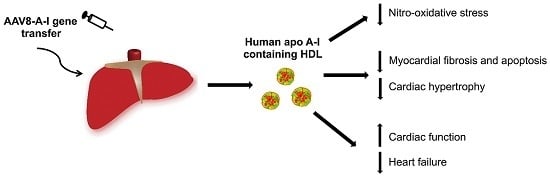
Selective HDL-Raising Human Apo A-I Gene Therapy Counteracts Cardiac Hypertrophy, Reduces Myocardial Fibrosis, and Improves Cardiac Function in Mice with Chronic Pressure Overload
Abstract
:1. Introduction
2. Results
2.1. Selective High-Density Lipoprotein (HDL)-Raising Gene Therapy Decreases Mortality after Transverse Aortic Constriction (TAC)
2.2. Atrial Hypertrophy, Lung Congestion, and Right Ventricular Hypertrophy in Control TAC Mice but Not in AAV8-A-I TAC Mice
2.3. AAV8-A-I Gene Transfer Counteracts Cardiac Hypertrophy, Increases Capillary Density and Relative Vascularity, and Reduces Interstitial and Perivascular Fibrosis after TAC
2.4. Selective HDL-Raising Gene Therapy Improves Cardiac Function in Both Sham Mice and TAC Mice
2.5. AAV8-A-I Gene Transfer Reduces Oxidative Stress and Myocardial Apoptosis after TAC
3. Discussion
4. Materials and Methods
4.1. In Vivo Experiments Evaluating the Effect of HDL-Raising Gene Transfer on the Development of Pressure Overload-Induced Cardiomyopathy
4.2. In Vivo Hemodynamic Measurements
4.3. Blood Sampling
4.4. Plasma Lipoprotein Analysis
4.5. Human Apo A-I Enzyme-Linked Immunosorbent Assay
4.6. Analysis of Lipid Peroxidation in Plasma
4.7. Histological and Morphometric Analysis
4.8. Statistical Analysis
Acknowledgments
Author Contributions
Conflicts of Interest
Abbreviations
| AAV | Adeno-associated viral |
| HDL | High-density lipoproteins |
| apo A-I | Apolipoprotein A-I |
| TAC | Transverse aortic constriction |
| LV | Left ventricle |
| TBARS | Thiobarbituric acid-reactive substances |
References
- Shah, A.S.; Tan, L.; Lu Long, J.; Davidson, W.S. The proteomic diversity of high density lipoproteins: Our emerging understanding of its importance in lipid transport and beyond. J. Lipid Res. 2013, 54, 2575–2585. [Google Scholar] [CrossRef] [PubMed]
- Gordts, S.C.; Singh, N.; Muthuramu, I.; de Geest, B. Pleiotropic effects of HDL: Towards new therapeutic areas for HDL-targeted interventions. Curr. Mol. Med. 2014, 14, 481–503. [Google Scholar] [CrossRef] [PubMed]
- Muthuramu, I.; Amin, R.; de Geest, B. New perspectives on biological HDL-targeted therapies. Expert Opin Biol. Ther. 2017, 17, 793–796. [Google Scholar] [CrossRef] [PubMed]
- Van Linthout, S.; Frias, M.; Singh, N.; de Geest, B. Therapeutic potential of HDL in cardioprotection and tissue repair. Handb. Exp. Pharmacol. 2015, 224, 527–565. [Google Scholar] [PubMed]
- Velagaleti, R.S.; Massaro, J.; Vasan, R.S.; Robins, S.J.; Kannel, W.B.; Levy, D. Relations of lipid concentrations to heart failure incidence: The Framingham heart study. Circulation 2009, 120, 2345–2351. [Google Scholar] [CrossRef] [PubMed]
- Van Linthout, S.; Spillmann, F.; Riad, A.; Trimpert, C.; Lievens, J.; Meloni, M.; Escher, F.; Filenberg, E.; Demir, O.; Li, J.; et al. Human apolipoprotein A-I gene transfer reduces the development of experimental diabetic cardiomyopathy. Circulation 2008, 117, 1563–1573. [Google Scholar] [CrossRef] [PubMed]
