A Critical Review on Health Promoting Benefits of Edible Mushrooms through Gut Microbiota
Abstract
:1. Introduction
2. Composition of Mushrooms
3. Medicinal Properties of Mushroom
3.1. Ganoderma
3.2. Chaga Mushroom
3.3. Coriolus versicolor
3.4. Maitake
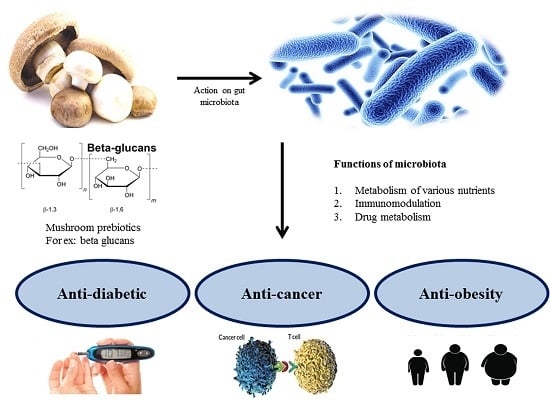
4. Gut Microbiota-Associated Health Benefits
5. Role of Mushrooms as Prebiotics in Improving the Host’s Health
6. Conclusions and Future Perspectives
Acknowledgments
Author Contributions
Conflicts of Interest
Abbreviations
| DM | Diabetes Mellitus |
| UV-C | Ultraviolet-C |
| DMBA | Dimethylbenzanthracene |
| MCF-7 | Michigan Cancer Foundation-7 Cells |
| GLPS3 | G. lucidum Polysaccharide Strain S3 |
| IL-2 | Interleukin-2 |
| TNF-α | Tumor Necrosis Factor- α |
| IFN-γ | Interferon-γ |
| NAFLD | Non-Alcoholic Fatty Liver Disease |
| GI | Gastrointestinal |
| WB mushroom | White Button Mushroom |
References
- Rathee, S.; Rathee, D.; Rathee, D.; Kumar, V.; Rathee, P. Mushrooms as therapeutic agents. Rev. Bras. Farmacogn. 2012, 22, 457–474. [Google Scholar] [CrossRef]
- Abugri, D.; McElhenney, W.H.; Willian, K.R. Fatty acid profiling in selected cultivated edible and wild medicinal mushrooms in the Southern United States. J. Exp. Food Chem. 2016, 2, 1–7. [Google Scholar] [CrossRef]
- Mhanda, F.N.; Kadhila-Muandingi, N.P.; Ueitele, I.S.E. Minerals and trace elements in domesticated Namibian Ganoderma species. Afr. J. Biotechnol. 2015, 14, 3216–3218. [Google Scholar] [CrossRef]
- De Sousa, V.M.C.; Dos Santos, E.F.; Sgarbieri, V.C. The importance of prebiotics in functional foods and clinical practice. Food Nutr. Sci. 2011, 2, 4. [Google Scholar] [CrossRef]
- Bhakta, M.; Kumar, P. Mushroom polysaccharides as a potential prebiotics. Int. J. Health Sci. Res. 2013, 3, 77–84. [Google Scholar] [CrossRef]
- Cani, P.D.; Delzenne, N.M. The role of the gut microbiota in energy metabolism and metabolic disease. Curr. Pharm. Des. 2009, 15, 1546–1558. [Google Scholar] [CrossRef] [PubMed]
- Petrovska, B. Protein fraction of edible Macedonian mushrooms. Eur. Food Sci. Technol. 2001, 212, 469–472. [Google Scholar] [CrossRef]
- Batbayar, S.; Lee, D.H.; Kim, H.W. Immunomodulation of fungal β-glucan in host defense signaling by dectin-1. Biomol. Ther. 2012, 20, 433–445. [Google Scholar] [CrossRef] [PubMed]
- Huan, G.; Cai, W.; Xu, B. Vitamin D2, ergosterol, and vitamin B2 content in commercially dried mushrooms marketed in China and increased vitamin D2 content following UV-C irradiation. Int. J. Vitam. Nutr. Res. 2016, 21, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Robbins, R.J. Phenolic acids in foods: An overview of analytical methodology. J. Agric. Food Chem. 2003, 51, 2866–2887. [Google Scholar] [CrossRef] [PubMed]
- Islam, T.; Yu, X.; Xu, B. Phenolic profiles, antioxidant capacities and metal chelating ability of edible mushroom commonly consumed in China. LWT Food Sci. Technol. 2016, 72, 423–431. [Google Scholar] [CrossRef]
- Rai, M.; Tidke, G.; Wasser, S.P. Therapeutic potential of mushrooms. Nat. Prod. Radiance 2005, 4, 246–257. [Google Scholar]
