M.P.; Colacchio, T.A.; Yu, C.T.; Hildebrandt, L.; Howell, A.L.; Weiss, J.; Guyre, P.M. Morphine inhibits spontaneous and cytokine-enhanced natural killer cell cytotoxicity in volunteers. Anesthesiology 1995, 83, 500–508. [Google Scholar] [CrossRef] [PubMed]
- Yeager, M.P.; Procopio, M.A.; DeLeo, J.A.; Arruda, J.L.; Hildebrandt, L.; Howell, A.L. Intravenous fentanyl increases natural killer cell cytotoxicity and circulating CD16(+) lymphocytes in humans. Anesth. Anal. 2002, 94, 94–99. [Google Scholar]
- Wang, Y.L.; Jiang, B.; Yin, F.F.; Shi, H.-Q.; Xu, X.D.; Zheng, S.S.; Wu, S.; Hou, S.C. Perioperative Blood Transfusion Promotes Worse Outcomes of Bladder Cancer after Radical Cystectomy: A Systematic Review and Meta-Analysis. PLoS ONE 2015, 10, e0130122. [Google Scholar] [CrossRef] [PubMed]
- Cata, J.P.; Lasala, J.; Pratt, G.; Feng, L.; Shah, J.B. Association between Perioperative Blood Transfusions and Clinical Outcomes in Patients Undergoing Bladder Cancer Surgery: A Systematic Review and Meta-Analysis Study. J. Blood Transfus. 2016, 2016, 9876394. [Google Scholar] [CrossRef] [PubMed]
- Reeh, M.; Ghadban, T.; Dedow, J.; Vettorazzi, E.; Uzunoglu, F.G.; Nentwich, M.; Kluge, S.; Izbicki, J.R.; Vashist, Y.K. Allogenic Blood Transfusion is Associated with Poor Perioperative and Long-Term Outcome in Esophageal Cancer. World J. Surg. 2017, 41, 208–215. [Google Scholar] [CrossRef] [PubMed]
- Gharehbaghian, A.; Haque, K.M. G.; Truman, C.; Evans, R.; Morse, R.; Newman, J.; Bannister, G.; Rogers, C.; Bradley, B.A. Effect of autologous salvaged blood on postoperative natural killer cell precursor frequency. Lancet 2004, 363, 1025–1030. [Google Scholar] [CrossRef]
- Takemura, M.; Osugi, H.; Takada, N.; Kinoshita, H.; Higashino, M. Immunologic Effects of Allogeneic versus Autologous Blood Transfusion in Patients Undergoing Radical Oesophagectomy. Eur. Surg. Res. 2003, 35, 115–122. [Google Scholar] [CrossRef] [PubMed]
- Ulrych, J.; Kvasnicka, T.; Fryba, V.; Komarc, M.; Malikova, I.; Burget, F.; Brzezkova, R.; Kvasnicka, J.; Krska, Z.; Kvasnicka, J. 28 day post-operative persisted hypercoagulability after surgery for benign diseases: A prospective cohort study. BMC Surg. 2016, 16, 16. [Google Scholar] [CrossRef] [PubMed]
- Caine, G.J.; Stonelake, P.S.; Lip, G.Y. H.; Kehoe, S.T. The Hypercoagulable State of Malignancy: Pathogenesis and Current Debate. Neoplasia 2002, 4, 465–473. [Google Scholar] [CrossRef] [PubMed]
- Elyamany, G.; Alzahrani, A.M.; Bukhary, E. Cancer-Associated Thrombosis: An Overview. Clin. Med. Insights Oncol. 2014, 8, 129–137. [Google Scholar] [CrossRef]
- Zhang, S.; Pan, S.B.; Lyu, Q.H.; Wu, P.; Qin, G.M.; Wang, Q.; He, Z.L.; He, X.M.; Wu, M.; Chen, G. Postoperative Regulatory T-Cells and Natural Killer Cells in Stage I Nonsmall Cell Lung Cancer Underwent Video-assisted Thoracoscopic Lobectomy or Thoracotomy. Chin. Med. J. 2015, 128, 1502–1509. [Google Scholar] [CrossRef] [PubMed]