- Gordts, S.C.; van Craeyveld, E.; Muthuramu, I.; Singh, N.; Jacobs, F.; de Geest, B. Lipid lowering and HDL raising gene transfer increase endothelial progenitor cells, enhance myocardial vascularity, and improve diastolic function. PLoS ONE 2012, 7, e46849. [Google Scholar] [CrossRef] [PubMed]
- Gordts, S.C.; Muthuramu, I.; Nefyodova, E.; Jacobs, F.; van Craeyveld, E.; de Geest, B. Beneficial effects of selective HDL-raising gene transfer on survival, cardiac remodelling and cardiac function after myocardial infarction in mice. Gene Ther. 2013, 20, 1053–1061. [Google Scholar] [CrossRef] [PubMed]
- Muthuramu, I.; Singh, N.; Amin, R.; Nefyodova, E.; Debasse, M.; Van Horenbeeck, I.; Jacobs, F.; de Geest, B. Selective homocysteine-lowering gene transfer attenuates pressure overload-induced cardiomyopathy via reduced oxidative stress. J. Mol. Med. 2015, 93, 609–618. [Google Scholar] [CrossRef] [PubMed]
- Van Craeyveld, E.; Gordts, S.; Jacobs, F.; de Geest, B. Gene therapy to improve high-density lipoprotein metabolism and function. Curr. Pharm. Des. 2010, 16, 1531–1544. [Google Scholar] [CrossRef] [PubMed]
- De Geest, B.; van Linthout, S.; Collen, D. Sustained expression of human apo A-I following adenoviral gene transfer in mice. Gene Ther. 2001, 8, 121–127. [Google Scholar] [CrossRef] [PubMed]
- De Geest, B.R.; van Linthout, S.A.; Collen, D. Humoral immune response in mice against a circulating antigen induced by adenoviral transfer is strictly dependent on expression in antigen-presenting cells. Blood 2003, 101, 2551–2556. [Google Scholar] [CrossRef] [PubMed]
- Feng, Y.; Jacobs, F.; Van Craeyveld, E.; Lievens, J.; Snoeys, J.; Van Linthout, S.; de Geest, B. The impact of antigen expression in antigen-presenting cells on humoral immune responses against the transgene product. Gene Ther. 2010, 17, 288–293. [Google Scholar] [CrossRef] [PubMed]
- Gordts, S.C.; van Craeyveld, E.; Jacobs, F.; de Geest, B. Gene transfer for inherited metabolic disorders of the liver: Immunological challenges. Curr. Pharm. Des. 2011, 17, 2542–2549. [Google Scholar] [CrossRef] [PubMed]
- Feng, Y.; van Eck, M.; Van Craeyveld, E.; Jacobs, F.; Carlier, V.; Van Linthout, S.; Erdel, M.; Tjwa, M.; de Geest, B. Critical role of scavenger receptor-BI-expressing bone marrow-derived endothelial progenitor cells in the attenuation of allograft vasculopathy after human apo A-I transfer. Blood 2009, 113, 755–764. [Google Scholar] [CrossRef] [PubMed]
- Rubin, E.M.; Ishida, B.Y.; Clift, S.M.; Krauss, R.M. Expression of human apolipoprotein A-I in transgenic mice results in reduced plasma levels of murine apolipoprotein A-I and the appearance of two new high density lipoprotein size subclasses. Proc. Natl. Acad. Sci. USA 1991, 88, 434–438. [Google Scholar] [CrossRef] [PubMed]
