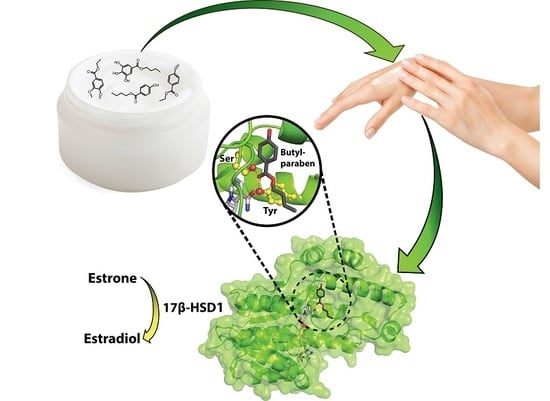
Interference of Paraben Compounds with Estrogen Metabolism by Inhibition of 17β-Hydroxysteroid Dehydrogenases
Abstract
:1. Introduction
2. Results
3. Discussion
4. Materials and Methods
4.1. Cosmetic Products Database
4.2. Virtual Screening and Property Calculation
4.3. In Vitro Testing
4.4. Docking
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
Appendix A

References
- Andersen, F.A. Final amended report on the safety assessment of methylparaben, ethylparaben, propylparaben, isopropylparaben, butylparaben, isobutylparaben, and benzylparaben as used in cosmetic products. Int. J. Toxicol. 2008, 27, 1–82. [Google Scholar]
- Daniel, J.W. Metabolic aspects of antioxidants and preservatives. Xenobiotica 1986, 16, 1073–1078. [Google Scholar] [CrossRef] [PubMed]
- Jackson, E.M. Moisturizers of today. J. Toxicol. Cutan. Ocul. Toxicol. 1992, 11, 173–184. [Google Scholar] [CrossRef]
- Soni, M.G.; Carabin, I.G.; Burdock, G.A. Safety assessment of esters of p-hydroxybenzoic acid (parabens). Food Chem. Toxicol. 2005, 43, 985–1015. [Google Scholar] [CrossRef] [PubMed]
- Sasseville, D.; Alfalah, M.; Lacroix, J.P. “Parabenoia” debunked, or “who’s afraid of parabens?”. Dermatitis 2015, 26, 254–259. [Google Scholar] [CrossRef] [PubMed]
- Darbre, P.D.; Harvey, P.W. Paraben esters: Review of recent studies of endocrine toxicity, absorption, esterase and human exposure, and discussion of potential human health risks. J. Appl. Toxicol. 2008, 28, 561–578. [Google Scholar] [CrossRef] [PubMed]
- Janjua, N.R.; Mortensen, G.K.; Andersson, A.M.; Kongshoj, B.; Skakkebaek, N.E.; Wulf, H.C. Systemic uptake of diethyl phthalate, dibutyl phthalate, and butyl paraben following whole-body topical application and reproductive and thyroid hormone levels in humans. Environ. Sci. Technol. 2007, 41, 5564–5570. [Google Scholar] [CrossRef] [PubMed]
- Sandanger, T.M.; Huber, S.; Moe, M.K.; Braathen, T.; Leknes, H.; Lund, E. Plasma concentrations of parabens in postmenopausal women and self-reported use of personal care products: The nowac postgenome study. J. Expo. Sci. Environ. Epidemiol. 2011, 21, 595–600. [Google Scholar] [CrossRef] [PubMed]