- Takabayashi, A.; Kanai, M.; Kawai, Y.; Iwata, S.; Sasada, T.; Obama, K.; Taki, Y. Change in Mitochondrial Membrane Potential in Peripheral Blood Lymphocytes, Especially in Natural Killer Cells, Is a Possible Marker for Surgical Stress on the Immune System. World J. Surg. 2003, 27, 659–665. [Google Scholar] [CrossRef] [PubMed]
- Bartal, I.; Melamed, R.; Greenfeld, K.; Atzil, S.; Glasner, A.; Domankevich, V.; Naor, R.; Beilin, B.; Yardeni, I.Z.; Ben-Eliyahu, S. Immune perturbations in patients along the perioperative period: Alterations in cell surface markers and leukocyte subtypes before and after surgery. Brain Behav. Immun. 2010, 24, 376–386. [Google Scholar] [CrossRef] [PubMed]
- Ng, C.S. H.; Lau, K.K. W. Surgical Trauma and Immune Functional Changes Following Major Lung Resection. Indian J. Surg. 2015, 77, 49–54. [Google Scholar] [CrossRef] [PubMed]
- Da Costa, M.L.; Redmond, H.P.; Finnegan, N.; Flynn, M.; Bouchier-Hayes, D. Laparotomy and laparoscopy differentially accelerate experimental flank tumour growth. Br. J. Surg. 1998, 85, 1439–1442. [Google Scholar] [CrossRef] [PubMed]
- Tsang, K.S.; Ng, M.H. L.; Leung, K.J.; Lai, P.B. S.; Lee, J.F. Y.; Lau, W.Y.; Leung, K.L. Lymphocyte subsets and natural killer cell cytotoxicity after laparoscopically assisted resection of rectosigmoid carcinoma. Surg. Endosc. 2003, 17, 1305–1310. [Google Scholar] [CrossRef] [PubMed]
- Lacy, A.M.; García-Valdecasas, J.C.; Delgado, S.; Castells, A.; Taurá, P.; Piqué, J.M.; Visa, J. Laparoscopy-assisted colectomy versus open colectomy for treatment of non-metastatic colon cancer: A randomised trial. Lancet 2002, 359, 2224–2229. [Google Scholar] [CrossRef]
- Bonjer, H.J.; Deijen, C.L.; Abis, G.A.; Cuesta, M.A.; van der Pas, M.H.G.M.; de Lange-de Klerk, E.S.M.; Lacy, A.M.; Bemelman, W.A.; Andersson, J.; Angenete, E.; et al. A Randomized Trial of Laparoscopic versus Open Surgery for Rectal Cancer. N. Engl. J. Med. 2015, 372, 1324–1332. [Google Scholar] [CrossRef] [PubMed]
- Janols, H.; Bergenfelz, C.; Allaoui, R.; Larsson, A.M.; Ryden, L.; Bjornsson, S.; Janciauskiene, S.; Wullt, M.; Bredberg, A.; Leandersson, K. A high frequency of MDSCs in sepsis patients, with the granulocytic subtype dominating in gram-positive cases. J. Leukoc. Biol. 2014, 96, 685–693. [Google Scholar] [CrossRef] [PubMed]
- Cuenca, A.; Delano, M.; Kelly-Scumpia, K.; Moreno, C.; Scumpia, P.; LaFace, D.; Heyworth, P.; Efron, P.; Moldawer, L. A Paradoxical Role for Myeloid-Derived Suppressor Cells in Sepsis and Trauma. Mol. Med. 2011, 17, 281–292. [Google Scholar] [CrossRef] [PubMed]
- Zhu, X.; Herrera, G.; Ochoa, J.B. Immunosupression and Infection After Major Surgery: A Nutritional Deficiency. Crit. Care Clin. 2010, 26, 491–500. [Google Scholar] [CrossRef] [PubMed]
- Mandruzzato, S.; Brandau, S.; Britten, C.M.; Bronte, V.; Damuzzo, V.; Gouttefangeas, C.; Maurer, D.; Ottensmeier, C.; van der Burg, S.H.; Welters, M.J. P.; et al. Toward harmonized phenotyping of human myeloid-derived suppressor cells by flow cytometry: Results from an interim study. Cancer Immunol. Immunother. 2016, 65, 161–169. [Google Scholar] [CrossRef] [PubMed]