- Golder-Novoselsky, E.; Forte, T.M.; Nichols, A.V.; Rubin, E.M. Apolipoprotein ai expression and high density lipoprotein distribution in transgenic mice during development. J. Biol. Chem. 1992, 267, 20787–20790. [Google Scholar] [PubMed]
- Feng, Y.; Van Craeyveld, E.; Jacobs, F.; Lievens, J.; Snoeys, J.; de Geest, B. Wild-type apo A-I and apo A-I(Milano) gene transfer reduce native and transplant arteriosclerosis to a similar extent. J. Mol. Med. 2009, 87, 287–297. [Google Scholar] [CrossRef] [PubMed]
- Perlino, E.; Cortese, R.; Ciliberto, G. The human α 1-antitrypsin gene is transcribed from two different promoters in macrophages and hepatocytes. EMBO J. 1987, 6, 2767–2771. [Google Scholar] [PubMed]
- Rouet, P.; Raguenez, G.; Ruminy, P.; Salier, J.P. An array of binding sites for hepatocyte nuclear factor 4 of high and low affinities modulates the liver-specific enhancer for the human α1-microglobulin/bikunin precursor. Biochem. J. 1998, 334, 577–584. [Google Scholar] [CrossRef] [PubMed]
- Rouet, P.; Raguenez, G.; Tronche, F.; Mfou′ou, V.; Salier, J.P. Hierarchy and positive/negative interplays of the hepatocyte nuclear factors HNF-1, -3 and -4 in the liver-specific enhancer for the human α-1-microglobulin/bikunin precursor. Nucleic Acids Res. 1995, 23, 395–404. [Google Scholar] [CrossRef] [PubMed]
- Rouet, P.; Raguenez, G.; Tronche, F.; Yaniv, M.; N′Guyen, C.; Salier, J.P. A potent enhancer made of clustered liver-specific elements in the transcription control sequences of human α 1-microglobulin/bikunin gene. J. Biol. Chem. 1992, 267, 20765–20773. [Google Scholar] [PubMed]
- Simonet, W.S.; Bucay, N.; Lauer, S.J.; Taylor, J.M. A far-downstream hepatocyte-specific control region directs expression of the linked human apolipoprotein E and C-I genes in transgenic mice. J. Biol. Chem. 1993, 268, 8221–8229. [Google Scholar] [PubMed]
- Van Linthout, S.; Spillmann, F.; Lorenz, M.; Meloni, M.; Jacobs, F.; Egorova, M.; Stangl, V.; de Geest, B.; Schultheiss, H.P.; Tschope, C. Vascular-protective effects of high-density lipoprotein include the downregulation of the angiotensin II type 1 receptor. Hypertension 2009, 53, 682–687. [Google Scholar] [CrossRef] [PubMed]
- Lin, L.; Gong, H.; Ge, J.; Jiang, G.; Zhou, N.; Li, L.; Ye, Y.; Zhang, G.; Ge, J.; Zou, Y. High density lipoprotein downregulates angiotensin II type 1 receptor and inhibits angiotensin II-induced cardiac hypertrophy. Biochem. Biophys. Res. Commun. 2011, 404, 28–33. [Google Scholar] [CrossRef] [PubMed]
- Lin, L.; Liu, X.; Xu, J.; Weng, L.; Ren, J.; Ge, J.; Zou, Y. High-density lipoprotein inhibits mechanical stress-induced cardiomyocyte autophagy and cardiac hypertrophy through angiotensin II type 1 receptor-mediated PI3K/Akt pathway. J. Cell. Mol. Med. 2015, 19, 1929–1938. [Google Scholar] [CrossRef] [PubMed]