- Nahata, A. Ganoderma lucidum: A potent medicinal mushroom with numerous health benefits. Pharm. Anal. Acta 2013, 4, 10. [Google Scholar] [CrossRef]
- Smina, T.P.; Nitha, B.; Devasagayam, T.P.; Janardhanan, K.K. Ganoderma lucidum total triterpenes induce apoptosis in MCF-7 cells and attenuate DMBA induced mammary and skin carcinomas in experimental animals. Mutat. Res. 2017, 813, 45–51. [Google Scholar] [CrossRef] [PubMed]
- Zeng, Q.; Zhou, F.; Lei, L.; Chen, J.; Lu, J.; Zhou, J.; Cao, K.; Gao, L.; Xia, F.; Ding, S.; et al. Ganoderma lucidum polysaccharides protect fibroblasts against UVB-induced photoaging. Mol. Med. Rep. 2016, 15, 111–116. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.S.; Ho, S.Y.; Nan, F.H. Ganoderma lucidum β 1,3/1,6 glucan as an immunomodulator in inflammation induced by a high-cholesterol diet. BMC Complement. Altern. Med. 2016, 16, 500. [Google Scholar] [CrossRef] [PubMed]
- Shuhaimi, Y.S.; Arbakariya, M.; Fatimah, A.; Khalilah, A.B.; Anas, A.K.; Yazid, A.M. Effect of Ganoderma lucidum polysaccharides on the growth of Bifidobacterium spp. as assessed using Real-time PCR. Int. Food Res. J. 2012, 19, 1199–1205. [Google Scholar]
- Li, K.; Zhuo, C.; Teng, C.; Yua, S.; Wang, X.; Hu, Y.; Ren, G.; Yu, M.; Qu, J. Effects of Ganoderma lucidum polysaccharides on chronic pancreatitis and intestinal microbiota in mice. Int. J. Biol. Macromol. 2016, 93, 904–912. [Google Scholar] [CrossRef] [PubMed]
- Tang, X.; Cai, W.; Xu, B. Comparison of the chemical profiles and antioxidant and antidiabetic activities of extracts from two Ganoderma species (Agaricomycetes). Int. J. Med. Mushrooms 2016, 18, 609–620. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.L.; Ding, Z.Y.; Liu, G.Q. Improved production and antitumor properties of triterpene acids from submerged culture of Ganoderma lingzhi. Molecules 2016, 21, 1395. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.; Tang, S.Z.; Huang, Q. Hepatoprotective effects and mechanisms of action of triterpenoids from lingzhi or reishi medicinal mushroom Ganoderma lucidum (Agaricomycetes) on α-amanitin-induced liver injury in mice. Int. J. Med. Mushrooms 2016, 18, 841–850. [Google Scholar] [CrossRef] [PubMed]
- Rajasekaran, M.; Kalaimagal, C. In vitro antioxidant activity of ethanolic extract of a medicinal mushroom, Ganoderma lucidum. J. Pharm. Sci. Res. 2011, 3, 1427–1433. [Google Scholar]
- Spinosa, R. The chaga storey. Mycophile 2006, 47, 1–8. [Google Scholar]
- Hartwell, J.L. Plants Used against Cancer; Quartermain Publishing: Lawrence, MA, USA, 1982; 694p. [Google Scholar]
- Hong, K.B.; Noh, D.O.; Park, Y.; Suh, H.J. Hepatoprotective activity of water extracts from chaga medicinal mushroom, Inonotus obliquus (higher Basidiomycetes) against tert-butyl hydroperoxide induced oxidative liver injury in primary cultured rat hepatocytes. Int. J. Med. Mushrooms 2015, 17, 1069–1076. [Google Scholar] [CrossRef] [PubMed]