- Bronte, V.; Brandau, S.; Chen, S.H.; Colombo, M.P.; Frey, A.B.; Greten, T.F.; Mandruzzato, S.; Murray, P.J.; Ochoa, A.; Ostrand-Rosenberg, S.; et al. Recommendations for myeloid-derived suppressor cell nomenclature and characterization standards. Nat. Commun. 2016, 7, 12150. [Google Scholar] [CrossRef] [PubMed]
- Mellqvist, U.H.; Hansson, M.; Brune, M.; Dahlgren, C.; Hermodsson, S.; Hellstrand, K. Natural killer cell dysfunction and apoptosis induced by chronic myelogenous leukemia cells: Role of reactive oxygen species and regulation by histamine. Blood 2000, 96, 1961–1968. [Google Scholar] [PubMed]
- Hoechst, B.; Voigtlaender, T.; Ormandy, L.; Gamrekelashvili, J.; Zhao, F.; Wedemeyer, H.; Lehner, F.; Manns, M.P.; Greten, T.F.; Korangy, F. Myeloid derived suppressor cells inhibit natural killer cells in patients with hepatocellular carcinoma via the NKp30 receptor. Hepatology 2009, 50, 799–807. [Google Scholar] [CrossRef] [PubMed]
- Goh, C.C.; Roggerson, K.M.; Lee, H.-C.; Golden-Mason, L.; Rosen, H.R.; Hahn, Y.S. Hepatitis C Virus-Induced Myeloid-Derived Suppressor Cells Suppress NK Cell IFN- Production by Altering Cellular Metabolism via Arginase-1. J. Immunol. 2016, 195, 2283–2292. [Google Scholar] [CrossRef] [PubMed]
- Yuan, L.; Xu, B.; Fan, H.; Yuan, P.; Zhao, P.; Suo, Z. Pre- and post-operative evaluation: Percentages of circulating myeloid-derived suppressor cells in rectal cancer patients. Neoplasma 2015, 62, 239–249. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Su, X.; Yang, L.; Qiao, F.; Fang, Y.; Yu, L.; Yang, Q.; Wang, Y.; Yin, Y.; Chen, R.; et al. The influence of myeloid-derived suppressor cells on angiogenesis and tumor growth after cancer surgery. Int. J. Cancer 2016, 138, 2688–2699. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Yang, L.; Wang, Y.Y.; Chen, R.; Qian, J.; Hong, Z.P.; Su, X.S. Surgery-induced monocytic myeloid-derived suppressor cells expand regulatory T cells in lung cancer. Oncotarget 2017, 8, 17050–17058. [Google Scholar] [CrossRef] [PubMed]
- Uchida, A.; Kolb, R.; Micksche, M. Generation of suppressor cells for natural killer activity in cancer patients after surgery. J. Natl. Cancer Inst. 1982, 68, 735–741. [Google Scholar] [PubMed]
- Ananth, A.A.; Tai, L.H.; Lansdell, C.; Alkayyal, A.A.; Baxter, K.E.; Angka, L.; Zhang, J.; Tanese de Souza, C.; Stephenson, K.B.; Parato, K.; et al. Surgical Stress Abrogates Pre-Existing Protective T Cell Mediated Anti-Tumor Immunity Leading to Postoperative Cancer Recurrence. PLoS ONE 2016, 11, e0155947. [Google Scholar] [CrossRef]
- Fortin, C.; Huang, X.; Yang, Y. NK Cell Response to Vaccinia Virus Is Regulated by Myeloid-Derived Suppressor Cells. J. Immunol. 2012, 189, 1843–1849. [Google Scholar] [CrossRef] [PubMed]
- Zhu, J.; Huang, X.; Yang, Y. Myeloid-derived Suppressor Cells Regulate Natural Killer Cell Response to Adenovirus-mediated Gene Transfer. J. Virol. 2012, 86, 13689–13696. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Han, Y.; Guo, Q.; Zhang, M.; Cao, X. Cancer-expanded myeloid-derived suppressor cells induce anergy of NK cells through membrane-bound TGF-beta 1. J. Immunol. 2009, 182, 240–249. [Google Scholar] [CrossRef] [PubMed]