- De Geest, B.; Stengel, D.; Landeloos, M.; Lox, M.; Le Gat, L.; Collen, D.; Holvoet, P.; Ninio, E. Effect of overexpression of human apo A-I in C57BL/6 and C57BL/6 apo E-deficient mice on 2 lipoprotein-associated enzymes, platelet-activating factor acetylhydrolase and paraoxonase. Comparison of adenovirus-mediated human apo A-I gene transfer and human apo A-I transgenesis. Arterioscler. Thromb. Vasc. Biol. 2000, 20, E68–E75. [Google Scholar] [PubMed]
- Shimizu, I.; Minamino, T. Physiological and pathological cardiac hypertrophy. J. Mol. Cell. Cardiol. 2016, 97, 245–262. [Google Scholar] [CrossRef] [PubMed]
- Hafstad, A.D.; Nabeebaccus, A.A.; Shah, A.M. Novel aspects of ros signalling in heart failure. Basic Res. Cardiol. 2013, 108, 359. [Google Scholar] [CrossRef] [PubMed]
- Den Ruijter, H.M.; Franssen, R.; Verkerk, A.O.; van Wijk, D.F.; Vaessen, S.F.; Holleboom, A.G.; Levels, J.H.; Opthof, T.; Sungnoon, R.; Stroes, E.S.; et al. Reconstituted high-density lipoprotein shortens cardiac repolarization. J. Am. Coll. Cardiol. 2011, 58, 40–44. [Google Scholar] [CrossRef] [PubMed]
- Wilcox, J.E.; Rosenberg, J.; Vallakati, A.; Gheorghiade, M.; Shah, S.J. Usefulness of electrocardiographic qt interval to predict left ventricular diastolic dysfunction. Am. J. Cardiol. 2011, 108, 1760–1766. [Google Scholar] [CrossRef] [PubMed]
- Ponikowski, P.; Voors, A.A.; Anker, S.D.; Bueno, H.; Cleland, J.G.; Coats, A.J.; Falk, V.; Gonzalez-Juanatey, J.R.; Harjola, V.P.; Jankowska, E.A.; et al. 2016 ESC guidelines for the diagnosis and treatment of acute and chronic heart failure: The task force for the diagnosis and treatment of acute and chronic heart failure of the european society of cardiology (ESC). Developed with the special contribution of the heart failure association (HFA) of the ESC. Eur. J. Heart Fail. 2016, 18, 891–975. [Google Scholar] [PubMed]
- Travers, J.G.; Kamal, F.A.; Robbins, J.; Yutzey, K.E.; Blaxall, B.C. Cardiac fibrosis: The fibroblast awakens. Circ. Res. 2016, 118, 1021–1040. [Google Scholar] [CrossRef] [PubMed]
- Kai, H.; Mori, T.; Tokuda, K.; Takayama, N.; Tahara, N.; Takemiya, K.; Kudo, H.; Sugi, Y.; Fukui, D.; Yasukawa, H.; et al. Pressure overload-induced transient oxidative stress mediates perivascular inflammation and cardiac fibrosis through angiotensin II. Hypertens. Res. 2006, 29, 711–718. [Google Scholar] [CrossRef] [PubMed]
- Spillmann, F.; de Geest, B.; Muthuramu, I.; Amin, R.; Miteva, K.; Pieske, B.; Tschope, C.; Van Linthout, S. Apolipoprotein A-I gene transfer exerts immunomodulatory effects and reduces vascular inflammation and fibrosis in ob/ob mice. J. Inflamm. 2016, 13, 25. [Google Scholar] [CrossRef] [PubMed]
- Spillmann, F.; Miteva, K.; Pieske, B.; Tschope, C.; van Linthout, S. High-density lipoproteins reduce endothelial-to-mesenchymal transition. Arterioscler. Thromb. Vasc. Biol. 2015, 35, 1774–1777. [Google Scholar] [CrossRef] [PubMed]