- Kang, J.H.; Jang, J.E.; Mishra, S.K.; Lee, H.J.; Nho, C.W.; Shin, D.; Jin, M.; Kim, M.K.; Choi, C.; Oh, S.H. Ergosterol peroxide from Chaga mushroom (Inonotus obliquus) exhibits anti-cancer activity by down-regulation of the β-catenin pathway in colorectal cancer. J. Ethnopharmacol. 2015, 15, 303–312. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.S.; Kim, E.J.; Kim, S.H. Ethanol extract of Innotus obliquus (chaga mushroom) induces G1 cell cycle arrest in HT-29 human colon cancer cells. Nutr. Res. Pract. 2015, 9, 111–116. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.; Teng, C.; Yu, S.; Wang, X.; Liang, J.; Bai, X.; Dong, L.; Song, T.; Yu, M.; Qu, J. Inonotus obliquus polysaccharide regulates gut microbiota of chronic pancreatitis in mice. AMB Express 2017, 7, 39. [Google Scholar] [CrossRef] [PubMed]
- Luo, K.W.; Yue, G.G.; Ko, C.H.; Lee, J.K.; Gao, S.; Li, L.F.; Li, G.; Fung, K.P.; Leung, P.C.; Lau, C.B. In vivo and in vitro anti-tumor and anti-metastasis effects of Coriolus versicolor aqueous extract on mouse mammary 4T1 carcinoma. Phytomedicine 2014, 21, 1078–1087. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, M.; Kawashima, H.; Takemori, K.; Ito, H.; Murai, A.; Masuda, S.; Yamada, K.; Uemura, D.; Horio, F. Ternatin, a cyclic peptide isolated from mushroom, and its derivative suppress hyperglycemia and hepatic fatty acid synthesis in spontaneously diabetic KK-A(y) mice. Biochem. Biophys. Res. Commun. 2012, 427, 299–304. [Google Scholar] [CrossRef] [PubMed]
- Yu, Z.T.; Liu, B.; Mukherjee, P.; Newburg, D.S. Trametes versicolor extract modifies human fecal microbiota composition in vitro. Plant Foods Hum. Nutr. 2013, 68, 107–112. [Google Scholar] [CrossRef] [PubMed]
- Pallav, K.; Dowd, S.E.; Villafuerte, J.; Yang, X.; Kabbani, T.; Hansen, J.; Dennis, M.; Leffler, D.A.; Newburg, D.S.; Kelly, C.P. Effects of polysaccharopeptide from Trametes versicolor and amoxicillin on the gut microbiome of healthy volunteers. Gut Microbes 2014, 5, 458–467. [Google Scholar] [CrossRef] [PubMed]
- Matijašević, D.; Pantić, M.; Rašković, B.; Pavlović, V.; Duvnjak, D.; Sknepnek, A.; Nikšić, M. The antibacterial activity of Coriolus versicolor methanol extract and its effect on ultrastructural changes of Staphylococcus aureus and Salmonella Enteritidis. Front. Microbiol. 2016, 7, 1226. [Google Scholar] [CrossRef] [PubMed]
- Alonso, E.N.; Ferronato, M.J.; Gandini, N.A.; Fermento, M.E.; Obiol, D.J.; Lopez Romero, A.; Arévalo, J.; Villegas, M.E.; Facchinetti, M.M.; Curino, A.C. Antitumoral effects of D-fraction from Grifola frondosa (maitake) mushroom in breast cancer. Nutr. Cancer 2017, 69, 29–43. [Google Scholar] [CrossRef] [PubMed]
- Lin, C.H.; Chang, C.Y.; Lee, K.R. Cold-water extracts of Grifola frondosa and its purified active fraction inhibit hepatocellular carcinoma in vitro and in vivo. Exp. Biol. Med. 2016, 241, 1374–1385. [Google Scholar] [CrossRef] [PubMed]
- Harhaji, L.J.; Mijatović, S.; Maksimović-Ivanić, D.; Stojanović, I.; Momcilović, M.; Maksimović, V.; Tufegdzić, S.; Marjanović, Z.; Mostarica-Stojković, M.; Vucinić, Z.; et al. Anti-tumor effect of Coriolus versicolor methanol extract against mouse B16 melanoma cells: In vitro and in vivo study. Food Chem. Toxicol. 2008, 46, 1825–1833. [Google Scholar] [CrossRef] [PubMed]