- Stahel, P.F.; Flierl, M.A.; Moore, E.E. “Metabolic staging” after major trauma–A guide for clinical decision making? Scand. J. Trauma Resusc. Emerg. Med. 2010, 18, 34. [Google Scholar] [CrossRef] [PubMed]
- López Hellín, J.; Baena-Fustegueras, J.; Sabín-Urkía, P.; Schwartz-Riera, S.; García-Arumí, E. Nutritional modulation of protein metabolism after gastrointestinal surgery. Eur. J. Clin. Nutr. 2008, 62, 254–262. [Google Scholar] [CrossRef] [PubMed]
- Finnerty, C.C.; Mabvuure, N.T.; Ali, A.; Kozar, R.A.; Herndon, D.N. The Surgically Induced Stress Response. J. Parenter. Enter. Nutr. 2013, 37, 21S–29S. [Google Scholar] [CrossRef] [PubMed]
- Simsek, T.; Uzelli Simsek, H.; Canturk, N.Z. Response to trauma and metabolic changes: Posttraumatic metabolism. Turk. J. Surg. 2014, 30, 153–159. [Google Scholar] [CrossRef] [PubMed]
- Drover, J.W.; Dhaliwal, R.; Weitzel, L.; Wischmeyer, P.E.; Ochoa, J.B.; Heyland, D.K. Perioperative Use of Arginine-supplemented Diets: A Systematic Review of the Evidence. J. Am. Coll. Surg. 2011, 212, 385–399. [Google Scholar] [CrossRef] [PubMed]
- Ochoa, J.B.; Bernard, A.C.; O’Brien, W.E.; Griffen, M.M.; Maley, M.E.; Rockich, A.K.; Tsuei, B.J.; Boulanger, B.R.; Kearney, P.A.; Morris Jr, S.M. Arginase I expression and activity in human mononuclear cells after injury. Ann. Surg. 2001, 233, 393–399. [Google Scholar] [CrossRef] [PubMed]
- Nijveldt, R.J.; Prins, H.A.; Siroen, M.P. C.; Rauwerda, J.A.; Teerlink, T.; van Leeuwen, P.A. M. Low arginine plasma levels in patients after thoracoabdominal aortic surgery. Eur. J. Clin. Nutr. 2000, 54, 615–617. [Google Scholar] [CrossRef] [PubMed]
- Vissers, Y.L. J.; Dejong, C.H. C.; Luiking, Y.C.; Fearon, K.C. H.; von Meyenfeldt, M.F.; Deutz, N.E. P. Plasma arginine concentrations are reduced in cancer patients: Evidence for arginine deficiency? Am. J. Clin. Nutr. 2005, 81, 1142–1146. [Google Scholar] [PubMed]
- Lu, Y.; Wang, W.; Wang, J.; Yang, C.; Mao, H.; Fu, X.; Wu, Y.; Cai, J.; Han, J.; Xu, Z.; et al. Overexpression of Arginine Transporter CAT-1 Is Associated with Accumulation of L-Arginine and Cell Growth in Human Colorectal Cancer Tissue. PLoS ONE 2013, 8, e73866. [Google Scholar] [CrossRef] [PubMed]
- Buijs, N.; van Bokhorst-de van der Schueren, M.A.; Langius, J.A.; Leemans, C.R.; Kuik, D.J.; Vermeulen, M.A.; van Leeuwen, P.A. Perioperative arginine-supplemented nutrition in malnourished patients with head and neck cancer improves long-term survival. Am. J. Clin. Nutr. 2010, 92, 1151–1156. [Google Scholar] [CrossRef] [PubMed]
- Cao, Y.; Feng, Y.; Zhang, Y.; Zhu, X.; Jin, F. L-Arginine supplementation inhibits the growth of breast cancer by enhancing innate and adaptive immune responses mediated by suppression of MDSCs in vivo. BMC Cancer 2016, 16, 343. [Google Scholar] [CrossRef] [PubMed]
- Yeh, C.L.; Pai, M.H.; Li, C.C.; Tsai, Y.L.; Yeh, S.L. Effect of arginine on angiogenesis induced by human colon cancer: In vitro and in vivo studies. J. Nutr. Biochem. 2010, 21, 538–543. [Google Scholar] [CrossRef] [PubMed]