- Witt, H.; Schubert, C.; Jaekel, J.; Fliegner, D.; Penkalla, A.; Tiemann, K.; Stypmann, J.; Roepcke, S.; Brokat, S.; Mahmoodzadeh, S.; et al. Sex-specific pathways in early cardiac response to pressure overload in mice. J. Mol. Med. 2008, 86, 1013–1024. [Google Scholar] [CrossRef] [PubMed]
- Kararigas, G.; Fliegner, D.; Forler, S.; Klein, O.; Schubert, C.; Gustafsson, J.A.; Klose, J.; Regitz-Zagrosek, V. Comparative proteomic analysis reveals sex and estrogen receptor β effects in the pressure overloaded heart. J. Proteome Res. 2014, 13, 5829–5836. [Google Scholar] [CrossRef] [PubMed]
- Nathwani, A.C.; Tuddenham, E.G.; Rangarajan, S.; Rosales, C.; McIntosh, J.; Linch, D.C.; Chowdary, P.; Riddell, A.; Pie, A.J.; Harrington, C.; et al. Adenovirus-associated virus vector-mediated gene transfer in hemophilia B. N. Engl. J. Med. 2011, 365, 2357–2365. [Google Scholar] [CrossRef] [PubMed]
- Van Craeyveld, E.; Lievens, J.; Jacobs, F.; Feng, Y.; Snoeys, J.; de Geest, B. Apolipoprotein A-I and lecithin: Cholesterol acyltransferase transfer induce cholesterol unloading in complex atherosclerotic lesions. Gene Ther. 2009, 16, 757–765. [Google Scholar] [CrossRef] [PubMed]
- Van Craeyveld, E.; Gordts, S.C.; Nefyodova, E.; Jacobs, F.; de Geest, B. Regression and stabilization of advanced murine atherosclerotic lesions: A comparison of LDL lowering and HDL raising gene transfer strategies. J. Mol. Med. 2011, 89, 555–567. [Google Scholar] [CrossRef] [PubMed]
- Li, R.; Chao, H.; Ko, K.W.; Cormier, S.; Dieker, C.; Nour, E.A.; Wang, S.; Chan, L.; Oka, K. Gene therapy targeting LDL cholesterol but not HDL cholesterol induces regression of advanced atherosclerosis in a mouse model of familial hypercholesterolemia. J. Genet. Syndr. Gene Ther. 2011, 2, 106. [Google Scholar] [CrossRef] [PubMed]
- De Geest, B.; Zhao, Z.; Collen, D.; Holvoet, P. Effects of adenovirus-mediated human apo A-I gene transfer on neointima formation after endothelial denudation in apo E-deficient mice. Circulation 1997, 96, 4349–4356. [Google Scholar] [CrossRef] [PubMed]
- Tangirala, R.K.; Tsukamoto, K.; Chun, S.H.; Usher, D.; Pure, E.; Rader, D.J. Regression of atherosclerosis induced by liver-directed gene transfer of apolipoprotein A-I in mice. Circulation 1999, 100, 1816–1822. [Google Scholar] [CrossRef] [PubMed]
- Jacobs, F.; Snoeys, J.; Feng, Y.; Van Craeyveld, E.; Lievens, J.; Armentano, D.; Cheng, S.H.; De Geest, B. Direct comparison of hepatocyte-specific expression cassettes following adenoviral and nonviral hydrodynamic gene transfer. Gene Ther. 2008, 15, 594–603. [Google Scholar] [CrossRef] [PubMed]
- Lock, M.; Alvira, M.; Vandenberghe, L.H.; Samanta, A.; Toelen, J.; Debyser, Z.; Wilson, J.M. Rapid, simple, and versatile manufacturing of recombinant adeno-associated viral vectors at scale. Hum. Gene Ther. 2010, 21, 1259–1271. [Google Scholar] [CrossRef] [PubMed]
- Muthuramu, I.; Amin, R.; Postnov, A.; Mishra, M.; Jacobs, F.; Gheysens, O.; van Veldhoven, P.P.; de Geest, B. Coconut oil aggravates pressure overload-induced cardiomyopathy without inducing obesity, systemic insulin resistance, or cardiac steatosis. Int. J. Mol. Sci. 2017, 18, 1565. [Google Scholar] [CrossRef] [PubMed]