- Boberg, J.; Taxvig, C.; Christiansen, S.; Hass, U. Possible endocrine disrupting effects of parabens and their metabolites. Reprod. Toxicol. 2010, 30, 301–312. [Google Scholar] [CrossRef] [PubMed]
- Ye, X.; Kuklenyik, Z.; Bishop, A.M.; Needham, L.L.; Calafat, A.M. Quantification of the urinary concentrations of parabens in humans by on-line solid phase extraction-high performance liquid chromatography-isotope dilution tandem mass spectrometry. J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 2006, 844, 53–59. [Google Scholar] [CrossRef] [PubMed]
- Lakeram, M.; Lockley, D.J.; Sanders, D.J.; Pendlington, R.; Forbes, B. Paraben transport and metabolism in the biomimetic artificial membrane permeability assay (bampa) and 3-day and 21-day caco-2 cell systems. J. Biomol. Screen. 2007, 12, 84–91. [Google Scholar] [CrossRef] [PubMed]
- Bledzka, D.; Gromadzinska, J.; Wasowicz, W. Parabens. From environmental studies to human health. Environ. Int. 2014, 67, 27–42. [Google Scholar] [CrossRef] [PubMed]
- Darbre, P.D.; Aljarrah, A.; Miller, W.R.; Coldham, N.G.; Sauer, M.J.; Pope, G.S. Concentrations of parabens in human breast tumours. J. Appl. Toxicol. 2004, 24, 5–13. [Google Scholar] [CrossRef] [PubMed]
- Holst, J.P.; Soldin, O.P.; Guo, T.; Soldin, S.J. Steroid hormones: Relevance and measurement in the clinical laboratory. Clin. Lab. Med. 2004, 24, 105–118. [Google Scholar] [CrossRef] [PubMed]
- Lin, C.Y.; Strom, A.; Vega, V.B.; Kong, S.L.; Yeo, A.L.; Thomsen, J.S.; Chan, W.C.; Doray, B.; Bangarusamy, D.K.; Ramasamy, A.; et al. Discovery of estrogen receptor α target genes and response elements in breast tumor cells. Genome Biol. 2004, 5, R66. [Google Scholar] [CrossRef] [PubMed]
- Miller, W.L. Steroidogenic enzymes. Endocr. Dev. 2008, 13, 1–18. [Google Scholar] [PubMed]
- Gunnarsson, C.; Hellqvist, E.; Stal, O. 17β-hydroxysteroid dehydrogenases involved in local oestrogen synthesis have prognostic significance in breast cancer. Br. J. Cancer 2005, 92, 547–552. [Google Scholar] [CrossRef] [PubMed]
- Vuorinen, A.; Engeli, R.; Meyer, A.; Bachmann, F.; Griesser, U.J.; Schuster, D.; Odermatt, A. Ligand-based pharmacophore modeling and virtual screening for the discovery of novel 17β-hydroxysteroid dehydrogenase 2 inhibitors. J. Med. Chem. 2014, 57, 5995–6007. [Google Scholar] [CrossRef] [PubMed]