- Park, K.G. M.; Hayes, P.D.; Eremin, O.; Sewell, H.; Park, K.G. M.; Garlick, P.J. Stimulation of lymphocyte natural cytotoxicity by L-arginine. Lancet 1991, 337, 645–646. [Google Scholar] [CrossRef]
- Xiao, L.; Eneroth, P.H.; Qureshi, G.A. Nitric oxide synthase pathway may mediate human natural killer cell cytotoxicity. Scand. J. Immunol. 1995, 42, 505–511. [Google Scholar] [CrossRef] [PubMed]
- Oberlies, J.; Watzl, C.; Giese, T.; Luckner, C.; Kropf, P.; Müller, I.; Ho, A.D.; Munder, M. Regulation of NK cell function by human granulocyte arginase. J. Immunol. 2009, 182, 5259–5267. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez, P.C.; Ochoa, A.C. Arginine regulation by myeloid derived suppressor cells and tolerance in cancer: Mechanisms and therapeutic perspectives. Immunol. Rev. 2008, 222, 180–191. [Google Scholar] [CrossRef] [PubMed]
- Fletcher, M.; Ramirez, M.E.; Sierra, R.A.; Raber, P.; Thevenot, P.; Al-Khami, A.A.; Sanchez-Pino, D.; Hernandez, C.; Wyczechowska, D.D.; Ochoa, A.C.; et al. L-Arginine Depletion Blunts Antitumor T-cell Responses by Inducing Myeloid-Derived Suppressor Cells. Cancer Res. 2015, 75, 275–283. [Google Scholar] [CrossRef] [PubMed]
- Oosterling, S.J.; van der Bij, G.J.; Mels, A.K.; Beelen, R.H. J.; Meijer, S.; van Egmond, M.; van Leeuwen, P.A. M. Perioperative IFN-alpha to avoid surgically induced immune suppression in colorectal cancer patients. Histol. Histopathol. 2006, 21, 753–760. [Google Scholar] [CrossRef] [PubMed]
- Böhm, M.; Ittenson, A.; Klatte, T.; Schierbaum, K.F.; Röhl, F.W.; Ansorge, S.; Allhoff, E.P. Pretreatment with interleukin-2 modulates perioperative immunodysfunction in patients with renal cell carcinoma. Folia Biol. 2003, 49, 63–68. [Google Scholar] [CrossRef]
- Klatte, T.; Ittenson, A.; Röhl, F.-W.; Ecke, M.; Allhoff, E.P.; McDougall, I. Perioperative immunomodulation with interleukin-2 in patients with renal cell carcinoma: Results of a controlled phase II trial. Br. J. Cancer 2006, 95, 1167–1173. [Google Scholar] [CrossRef] [PubMed]
- Brivio, F.; Lissoni, P.; Alderi, G.; Barni, S.; Lavorato, F.; Fumagalli, L. Preoperative interleukin-2 subcutaneous immunotherapy may prolong the survival time in advanced colorectal cancer patients. Oncology 1996, 53, 263–268. [Google Scholar] [CrossRef] [PubMed]
- Shaashua, L.; Shabat-Simon, M.; Haldar, R.; Matzner, P.; Zmora, O.; Shabtai, M.; Sharon, E.; Allweis, T.; Barshack, I.; Hayman, L.; et al. Perioperative COX-2 and β-Adrenergic Blockade Improves Metastatic Biomarkers in Breast Cancer Patients in a Phase-II Randomized Trial. Clin. Cancer Res. 2017. clincanres-0152. [Google Scholar] [CrossRef] [PubMed]
- Tai, L.H.; Zhang, J.; Scott, K.J.; De Souza, C.T.; Alkayyal, A.A.; Ananth, A.A.; Sahi, S.; Adair, R.A.; Mahmoud, A.B.; Sad, S.; et al. Perioperative influenza vaccination reduces postoperative metastatic disease by reversing surgery-induced dysfunction in natural killer cells. Clin. Cancer Res. 2013, 19, 5104–5115. [Google Scholar] [CrossRef] [PubMed]