- Phillip, A.; Green, E.S.J.; Voigt, R.M. The gastrointestinal microbiome alcohol effects on the composition of intestinal microbiota. Alcohol. Res. 2015, 37, 223–236. [Google Scholar]
- Guinane, C.M.; Cotter, P.D. Role of the gut microbiota in health and chronic gastrointestinal disease: Understanding a hidden metabolic organ. Ther. Adv. Gastroenterol. 2013, 6, 295–308. [Google Scholar] [CrossRef] [PubMed]
- Conlon, M.A.; Bird, A.R. The impact of diet and lifestyle on gut microbiota and human health. Nutrients 2014, 7, 17–44. [Google Scholar] [CrossRef] [PubMed]
- Houghton, D.; Stewart, C.J.; Christopher, P. Gut microbiota and lifestyle interventions in NAFLD. Int. J. Mol. Sci. 2016, 17, 447. [Google Scholar] [CrossRef] [PubMed]
- Clarke, S.F.; Murphy, E.F.; Nilaweera, K. The gut microbiota and its relationship to diet and obesity new insights. Gut Microbes 2012, 3, 186–202. [Google Scholar] [CrossRef] [PubMed]
- Finelli, C.; Tarantino, G. Non-alcoholic fatty liver disease, diet and gut microbiota. EXCLI J. 2014, 13, 461–490. [Google Scholar] [PubMed]
- Schuijt, T.J.; Lankelma, J.M.; Scicluna, B.P.; Schuijt, T.J.; Lankelma, J.M.; Scicluna, B.P.; de Sousa e Melo, F.; Roelofs, J.J.; de Boer, J.D.; Hoogendijk, A.J.; et al. The gut microbiota plays a protective role in the host defence against pneumococcal pneumonia. Gut 2016, 65, 575–583. [Google Scholar] [CrossRef] [PubMed]
- Vyas, U.; Ranganathan, N. Probiotics, prebiotics, and synbiotics: Gut and beyond. Gastroenterol. Res. Pract. 2012, 872716. [Google Scholar] [CrossRef] [PubMed]
- Jandhyala, S.M.; Talukdar, R.; Subramanyam, C.; Vuyyuru, H.; Sasikala, M.; Reddy, D.N. Role of the normal gut microbiota. World J. Gastroenterol. 2015, 21, 8787–8803. [Google Scholar] [CrossRef] [PubMed]
- Le Blanc, J.G.; Milani, C.; de Giori, G.S. Bacteria as vitamin suppliers to their host: A gut microbiota perspective. Curr. Opin. Biotechnol. 2013, 24, 160–168. [Google Scholar] [CrossRef] [PubMed]
- Kang, M.J.; Kim, H.G.; Kim, J.S.; Oh, D.G.; Um, Y.J.; Seo, C.S.; Han, J.W.; Cho, H.J.; Kim, G.H.; Jeong, T.C.; et al. The effect of gut microbiota on drug metabolism. Expert Opin. Drug Metab. Toxicol. 2013, 9, 1295–1308. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.J.; Wu, E. The role of gut microbiota in immune homeostasis and autoimmunity. Gut Microbes 2012, 3, 4–14. [Google Scholar] [CrossRef] [PubMed]
- Okumura, R.; Takeda, K. Maintenance of gut homeostasis by the mucosal immune system. Proc. Jpn. Acad. Ser. B Phys. Biol. Sci. 2016, 92, 423–435. [Google Scholar] [CrossRef] [PubMed]
- Goto, Y.; Ivanov, I.I. Intestinal epithelial cells as mediators of the commensal–host immune crosstalk. Immunol. Cell Biol. 2013, 91, 204–214. [Google Scholar] [CrossRef] [PubMed]