- Vuorinen, A.; Engeli, R.T.; Leugger, S.; Bachmann, F.; Akram, M.; Atanasov, A.G.; Waltenberger, B.; Temml, V.; Stuppner, H.; Krenn, L.; et al. Potential antiosteoporotic natural product lead compounds that inhibit 17β-hydroxysteroid dehydrogenase type 2. J. Nat. Prod. 2017, 80, 965–974. [Google Scholar] [CrossRef] [PubMed]
- Schuster, D.; Nashev, L.G.; Kirchmair, J.; Laggner, C.; Wolber, G.; Langer, T.; Odermatt, A. Discovery of nonsteroidal 17β-hydroxysteroid dehydrogenase 1 inhibitors by pharmacophore-based screening of virtual compound libraries. J. Med. Chem. 2008, 51, 4188–4199. [Google Scholar] [CrossRef] [PubMed]
- Routledge, E.J.; Parker, J.; Odum, J.; Ashby, J.; Sumpter, J.P. Some alkyl hydroxy benzoate preservatives (parabens) are estrogenic. Toxicol. Appl. Pharmacol. 1998, 153, 12–19. [Google Scholar] [CrossRef] [PubMed]
- Miller, D.; Wheals, B.B.; Beresford, N.; Sumpter, J.P. Estrogenic activity of phenolic additives determined by an in vitro yeast bioassay. Environ. Health Perspect. 2001, 109, 133–138. [Google Scholar] [PubMed]
- Byford, J.R.; Shaw, L.E.; Drew, M.G.; Pope, G.S.; Sauer, M.J.; Darbre, P.D. Oestrogenic activity of parabens in MCF7 human breast cancer cells. J. Steroid Biochem. Mol. Biol. 2002, 80, 49–60. [Google Scholar] [CrossRef]
- Okubo, T.; Yokoyama, Y.; Kano, K.; Kano, I. Er-dependent estrogenic activity of parabens assessed by proliferation of human breast cancer MCF-7 cells and expression of eralpha and pr. Food Chem. Toxicol. 2001, 39, 1225–1232. [Google Scholar] [CrossRef]
- Pugazhendhi, D.; Pope, G.S.; Darbre, P.D. Oestrogenic activity of p-hydroxybenzoic acid (common metabolite of paraben esters) and methylparaben in human breast cancer cell lines. J. Appl. Toxicol. 2005, 25, 301–309. [Google Scholar] [CrossRef] [PubMed]
- Prusakiewicz, J.J.; Harville, H.M.; Zhang, Y.; Ackermann, C.; Voorman, R.L. Parabens inhibit human skin estrogen sulfotransferase activity: Possible link to paraben estrogenic effects. Toxicology 2007, 232, 248–256. [Google Scholar] [CrossRef] [PubMed]
- Bando, H.; Mohri, S.; Yamashita, F.; Takakura, Y.; Hashida, M. Effects of skin metabolism on percutaneous penetration of lipophilic drugs. J. Pharm. Sci. 1997, 86, 759–761. [Google Scholar] [CrossRef] [PubMed]