- Serafini, P.; Meckel, K.; Kelso, M.; Noonan, K.; Califano, J.; Koch, W.; Dolcetti, L.; Bronte, V.; Borrello, I. Phosphodiesterase-5 inhibition augments endogenous antitumor immunity by reducing myeloid-derived suppressor cell function. J. Exp. Med. 2006, 203, 2691–2702. [Google Scholar] [CrossRef] [PubMed]
- Tai, L.-H.; Alkayyal, A.A.; Leslie, A.; Sahi, S.; Bennett, S.; de Souza, C.T.; Baxter, K.E.; Angka, L.; Xu, R.; Kennedy, M.A.; et al. Phosphodiesterase-5 inhibition reduces postoperative metastatic disease by targeting surgery-induced myeloid derived suppressor cells. OncoImmunology 2017. under review. [Google Scholar]
- Nichols, P.H.; Ramsden, C.W.; Ward, U.; Sedman, P.C.; Primrose, J.N. Perioperative immunotherapy with recombinant interleukin 2 in patients undergoing surgery for colorectal cancer. Cancer Res. 1992, 52, 5765–5769. [Google Scholar] [PubMed]
- Nichols, P.H.; Ramsden, C.W.; Ward, U.; Trejdosiewicz, L.K.; Ambrose, N.S.; Primrose, J.N. Peri-operative modulation of cellular immunity in patients with colorectal cancer. Clin. Exp. Immunol. 1993, 94, 4–10. [Google Scholar] [CrossRef] [PubMed]
- Brivio, F.; Fumagalli, L.; Lissoni, P.; Nardone, A.; Nespoli, L.; Fattori, L.; Denova, M.; Chiarelli, M.; Nespoli, A. Pre-operative immunoprophylaxis with interleukin-2 may improve prognosis in radical surgery for colorectal cancer stage B-C. Anticancer Res. 2006, 26, 599–603. [Google Scholar] [PubMed]
- Melnyk, M.; Casey, R.G.; Black, P.; Koupparis, A.J. Enhanced recovery after surgery (ERAS) protocols: Time to change practice? Can. Urol. Assoc. J. 2011, 342–348. [Google Scholar] [CrossRef] [PubMed]
- Gustafsson, U.O.; Scott, M.J.; Schwenk, W.; Demartines, N.; Roulin, D.; Francis, N.; McNaught, C.E.; MacFie, J.; Liberman, A.S.; Soop, M.; et al. Guidelines for Perioperative Care in Elective Colonic Surgery: Enhanced Recovery After Surgery (ERAS®) Society Recommendations. World J. Surg. 2013, 37, 259–284. [Google Scholar] [CrossRef] [PubMed]
- Ljungqvist, O.; Scott, M.; Fearon, K.C. Enhanced Recovery After Surgery. JAMA Surg. 2017, 152, 292. [Google Scholar] [CrossRef] [PubMed]

| Peri-Operative Target | Trial ID, Phase | Intervention | Study Title |
|---|---|---|---|
| Enhance Immune Function Trials | |||
| Adoptive Cell Transfer | NCT02725996, Phase II | NK cells | By Using Adoptive Transfer of Autologous NK Cells to Prevent Recurrence of Hepatocellular Carcinoma After Curative Therapy |
| Cytokine Therapy | [127] | IFNα | Peri-operative IFN-alpha to avoid surgically induced immune suppression in colorectal cancer patients |
| [128,129] Phase II | IL-2 | Peri-operative immunomodulation with interleukin-2 in patients with renal cell carcinoma | |
| [130] | IL-2 | Preoperative interleukin-2 subcutaneous immunotherapy may prolong the survival time in advanced colorectal cancer patients. | |
| Innate Immune Stimulation and PDE5 Inhibition a | NCT02998736, Phase I | Cialis | Trial of Peri-operative Tadalafil and Influenza Vaccination in Cancer Patients Undergoing Major Surgical Resection of a Primary Abdominal Malignancy (PERIOP-04) |