- Weiss, J.L.; Frederiksen, J.W.; Weisfeldt, M.L. Hemodynamic determinants of the time-course of fall in canine left ventricular pressure. J. Clin. Investig. 1976, 58, 751–760. [Google Scholar] [CrossRef] [PubMed]
- McLaren, D.G.; Wang, S.P.; Stout, S.J.; Xie, D.; Miller, P.L.; Mendoza, V.; Rosa, R.; Castro-Perez, J.; Previs, S.F.; Johns, D.G.; et al. Tracking fatty acid kinetics in distinct lipoprotein fractions in vivo: A novel high-throughput approach for studying dyslipidemia in rodent models. J. Lipid Res. 2013, 54, 276–281. [Google Scholar] [CrossRef] [PubMed]
- Van Craeyveld, E.; Jacobs, F.; Gordts, S.C.; de Geest, B. Low-density lipoprotein receptor gene transfer in hypercholesterolemic mice improves cardiac function after myocardial infarction. Gene Ther. 2012, 19, 860–871. [Google Scholar] [CrossRef] [PubMed]
- Junqueira, L.C.; Bignolas, G.; Brentani, R.R. Picrosirius staining plus polarization microscopy, a specific method for collagen detection in tissue sections. Histochem. J. 1979, 11, 447–455. [Google Scholar] [CrossRef] [PubMed]




| Parameter | Control Sham | AAV8-A-I Sham | Control TAC | AAV8-A-I TAC |
|---|---|---|---|---|
| Number of mice | 10 | 10 | 10 | 10 |
| Total cholesterol | 5.56 ± 0.33 | 5.96 ± 0.21 | 5.84 ± 0.34 | 6.01 ± 0.23 |
| Non-HDL cholesterol | 4.42 ± 0.33 | 4.28 ± 0.25 | 4.70 ± 0.30 | 4.36 ± 0.25 |
| HDL cholesterol | 1.14 ± 0.05 | 1.68 ± 0.08 °°° | 1.14 ± 0.07 | 1.66 ± 0.09 *** |
| Parameter | Control Sham | AAV8-A-I Sham | Control TAC | AAV8-A-I TAC |
|---|---|---|---|---|
| Number of mice | 10 | 10 | 11 | 9 |
| Body weight (g) | 28.8 ± 0.6 | 29.7 ± 0.5 | 27.5 ± 0.3 | 29.2 ± 0.9 |
| Heart weight (mg) | 135 ± 3 | 133 ± 7 | 224 ±18 §§§ | 201 ± 7 §§§ |
| Heart weight/tibia length (mg/mm) | 7.78 ± 0.12 | 7.66 ± 0.40 | 12.7 ± 1.0 §§§ | 11.5 ± 0.4 §§§ |
| Left ventricular weight (mg) | 86.9 ± 2.6 | 86.9 ± 5.0 | 163 ± 15 §§§ | 143 ± 8 §§ |
| Right ventricular weight (mg) | 22.7 ± 0.8 | 24.2 ± 0.9 | 30.1 ± 2.0 §§§ | 27.9 ± 2.7 |
| Atrial weight (mg) | 9.10 ± 0.85 | 8.10 ± 0.92 | 18.6 ± 2.3 §§§ | 10.1 ± 0.7 * |
| Lung weight (mg) | 152 ± 3 | 151 ± 2 | 171 ± 8 § | 149 ± 3 * |
| Tibia length (mm) | 17.4 ± 0.1 | 17.3 ± 0.1 | 17.5 ± 0.1 | 17.6 ± 0.1 |
| Parameter | Control Sham | AAV8-A-I Sham | Control TAC | AAV8-A-I TAC |
|---|---|---|---|---|
| Number of mice | 12 | 12 | 39 | 24 |
| Left ventricular wall area (mm2) | 10.4 ± 0.3 | 9.93 ± 0.30 | 14.2 ± 0.3 §§§ | 13.1 ± 0.4 §§§,* |
| Septal wall thickness (µm) | 1110 ± 20 | 990 ± 20 | 1430 ± 30 §§§ | 1190 ± 30 §§,*** |
| Anterior wall thickness (µm) | 1130 ± 30 | 1090 ± 20 | 1420 ± 30 §§§ | 1270 ± 30 §§,* |
| Cardiomyocyte cross-sectional area (µm2) | 228 ± 8 | 228 ± 12 | 502 ± 13 §§§ | 433 ± 21 §§§,** |