- Pedersen, S.; Marra, F.; Nicoli, S.; Santi, P. In vitro skin permeation and retention of parabens from cosmetic formulations. Int. J. Cosmet. Sci. 2007, 29, 361–367. [Google Scholar] [CrossRef] [PubMed]
- Ishiwatari, S.; Suzuki, T.; Hitomi, T.; Yoshino, T.; Matsukuma, S.; Tsuji, T. Effects of methyl paraben on skin keratinocytes. J. Appl. Toxicol. 2007, 27, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Frederiksen, H.; Jorgensen, N.; Andersson, A.M. Parabens in urine, serum and seminal plasma from healthy Danish men determined by liquid chromatography-tandem mass spectrometry (LC-MS/MS). J. Expo. Sci. Environ. Epidemiol. 2011, 21, 262–271. [Google Scholar] [CrossRef] [PubMed]
- Schlumpf, M.; Kypke, K.; Wittassek, M.; Angerer, J.; Mascher, H.; Mascher, D.; Vokt, C.; Birchler, M.; Lichtensteiger, W. Exposure patterns of uv filters, fragrances, parabens, phthalates, organochlor pesticides, pbdes, and pcbs in human milk: Correlation of uv filters with use of cosmetics. Chemosphere 2010, 81, 1171–1183. [Google Scholar] [CrossRef] [PubMed]
- The European Commission Homepage. Available online: http://ec.europa.eu/growth/tools-databases/cosing/ (accessed on 28 May 2014).
- Kim, S.; Thiessen, P.A.; Bolton, E.E.; Chen, J.; Fu, G.; Gindulyte, A.; Han, L.; He, J.; He, S.; Shoemaker, B.A.; et al. Pubchem substance and compound databases. Nucleic Acids Res. 2016, 44, D1202–D1213. [Google Scholar] [CrossRef] [PubMed]
- Hawkins, P.C.; Skillman, A.G.; Warren, G.L.; Ellingson, B.A.; Stahl, M.T. Conformer generation with OMEGA: Algorithm and validation using high quality structures from the protein databank and Cambridge structural database. J. Chem. Inform. Model. 2010, 50, 572–584. [Google Scholar] [CrossRef] [PubMed]
- Jones, G.; Willett, P.; Glen, R.C.; Leach, A.R.; Taylor, R. Development and validation of a genetic algorithm for flexible docking. J. Mol. Biol. 1997, 267, 727–748. [Google Scholar] [CrossRef] [PubMed]
- Mazumdar, M.; Fournier, D.; Zhu, D.W.; Cadot, C.; Poirier, D.; Lin, S.X. Binary and ternary crystal structure analyses of a novel inhibitor with 17β-HSD type 1: A lead compound for breast cancer therapy. Biochem. J. 2009, 424, 357–366. [Google Scholar] [CrossRef] [PubMed]