| Immuno-nutrition | NCT02987296, Phase Ib | Dietary Supplemental Arginine | Peri-operative Immunonutrition in Colorectal Cancer Patients Undergoing Abdominal Surgery (PERIOP-02) |
| Prevent Immune Suppression Trials | |||
| Anaesthetic/ Analgesic | NCT01841294, Phase IV | Lidocaine | NK Activity Modulation Induced by Intravenous Lidocaine During Colorectal Laparoscopic Surgery |
| NCT01367418, Phase III b | Epidural (Bupivacaine; Ropivacaine; Sufentanil) | Effects of Anesthetic Technique on Immune and Inflammatory Systems Following Radical Prostatectomy (AIMS) | |
| NCT01929915 | Epidural | Analgesia and Pancreatic Cancer Surgery | |
| NCT02326727 | Epidural Ropivacaine | Influence of Epidural Analgesia on Natural Killer Cell (NK) Activity After Colonic Cancer Surgery | |
| NCT02567942 | Propofol vs. Sevoflurane | Assessment of the Anesthetic Effect on the Activity of Immune Cell in Patient With Colon Cancer | |
| NCT02896413 | Dexmedeto-midine | The Effects of Peri-operative Dexmedetomidine Administration on Immune Suppression and Outcomes in Patients With Uterine Cancer Undergoing Radical Resection | |
| NCT03109990 | Dexmedeto-midine | Impact of Dexmedetomidine on Breast Cancer Recurrence After Surgery | |
| Complementary Therapy | NCT02620033 c | Yoga Therapy | Impact of Yoga As Complementary Therapy in Patients Undergoing Radical Prostatectomy |
| COX2 Inhibitor | NCT00502684 Phase II [131] | Propranolol, etodolac | Peri-operative Administration of COX 2 Inhibitors and Beta Blockers to Women Undergoing Breast Cancer Surgery |
| Non-Immune Augmenting Trials | |||
| Hyper-coagulation | NCT01455831, Phase III | Tinzaparin | Extended Peri-operative Tinzaparin to Improve Disease-free Survival in Patients With Resectable Colorectal Cancer (PERIOP-01) |
| Exploratory | NCT02661776 | None | The Change of NK Cell Activity After Head and Neck Cancer Surgery |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Angka, L.; Khan, S.T.; Kilgour, M.K.; Xu, R.; Kennedy, M.A.; Auer, R.C. Dysfunctional Natural Killer Cells in the Aftermath of Cancer Surgery. Int. J. Mol. Sci. 2017, 18, 1787. https://doi.org/10.3390/ijms18081787
Angka L, Khan ST, Kilgour MK, Xu R, Kennedy MA, Auer RC. Dysfunctional Natural Killer Cells in the Aftermath of Cancer Surgery. International Journal of Molecular Sciences. 2017; 18(8):1787. https://doi.org/10.3390/ijms18081787
Chicago/Turabian StyleAngka, Leonard, Sarwat T. Khan, Marisa K. Kilgour, Rebecca Xu, Michael A. Kennedy, and Rebecca C. Auer. 2017. "Dysfunctional Natural Killer Cells in the Aftermath of Cancer Surgery" International Journal of Molecular Sciences 18, no. 8: 1787. https://doi.org/10.3390/ijms18081787
APA StyleAngka, L., Khan, S. T., Kilgour, M. K., Xu, R., Kennedy, M. A., & Auer, R. C. (2017). Dysfunctional Natural Killer Cells in the Aftermath of Cancer Surgery. International Journal of Molecular Sciences, 18(8), 1787. https://doi.org/10.3390/ijms18081787