| Cardiomyocyte density (number/mm2) | 4530 ± 180 | 4400 ± 160 | 2240 ± 60 §§§ | 2680 ± 120 §§,* |
| Capillary density (number/mm2) | 6400 ± 170 | 6020 ± 290 | 5330 ± 150 §§ | 5910 ± 220 * |
| Relative vascularity (µm−2) | 0.00632 ± 0.00020 | 0.00610 ± 0.00026 | 0.00487 ± 0.00014 §§§ | 0.00536 ± 0.00020 §,* |
| Interstitial fibrosis (%) | 0.939 ± 0.073 | 0.892 ± 0.083 | 9.98 ± 0.70 §§§ | 5.46 ± 0.41 §§,** |
| Perivascular fibrosis (ratio) | 0.291 ± 0.010 | 0.287 ± 0.019 | 0.880 ± 0.022 §§§ | 0.562 ± 0.031 §,*** |
| Parameter | Control Sham | AAV8-A-I Sham | Control TAC | AAV8-A-I TAC |
|---|---|---|---|---|
| Number of mice | 10 | 12 | 29 | 16 |
| LEFT VENTRICLE | ||||
| Peak systolic pressure (mm Hg) | 100 ± 2 | 102 ± 3 | 175 ± 5 §§§ | 177 ± 6 §§§ |
| End-diastolic pressure (mm Hg) | 2.91 ± 0.32 | 2.63 ± 0.34 | 4.04 ± 0.48 | 2.12 ± 0.39 * |
| dP/dtmax (mm Hg/ms) | 11.9 ± 1.1 | 12.5 ± 0.7 | 10.2 ± 0.3 | 12.1 ± 0.4 ** |
| dP/dtmin (mmHg/ms) | −9.32 ± 0.70 | −11.1 ± 0.3 ° | −10.3 ± 0.5 | −12.1 ± 0.4 * |
| Tau (ms) | 6.39 ± 0.43 | 5.47 ± 0.12 ° | 6.32 ± 0.27 | 5.35 ± 0.14 * |
| Heart rate (bpm) | 559 ± 19 | 601 ± 14 | 597 ± 9 | 629 ± 8 |
| AORTA | ||||
| Systolic pressure (mm Hg) | 99.6 ± 2.5 | 101 ± 5 | 174 ± 6 §§§ | 177 ± 7 §§§ |
| Diastolic pressure (mm Hg) | 61.9 ± 1.9 | 63.5 ± 2.0 | 58.2 ± 2.9 | 64.4 ± 3.6 |
| Mean pressure (mm Hg) | 79.8 ± 2.1 | 81.1 ± 2.3 | 98.8 ± 2.9 §§ | 105 ± 3 §§§ |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Amin, R.; Muthuramu, I.; Aboumsallem, J.P.; Mishra, M.; Jacobs, F.; De Geest, B. Selective HDL-Raising Human Apo A-I Gene Therapy Counteracts Cardiac Hypertrophy, Reduces Myocardial Fibrosis, and Improves Cardiac Function in Mice with Chronic Pressure Overload. Int. J. Mol. Sci. 2017, 18, 2012. https://doi.org/10.3390/ijms18092012
Amin R, Muthuramu I, Aboumsallem JP, Mishra M, Jacobs F, De Geest B. Selective HDL-Raising Human Apo A-I Gene Therapy Counteracts Cardiac Hypertrophy, Reduces Myocardial Fibrosis, and Improves Cardiac Function in Mice with Chronic Pressure Overload. International Journal of Molecular Sciences. 2017; 18(9):2012. https://doi.org/10.3390/ijms18092012
Chicago/Turabian StyleAmin, Ruhul, Ilayaraja Muthuramu, Joseph Pierre Aboumsallem, Mudit Mishra, Frank Jacobs, and Bart De Geest. 2017. "Selective HDL-Raising Human Apo A-I Gene Therapy Counteracts Cardiac Hypertrophy, Reduces Myocardial Fibrosis, and Improves Cardiac Function in Mice with Chronic Pressure Overload" International Journal of Molecular Sciences 18, no. 9: 2012. https://doi.org/10.3390/ijms18092012
APA StyleAmin, R., Muthuramu, I., Aboumsallem, J. P., Mishra, M., Jacobs, F., & De Geest, B. (2017). Selective HDL-Raising Human Apo A-I Gene Therapy Counteracts Cardiac Hypertrophy, Reduces Myocardial Fibrosis, and Improves Cardiac Function in Mice with Chronic Pressure Overload. International Journal of Molecular Sciences, 18(9), 2012. https://doi.org/10.3390/ijms18092012