- Hutkins, R.W.; Krumbeck, J.A.; Bindels, L.B.; Cani, P.D.; Fahey, G., Jr.; Goh, Y.J.; Hamaker, B.; Martens, E.C.; Mills, D.A.; Rastal, R.A.; et al. Prebiotics: Why definitions matter. Curr. Opin. Biotechnol. 2016, 37, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Singdevsachan, S.K.; Mishra, P.A.J.; Baliyarsingh, B.; Tayung, K.; Thatoi, H. Mushroom polysaccharides as potential prebiotics with their antitumor and immunomodulating properties: A review. Bioact. Carbohydr. Diet. Fibre 2015, 7, 1–14. [Google Scholar] [CrossRef]
- Varshney, J.; Ooi, J.H.; Jayarao, B.M. White button mushrooms increase microbial diversity and accelerate the resolution of Citrobacterrodentium infection in mice. J. Nutr. 2013, 143, 526–532. [Google Scholar] [CrossRef] [PubMed]
- Meneses, M.E.; Carrera, M.D.; Torres, N. Hypocholesterolemic properties and prebiotic effects of Mexican Ganoderma lucidum in C57BL/6 Mice. PLoS ONE 2016, 11, e0159631. [Google Scholar] [CrossRef] [PubMed]
- Giannenasa, I.; Tsalie, E.B.; Chronisc, E.F. Consumption of Agaricus bisporus mushroom affects the performance, intestinal microbiota composition and morphology, and antioxidant status of turkey poults. Anim. Feed Sci. Technol. 2011, 165, 218–229. [Google Scholar] [CrossRef]
- Geurts, L.; Neyrinck, A.M.; Delzenne, N.M. Gut microbiota controls adipose tissue expansion, gut barrier and glucose metabolism: Novel insights into molecular targets and interventions using prebiotics. Benef. Microbes 2014, 5, 3–17. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Zhang, X. Lentinula edodes-derived polysaccharide alters the spatial structure of gut microbiota in mice. PLoS ONE 2015, 10, e0115037. [Google Scholar] [CrossRef] [PubMed]
- Saman, P.; Chaiongkarn, A.; Moonmangmee, S.; Sukcharoen, J.; Kuancha, C.; Fungsin, B. Evaluation of prebiotic property in edible mushrooms. Biol. Chem. Res. 2016, 3, 75–85. [Google Scholar]
- Pandeya, D.R.; Souza, R.D.; Rahman, M.M. Host-microbial interaction in the mammalian intestine and their metabolic role inside. Biomed. Res. 2011, 2, 1–8. [Google Scholar]
- Gerritsen, J.; Smidt, H.; Rijkers, G.T.; de Vos, W.M. Intestinal microbiota in human health and disease: The impact of probiotics. Genes Nutr. 2011, 6, 209–240. [Google Scholar] [CrossRef] [PubMed]
- Khoruts, A.; Dicksved, J.; Jansson, J.K.; Sadowsky, M.J. Changes in the composition of the human fecal microbiome after bacteriotherapy for recurrent Clostridium difficile-associated diarrhea. J. Clin. Gastroenterol. 2010, 44, 354–360. [Google Scholar] [CrossRef] [PubMed]
- Hetland, G.; Dag, M.; Eide, M.; Haugen, M.H.; Mirlashari, M.R.; Paulsen, J.E. The Agaricus blazei-based mushroom extract, andosan, protects against intestinal tumorigenesis in the A/J Min/+ mouse. PLoS ONE 2016, 11, e0167754. [Google Scholar] [CrossRef] [PubMed]
- Huang, H.Y.; Korivi, M.; Chaing, Y.Y.; Chien, T.Y.; Tsai, Y.C. Pleurotus tuber-regium polysaccharides attenuate hyperglycemia and oxidative stress in experimental diabetic rats. J. Evid. Based Complement. Altern. Med. 2012, 856381. [Google Scholar] [CrossRef]