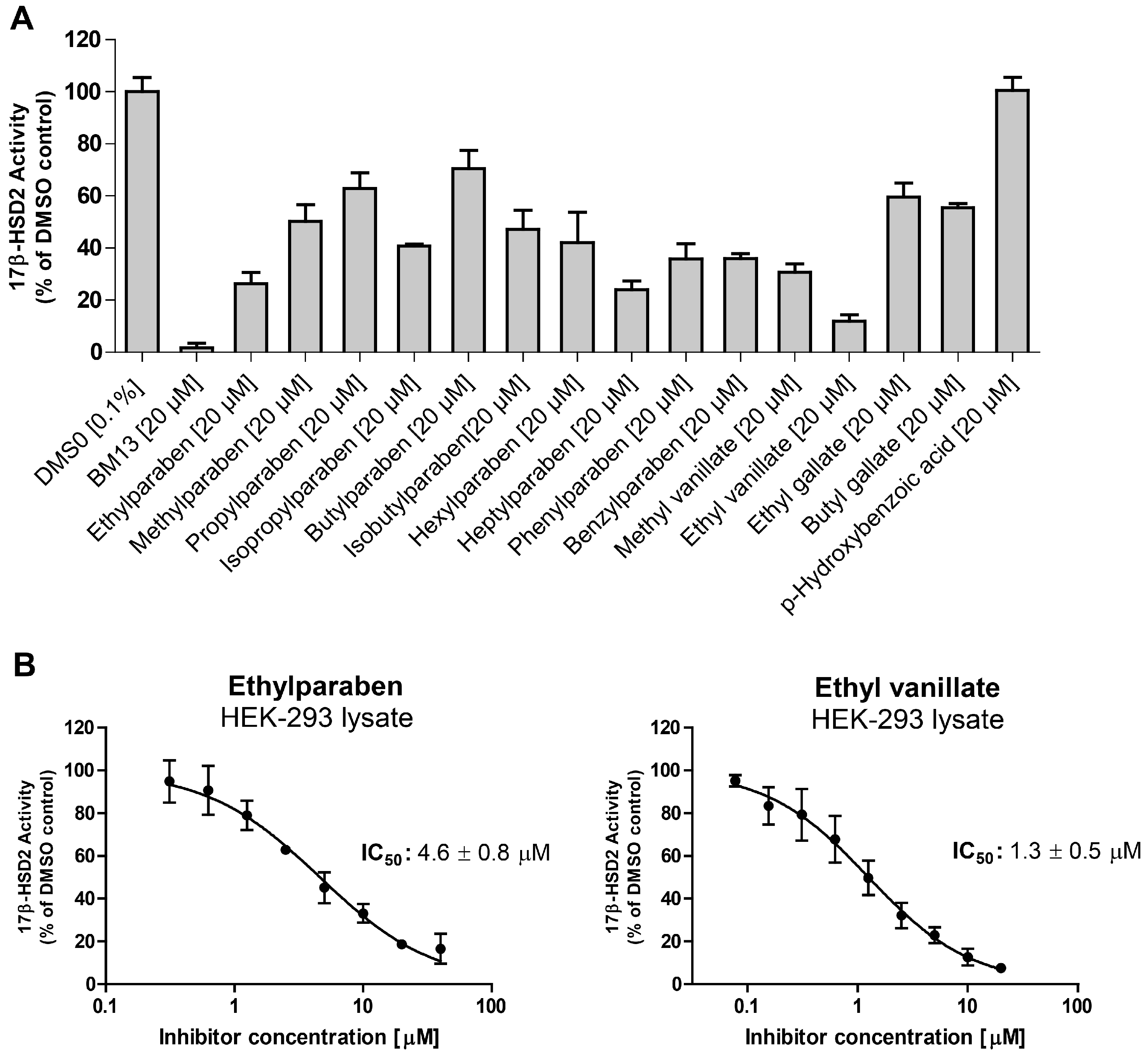


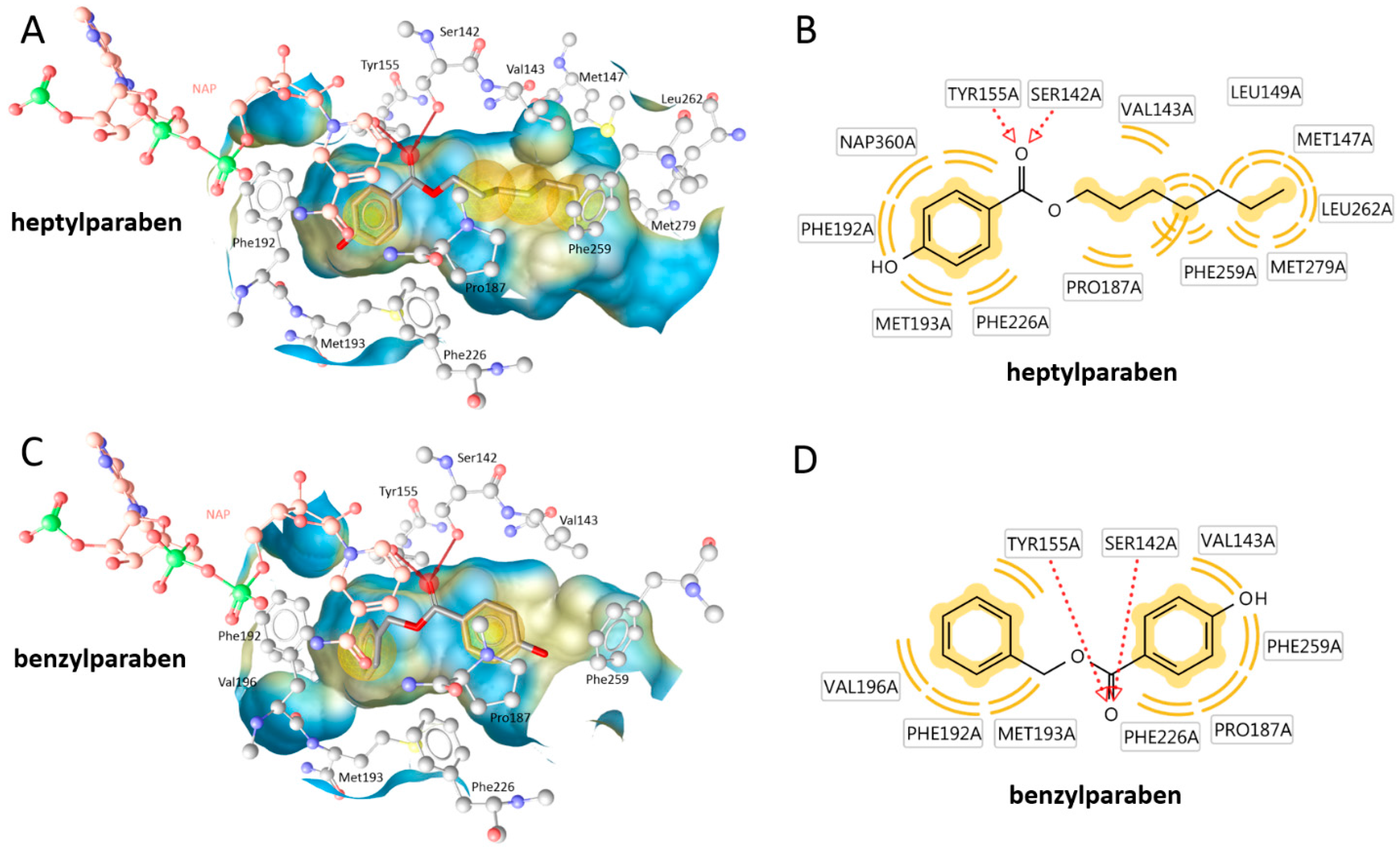
| Compound | Molecular Weight (g) | 17β-HSD2 % Inhibition at 20 µM | 17β-HSD1 % Inhibition at 20 µM | cLogP |
|---|---|---|---|---|
| p-hydroxybenzoic acid | 138.12 | 0 ± 5 | 7 ± 11 | 0.86 |
| methylparaben | 152.15 | 50 ± 6 | 0 ± 7 | 0.95 |
| ethylparaben | 166.18 | 74 ± 4 | 7 ± 6 | 1.34 |
| propylparaben | 180.20 | 37 ± 6 | 37 ± 5 | 1.73 |
| isopropylparaben | 180.20 | 60 ± 1 | 32 ± 9 | 1.73 |
| butylparaben | 194.23 | 30 ± 7 | 52 ± 10 | 2.12 |
| isobutylparaben | 194.23 | 53 ± 7 | 65 ± 4 | 1.97 |
| phenylparaben | 214.22 | 64 ± 6 | 35 ± 1 | 2.38 |
| hexylparaben | 222.28 | 58 ± 12 | 80 ± 5 | 2.90 |
| benzylparaben | 228.25 | 64 ± 6 | 67 ± 9 | 2.23 |
| heptylparaben | 236.31 | 76 ± 3 | 83 ± 6 | 3.29 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Engeli, R.T.; Rohrer, S.R.; Vuorinen, A.; Herdlinger, S.; Kaserer, T.; Leugger, S.; Schuster, D.; Odermatt, A. Interference of Paraben Compounds with Estrogen Metabolism by Inhibition of 17β-Hydroxysteroid Dehydrogenases. Int. J. Mol. Sci. 2017, 18, 2007. https://doi.org/10.3390/ijms18092007
Engeli RT, Rohrer SR, Vuorinen A, Herdlinger S, Kaserer T, Leugger S, Schuster D, Odermatt A. Interference of Paraben Compounds with Estrogen Metabolism by Inhibition of 17β-Hydroxysteroid Dehydrogenases. International Journal of Molecular Sciences. 2017; 18(9):2007. https://doi.org/10.3390/ijms18092007
Chicago/Turabian StyleEngeli, Roger T., Simona R. Rohrer, Anna Vuorinen, Sonja Herdlinger, Teresa Kaserer, Susanne Leugger, Daniela Schuster, and Alex Odermatt. 2017. "Interference of Paraben Compounds with Estrogen Metabolism by Inhibition of 17β-Hydroxysteroid Dehydrogenases" International Journal of Molecular Sciences 18, no. 9: 2007. https://doi.org/10.3390/ijms18092007
APA StyleEngeli, R. T., Rohrer, S. R., Vuorinen, A., Herdlinger, S., Kaserer, T., Leugger, S., Schuster, D., & Odermatt, A. (2017). Interference of Paraben Compounds with Estrogen Metabolism by Inhibition of 17β-Hydroxysteroid Dehydrogenases. International Journal of Molecular Sciences, 18(9), 2007. https://doi.org/10.3390/ijms18092007