- Chang, C.J.; Lin, C.S.; Lu, C.C.; Martel, J.; Ko, Y.F.; Ojcius, D.M.; Tseng, S.F.; Wu, T.R.; Chen, Y.Y.; Young, J.D.; et al. Ganoderma lucidum reduces obesity in mice by modulating the composition of the gut microbiota. Nat. Commun. 2015, 6, 7489. [Google Scholar] [CrossRef] [PubMed]
- Stamets, P.E. Integrative Fungal Solutions for Protecting Bees and Overcoming Colony Collapse Disorder (CCD): Methods and Compositions. U.S. Patent 20140220150 A1, 7 August 2014. [Google Scholar]
- Kim, H.; Han, S.; Lee, C.; Lee, K.; Hong, D. Compositions Containing Polysaccharides from Phellinus linteus and Methods for Treating Diabetes Mellitus Using Same. U.S. Patent 6,809,084 B1, 26 October 2004. [Google Scholar]
- Kuo, H.C.; Lu, C.C.; Shen, C.H.; Tung, S.Y.; Hsieh, M.C.; Lee, K.C.; Lee, L.Y.; Chen, C.C.; Teng, C.C.; Huang, W.S.; et al. Hericium erinaceus mycelium and its isolated erinacine A protection from MPTP-induced neurotoxicity through the ER stress, triggering an apoptosis cascade. J. Transl. Med. 2016, 14, 78. [Google Scholar] [CrossRef] [PubMed]
- Lindequist, U.; Niedermeyer, T.H.J.; Julich, W.D. The pharmacological potential of mushrooms. Evid. Based Complement. Altern. Med. 2005, 2, 285–299. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Yang, J.; Ning, Z. Lentinula edodes-derived polysaccharide rejuvenates mice in terms of immune responses and gut microbiota. Food Funct. 2015, 6, 2653–2663. [Google Scholar] [CrossRef] [PubMed]
- Grienke, U.; Zoll, M.; Peintner, U. European medicinal polypores—A modern view on traditional uses. J. Ethnopharmacol. 2014, 154, 564–583. [Google Scholar] [CrossRef] [PubMed]
- Lemieszek, M.; Rzeski, W. Anticancer properties of polysaccharides isolated from fungi of the Basidiomycetes class. Contemp. Oncol. 2012, 16, 285–289. [Google Scholar] [CrossRef] [PubMed]
| Medicinal Mushroom | Active Immunomodulators | Health Benefits | Gut Microbiota Regulation |
|---|---|---|---|
| Grifola frondosa | MD-fraction Grifolan | The Agaricus blazei-based mushroom extract, andosan, protects against intestinal tumorigenesis in A/J Min/+ mice [62]. | Andosan may also have influenced the composition and activity of microbiota in the A/J Min/+ mice. |
| Pleurotus tuberregium | Polysaccharides | Pleurotus tuberregium possesses antihyperglycemic properties and attenuated oxidative stress in diabetic rats on a high-fat diet [63]. | There are possible roles of gut microbiota in the polysaccharide-induced attenuation of obesity and hyperglycemia. |
| Ganoderma lucidum | GLP(AI), Ganopoly, Ganoderans | Ganoderma lucidum reduces obesity in mice by modulating the composition of the gut microbiota [64]. | GL has decreased Firmicutes-to-Bacteroidetes ratios. Reduced endotoxin-bearing Proteobacteria levels. It also maintains intestinal barrier integrity and reduces metabolic endotoxemia. |
| Polyporus umbellatus | Polysaccharides | Integrative fungal solutions for protecting bees [65]. | Increases the intestinal microbiome to regulate host health. |
| Phellinus linteus | Polysaccharides | Anti-diabetic potential [66]. | Phellinus linteus induces changes in the composition and activity of the gastrointestinal tract microbiota that confer nutritional and health benefits to the host. |
| Trametes versicolor | Krestin (PSK), PSP | Prevents host from diarrhea, Clostridium difficile infection, and inflammatory bowel disease [32]. | Polysaccharopeptide from Trametes versicolor regulates the gut microbiota to maintain the host health. |
| Hericum erinaceus | Galactoxyloglucan–protein complex | Hericum erinaceus possesses anti-cancer, immuno-modulating, hypolipidemic, antioxidant and neuro-protective activities [67]. | Hericum erinaceus renders changes in the composition and activity of the gastrointestinal tract microbiota that confer nutritional and health benefits to the host. |
| Agaricus bisporus | Polysaccharides | Anti-bacterial property [53]. | White button mushrooms increase microbial diversity and accelerate the resolution of citrobacter rodentium infection in mice. |
| Fomitopsis officinalis | Polysaccharides | Fomitopsis officinalis acts as an insulin sensitizer in glucose tolerance tests and regulates hyperglycemia in mice with non-insulin-dependent diabetes [68]. | Exact action on gut microbiota is yet to be discovered. |
| Lentinula edodes | Lentinan, KS-2 | Lentinula edodes-derived polysaccharide rejuvenates mice in terms of immune responses and gut microbiota [69]. | L2 reverses the gut microbiota structure, such as the reduced ratio Firmicutes/Bacteroidetes, the increased Bacteroidia, the decreased Bacilli and Betaproteobacteria, the increased Bacteroidaceae, the decreased Lactobacillaceae, and Alcaligenaceae. |
| Fomes fomentarius | Polysaccharides | Fomes fomentarius is used to cure various ailments such as dysmenorrhoea, hemorrhoids, bladder disorders, pyretic diseases, treatment of coughs, cancer, and rheumatism [70]. | The exact role in regulating gut microbiota is not yet elucidated well. |
| Schizophyllum commune | Schizophyllan, Sonifilan, SPG | Used as an immune modulator [71]. | The exact role in regulating gut microbiota is not yet elucidated well. |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jayachandran, M.; Xiao, J.; Xu, B. A Critical Review on Health Promoting Benefits of Edible Mushrooms through Gut Microbiota. Int. J. Mol. Sci. 2017, 18, 1934. https://doi.org/10.3390/ijms18091934
Jayachandran M, Xiao J, Xu B. A Critical Review on Health Promoting Benefits of Edible Mushrooms through Gut Microbiota. International Journal of Molecular Sciences. 2017; 18(9):1934. https://doi.org/10.3390/ijms18091934
Chicago/Turabian StyleJayachandran, Muthukumaran, Jianbo Xiao, and Baojun Xu. 2017. "A Critical Review on Health Promoting Benefits of Edible Mushrooms through Gut Microbiota" International Journal of Molecular Sciences 18, no. 9: 1934. https://doi.org/10.3390/ijms18091934
APA StyleJayachandran, M., Xiao, J., & Xu, B. (2017). A Critical Review on Health Promoting Benefits of Edible Mushrooms through Gut Microbiota. International Journal of Molecular Sciences, 18(9), 1934. https://doi.org/10.3390/ijms18091934